Published online by Cambridge University Press: 05 July 2018
Fluoro-aluminoleakeite, ideally 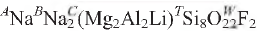 , is a new mineral of the amphibole group from Norra Kärr, Sweden (IMA-CNMMNC 2009-012). It occurs in a proterozoic alkaline intrusion that mainly comprises a fine-grained schistose agpaitic nepheline-syenite (grennaite). Fluoro- aluminoleakeite occurs as isolated prismatic crystals 0.10–2 mm long in a syenitic matrix. Crystals are light greenish-blue with a greenish-blue streak. It is brittle, has a Mohs hardness of 6 and a splintery fracture; it is non-fluorescent with perfect {110} cleavage, no observable parting, and has a calculated density of 3.14 g cm–3. In plane-polarized light, it is pleochroic, X = pale green, Y = dark green, Z = pale green; X ^ a = 62.9° (in β obtuse), Y || b. Fluoro-aluminoleakeite is biaxial negative, α = 1.632(1), β = 1.638(1), γ = 1.643(1); 2Vobs. = 98.0(4)°, 2Vcalc. = 95.5°.M
, is a new mineral of the amphibole group from Norra Kärr, Sweden (IMA-CNMMNC 2009-012). It occurs in a proterozoic alkaline intrusion that mainly comprises a fine-grained schistose agpaitic nepheline-syenite (grennaite). Fluoro- aluminoleakeite occurs as isolated prismatic crystals 0.10–2 mm long in a syenitic matrix. Crystals are light greenish-blue with a greenish-blue streak. It is brittle, has a Mohs hardness of 6 and a splintery fracture; it is non-fluorescent with perfect {110} cleavage, no observable parting, and has a calculated density of 3.14 g cm–3. In plane-polarized light, it is pleochroic, X = pale green, Y = dark green, Z = pale green; X ^ a = 62.9° (in β obtuse), Y || b. Fluoro-aluminoleakeite is biaxial negative, α = 1.632(1), β = 1.638(1), γ = 1.643(1); 2Vobs. = 98.0(4)°, 2Vcalc. = 95.5°.M
Fluoro-aluminoleakeite is monoclinic, space group C2/m, a = 9.7043(5) Å, b = 17.7341(8) Å, c = 5.2833(3) Å, β = 104.067(4)°, V = 882.0(2) Å3, Z = 2. The eight strongest X-ray diffraction lines in the powder-diffraction pattern are [d in Å, (I), (hkl)]: 2.687, (100), (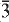 31, 151); 4.435, (80), (021, 040); 3.377, (80), (131); 2.527, (60), (
31, 151); 4.435, (80), (021, 040); 3.377, (80), (131); 2.527, (60), ( 02); 8.342, (50), (110); 3.096, (40), (310); 2.259, (40), (
02); 8.342, (50), (110); 3.096, (40), (310); 2.259, (40), (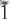 71,
71, 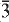 12) and 2.557, (30), (002, 061). Analysis, by a combination of electron microprobe and crystal-structure refinement, gives SiO2 58.61, Al2O3 7.06, TiO2 0.32, FeO 3.27, Fe2O3 6.05, MgO 8.61, MnO 0.73, ZnO 0.43, CaO 0.05, Na2O 9.90, K2O 2.43, Li2O 1.62, F 3.37, H2Ocalc. 0.50, sum 101.08 wt.%. The formula unit, calculated on the basis of 24 (O,OH,F,Cl) p.f.u. with (OH) + F = 2 a.p.f.u., is A(Na0.65
12) and 2.557, (30), (002, 061). Analysis, by a combination of electron microprobe and crystal-structure refinement, gives SiO2 58.61, Al2O3 7.06, TiO2 0.32, FeO 3.27, Fe2O3 6.05, MgO 8.61, MnO 0.73, ZnO 0.43, CaO 0.05, Na2O 9.90, K2O 2.43, Li2O 1.62, F 3.37, H2Ocalc. 0.50, sum 101.08 wt.%. The formula unit, calculated on the basis of 24 (O,OH,F,Cl) p.f.u. with (OH) + F = 2 a.p.f.u., is A(Na0.65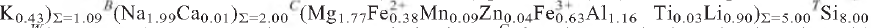 O22W(F1.47OH0.53)Σ=2.00. Crystal-structure analysis shows CLi to be completely ordered at the M(3) site, and provided reliable site populations. Fluoro-aluminoleakeite is related to the end-member leakeite,
O22W(F1.47OH0.53)Σ=2.00. Crystal-structure analysis shows CLi to be completely ordered at the M(3) site, and provided reliable site populations. Fluoro-aluminoleakeite is related to the end-member leakeite, 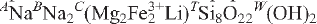 , by the substitutions CFe3+ → CAl and WF → W(OH).
, by the substitutions CFe3+ → CAl and WF → W(OH).