Published online by Cambridge University Press: 05 July 2018
Decrespignyite-(Y) is a new copper yttrium rare earth carbonate chloride hydrate from the Paratoo copper mine, near Yunta, Olary district, South Australia. Decrespignyite-(Y) occurs as blue crusts, coatings and fillings in thin fissures on the slatey country rock. Individual pseudohexagonal platelets are typically 10–50 µm in maximum dimension and are often curved. Associated minerals include caysichite-(Y), donnayite-(Y), malachite and kamphaugite-(Y). Electron microprobe and CHN analyses gave: Y2O3 42.2; La2O3 0.1; Pr2O3 0.1; Nd2O3 1.3; Sm2O3 1.0; Gd2O3 4; Tb2O3 0.4; Dy2O3 3.7; Ho2O3 2.6; Er2O3 2.5; CaO 0.5; CuO 10.9; Cl 3.0; CO2 19.8; H2O 10.8, yielding an empirical formula of (Y3.08Gd0.22Dy0.16Ho0.11Er0.10Nd0.06Sm0.05Tb0.02La0.02Pr0.01Ca0.08)∑3.91Cu1.12(CO3)3.70-Cl0.7(OH)5.79·2.4H2O. The simplified formula is (Y,REE)4Cu(CO3)4Cl(OH)5·2H2O. The mineral is royal blue to turquoise-blue in colour, transparent, with a pearly to vitreous lustre and a pale blue streak. No cleavage was observed but the morphology suggests that cleavage would be on [010]. The Mohs' hardness is estimated to be 4. The strongest lines in the X-ray powder pattern are {dobs (Iobs) (hkl)} 22.79 (30) (010); 7.463 (30) (001); 7.086 (50) (011); 6.241 (100) (021); 4.216 (30) (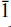 12); 3.530 (40) (022); 3.336 (30) (032); 2.143 (30) (222,
12); 3.530 (40) (022); 3.336 (30) (032); 2.143 (30) (222, 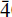 01). The powder diffraction pattern was indexed on a monoclinic cell with a = 8.899(6), b = 22.77(2), c = 8.589(6)Å, β = 120.06(5)°, V = 1506.3(7) Å3 and Z = 4. The structure of the new mineral could not be determined but powder diffraction data indicate the space group is P2, Pm or P2/m. The measured density is 3.64(2) g/cm3 and the calculated density is 3.645 g/cm3. Decrespignyite-(Y) is biaxial negative with α = 1.604(4) and γ = 1.638(3) with β very close to γ pleochroism is medium strong; X very pale bluish, Y and Z bluish (with greenish tint). Decrespignyite-(Y) is a supergene mineral which precipitated from mildly basic carbonated ground waters. The mineral is named after Robert Champion de Crespigny, a prominent figure in the Australian mining industry and chancellor of the University of Adelaide.
01). The powder diffraction pattern was indexed on a monoclinic cell with a = 8.899(6), b = 22.77(2), c = 8.589(6)Å, β = 120.06(5)°, V = 1506.3(7) Å3 and Z = 4. The structure of the new mineral could not be determined but powder diffraction data indicate the space group is P2, Pm or P2/m. The measured density is 3.64(2) g/cm3 and the calculated density is 3.645 g/cm3. Decrespignyite-(Y) is biaxial negative with α = 1.604(4) and γ = 1.638(3) with β very close to γ pleochroism is medium strong; X very pale bluish, Y and Z bluish (with greenish tint). Decrespignyite-(Y) is a supergene mineral which precipitated from mildly basic carbonated ground waters. The mineral is named after Robert Champion de Crespigny, a prominent figure in the Australian mining industry and chancellor of the University of Adelaide.