Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Mitchell, Richard S.
1980.
Who's Who in Mineral Names.
Rocks & Minerals,
Vol. 55,
Issue. 1,
p.
18.
Mitchell, Richard S.
1981.
Who's Who in Mineral Names.
Rocks & Minerals,
Vol. 56,
Issue. 6,
p.
250.
Segeler, Curt G.
Kampf, Anthony R.
Ulrich, William
and
Whitmore, Robert W.
1981.
Phosphate Minerals of the: Palermo No.1 Pegmatite.
Rocks & Minerals,
Vol. 56,
Issue. 5,
p.
197.
Cares, Janet W.
1990.
Minerals of New HampshireA Checklist.
Rocks & Minerals,
Vol. 65,
Issue. 4,
p.
297.
Hawthorne, Frank C.
1997.
Modular Aspects of Minerals.
p.
373.
Hawthorne, Frank C.
1998.
Structure and chemistry of phosphate minerals.
Mineralogical Magazine,
Vol. 62,
Issue. 2,
p.
141.
Hawthorne, Frank C.
Cooper, Mark A.
and
Paar, Werner H.
2008.
The crystal structure of braithwaiteite.
Journal of Coordination Chemistry,
Vol. 61,
Issue. 1,
p.
15.
Grey, I. E.
Mumme, W. G.
Neville, S. M.
Wilson, N. C.
and
Birch, W. D.
2010.
Jahnsite—whiteite solid solutions and associated minerals in the phosphate pegmatite at Hagendorf-Süd, Bavaria, Germany.
Mineralogical Magazine,
Vol. 74,
Issue. 6,
p.
969.
Capitelli, Francesco
Chita, Giuseppe
Cavallo, Andrea
Bellatreccia, Fabio
and
Della Ventura, Giancarlo
2011.
Crystal structure of whiteite-(CaFeMg) from Crosscut Creek, Canada.
Zeitschrift für Kristallographie,
Vol. 226,
Issue. 9,
p.
731.
Grey, I. E.
Macrae, C. M.
Keck, E.
and
Birch, W. D.
2012.
Aluminium-bearing strunzite derived from jahnsite at the Hagendorf-Süd pegmatite, Germany.
Mineralogical Magazine,
Vol. 76,
Issue. 5,
p.
1165.
Yakovenchuk, V. N.
Keck, E.
Krivovichev, S. V.
Pakhomovsky, Y. A.
Selivanova, E. A.
Mikhailova, J. A.
Chernyatieva, A. P.
and
Yu. Ivanyuk, G.
2012.
Whiteite-(CaMnMn), CaMnMn2Al2[PO4]4(OH)2·8H2O, a new mineral from the Hagendorf-Süd granitic pegmatite, Germany.
Mineralogical Magazine,
Vol. 76,
Issue. 7,
p.
2761.
Frost, Ray L.
Scholz, Ricardo
López, Andrés
and
Xi, Yunfei
2014.
A vibrational spectroscopic study of the phosphate mineral whiteite CaMn++Mg2Al2(PO4)4(OH)2·8(H2O).
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy,
Vol. 124,
Issue. ,
p.
243.
Mills, S. J.
and
Grey, I. E.
2015.
Nomenclature for the laueite supergroup.
Mineralogical Magazine,
Vol. 79,
Issue. 2,
p.
243.
Grey, I. E.
Keck, E.
Mumme, W. G.
MacRae, C. M.
Price, J. R.
Glenn, A. M.
and
Davidson, C. J.
2015.
Crystallographic ordering of aluminium in laueite at Hagendorf-Süd.
Mineralogical Magazine,
Vol. 79,
Issue. 2,
p.
309.
Atencio, Daniel
2015.
The discovery of new mineral species and type minerals from Brazil.
Brazilian Journal of Geology,
Vol. 45,
Issue. 1,
p.
143.
Zaarour, Moussa
Perez, Olivier
Boullay, Philippe
Martens, Jörn
Mihailova, Boriana
Karaghiosoff, Konstantin
Palatinus, Lukáš
and
Mintova, Svetlana
2017.
Synthesis of new cobalt aluminophosphate framework by opening a cobalt methylphosphonate layered material.
CrystEngComm,
Vol. 19,
Issue. 34,
p.
5100.
Elliott, Peter
and
Willis, Anthony C.
2019.
Whiteite-(mnmnmg), a New Jahnsite-Group Mineral from Iron Monarch, South Australia: Description and Crystal Structure.
The Canadian Mineralogist,
Vol. 57,
Issue. 2,
p.
215.
Vignola, Pietro
Hatert, Frédéric
Rotiroti, Nicola
Nestola, Fabrizio
Risplendente, Andrea
and
Vanini, Francesco
2019.
Jahnsite-(mnmnfe), Mn2+Mn2+Fe2+2Fe3+2(PO4)4(OH)2·8H2O, a New Phosphate Mineral from the Malpensata Pegmatite, Olgiasca, Colico Municipality, Lecco Province, Italy.
The Canadian Mineralogist,
Vol. 57,
Issue. 2,
p.
225.
Grey, Ian E.
Keck, Erich
Kampf, Anthony R.
MacRae, Colin M.
Cashion, John D.
and
Glenn, A. Matt
2020.
Jahnsite-(CaMnZn) from the Hagendorf-Süd pegmatite, Oberpfalz, Bavaria, and structural flexibility of jahnsite-group minerals.
Mineralogical Magazine,
Vol. 84,
Issue. 4,
p.
547.
Grey, Ian E.
Smith, Jason B.
Kampf, Anthony R.
Mumme, W. Gus
MacRae, Colin M.
Riboldi-Tunnicliffe, Alan
Boer, Stephanie
Glenn, Alexander M.
and
Gable, Robert W.
2021.
Whiteite-(MnMnMn), a new jahnsite-group mineral species from the Foote mine, North Carolina, USA, and chemical pressure effects in jahnsite-group minerals..
Mineralogical Magazine,
Vol. 85,
Issue. 6,
p.
862.
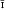 or P1, a 6·70(4) Å, b8·85(4) Å, c 6·54(3) Å, α 92·1(2)°, β 110·2(2)°, γ 93·2(2)°, Z = 1 for composition
or P1, a 6·70(4) Å, b8·85(4) Å, c 6·54(3) Å, α 92·1(2)°, β 110·2(2)°, γ 93·2(2)°, Z = 1 for composition  .
.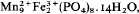 from Pala, California, is shown to be an intimate mixture of hureaulite and jahnsite on the basis of calculated and observed powder patterns and on reinterpretation of the original chemical analysis published by Schaller (1912). It is a breakdown product resulting from oxidation of Fe2+ in the original hureaulite (‘palaite’) along with further aquation followed by fine-grained recrystallization. The reaction proposed is:
from Pala, California, is shown to be an intimate mixture of hureaulite and jahnsite on the basis of calculated and observed powder patterns and on reinterpretation of the original chemical analysis published by Schaller (1912). It is a breakdown product resulting from oxidation of Fe2+ in the original hureaulite (‘palaite’) along with further aquation followed by fine-grained recrystallization. The reaction proposed is: