Published online by Cambridge University Press: 29 June 2022
Grandviewite is redefined on the basis of a reinvestigation of the holotype specimen from the Grandview mine, Arizona, USA, and additional specimens found in the Restauradora vein at the Capillitas mine, northwestern Argentina. Grandviewite from the Capillitas mine forms globular masses up to a couple of millimetres in diameter, formed by very thin platy to acicular lath-like crystals, greenish-pale blue in colour, pleochroic (X = colourless, Y = very pale blue and Z = greenish-pale blue), with pale blue streak and silky to a satiny lustre. Results of an electron-microprobe study and crystal-structure determination lead to the new ideal formula Cu3Al2(SO4)(OH)10⋅H2O, which requires (in wt.%) CuO 45.13, Al2O3 19.28, SO3 15.14, and H2O 20.45, total 100.00. Its empirical formulas are (Cu2.96Mn0.01)Σ2.97Al2.03(SO4)0.97(SiO4)0.03(AsO4)0.01(OH)9.97Cl0.01⋅H2O (Grandview mine) and Cu2.97(Al2.03Fe0.01)Σ2.04(SO4)0.95(SiO4)0.03(AsO4)0.01(PO4)0.01(OH)9.97Cl0.01⋅H2O (Capillitas mine). Grandviewite is triclinic, P$\bar{1}$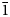 , with unit-cell parameters refined from powder X-ray diffraction data: a = 5.713(2), b = 10.1374(8), c = 10.9791(9) Å, α = 72.240(6)°, β = 82.79(2)°, γ = 86.07(2)°, V = 600.5(3) Å3 and Z = 2 (Grandview mine); and a = 5.749(3), b = 10.1388(13), c = 10.9656(16) Å, α = 72.344(1)°, β = 82.83(4)°, γ = 86.77(3)°, V = 604.2(3) Å3 and Z = 2 (Capillitas mine). The crystal structure of grandviewite from the Capillitas mine was solved by 3-dimensional electron diffraction analysis (R(obs)/wR(obs) = 0.1304/0.1316 for 6401/31007 observed reflections with I ≥ 3σ(I). Grandviewite contains infinite AlO6–Cu1–AlO6 slabs along a connected on both ends to Cu2 [4 + 1]–SO4 chains. The SO4 tetrahedra form a disordered chain along a connected to the Cu2 pyramids on one side and are otherwise stabilised by strong hydrogen bonds to surrounding units. The Raman and infrared spectra for samples from both occurrences are identical. The redefinition (new chemical formula and triclinic symmetry) has been approved by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (number 21-K).
, with unit-cell parameters refined from powder X-ray diffraction data: a = 5.713(2), b = 10.1374(8), c = 10.9791(9) Å, α = 72.240(6)°, β = 82.79(2)°, γ = 86.07(2)°, V = 600.5(3) Å3 and Z = 2 (Grandview mine); and a = 5.749(3), b = 10.1388(13), c = 10.9656(16) Å, α = 72.344(1)°, β = 82.83(4)°, γ = 86.77(3)°, V = 604.2(3) Å3 and Z = 2 (Capillitas mine). The crystal structure of grandviewite from the Capillitas mine was solved by 3-dimensional electron diffraction analysis (R(obs)/wR(obs) = 0.1304/0.1316 for 6401/31007 observed reflections with I ≥ 3σ(I). Grandviewite contains infinite AlO6–Cu1–AlO6 slabs along a connected on both ends to Cu2 [4 + 1]–SO4 chains. The SO4 tetrahedra form a disordered chain along a connected to the Cu2 pyramids on one side and are otherwise stabilised by strong hydrogen bonds to surrounding units. The Raman and infrared spectra for samples from both occurrences are identical. The redefinition (new chemical formula and triclinic symmetry) has been approved by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (number 21-K).
Associate Editor: Charles A Geiger