Article contents
Cámaraite, Ba3NaTi4(Fe2+,Mn)8(Si2O7)4O4(OH,F)7. II. The crystal structure and crystal chemistry of a new group-II Ti-disilicate mineral
Published online by Cambridge University Press: 05 July 2018
Abstract
Cámaraite — ideally Ba3NaTi4Fe82+(Si2O7)4O4(OH)4F3 — is triclinic, space group C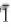 , a = 10.6965(7) Å, b = 13.7861(9) Å, c = 21.478(2) Å, α = 99.345(1)°, β = 92.315(2)°, γ = 89.993(2)°, V = 3122.6(4) Å3, Z = 4, Dcalc. = 4.018 g cm–3, from the Verkhnee Espe alkaline deposit, Akjailyautas Mountains, Kazakhstan, has been solved and refined to R1 5.87% on the basis of 6682 unique reflections (Fo >4σF). The crystal structure of cámaraite can be described as a combination of a TS block and an intermediate (I) block. The TS (titanium silicate) block consists of HOH sheets (H-heteropolyhedral, O-octahedral), and is characterized by a minimal cell based on translation vectors t1 and t2, with t1 ~5.5 and t2 ~7 Å and t1 ^ t2 close to 90°. We describe the crystal structure of cámaraite using a double minimal cell, with 2t1 and 2t2 translations. In the O sheet, there are eight [6]-coordinated MO sites occupied mainly by Fe2+ and Mn, with minor Fe3+, Mg, Zr, Ca and Zn with <MO–ϕ> = 2.185 Å. Eight MO sites give, ideally Fe82+ p.f.u. In the H sheet, there are four [6]-coordinated MH sites occupied almost solely by Ti (Ti = 4 a.p.f.u.), with <MH–ϕ= = 1.963 Å, and eight [4]-coordinated Si sites occupied solely by Si, with <Si—0> = 1.621 Å. The topology of the TS block is as in Group II of the Ti-disilicates (Ti = 2 a.p.f.u. per minimal cell) in the structure hierarchy of Sokolova (2006). There are six peripheral (P) sites, four [8–12]-coordinated Ba-dominant AP sites, giving ideally 3 Ba p.f.u., and two [10]-coordinated Na-dominant BP sites, giving ideally 1 Na p.f.u. There are two I blocks: the I1 block is a layer of Ba atoms (two AP sites); the I2 block is a layer of Ba (two AP sites) and Na atoms (two BP sites). Along c, there are two types of linkage of TS blocks: (1) TS blocks link via AP cations which constitute the I1 block, and (2) TS blocks link via common vertices of MH octahedra (as in astrophyllite-group minerals) and AP and BP cations which constitute the I2 block. Cámaraite is the only mineral of Group II with two types of linkage of TS blocks and two types of I blocks in its structure. The relation of cámaraite to the Group-II minerals is discussed.
, a = 10.6965(7) Å, b = 13.7861(9) Å, c = 21.478(2) Å, α = 99.345(1)°, β = 92.315(2)°, γ = 89.993(2)°, V = 3122.6(4) Å3, Z = 4, Dcalc. = 4.018 g cm–3, from the Verkhnee Espe alkaline deposit, Akjailyautas Mountains, Kazakhstan, has been solved and refined to R1 5.87% on the basis of 6682 unique reflections (Fo >4σF). The crystal structure of cámaraite can be described as a combination of a TS block and an intermediate (I) block. The TS (titanium silicate) block consists of HOH sheets (H-heteropolyhedral, O-octahedral), and is characterized by a minimal cell based on translation vectors t1 and t2, with t1 ~5.5 and t2 ~7 Å and t1 ^ t2 close to 90°. We describe the crystal structure of cámaraite using a double minimal cell, with 2t1 and 2t2 translations. In the O sheet, there are eight [6]-coordinated MO sites occupied mainly by Fe2+ and Mn, with minor Fe3+, Mg, Zr, Ca and Zn with <MO–ϕ> = 2.185 Å. Eight MO sites give, ideally Fe82+ p.f.u. In the H sheet, there are four [6]-coordinated MH sites occupied almost solely by Ti (Ti = 4 a.p.f.u.), with <MH–ϕ= = 1.963 Å, and eight [4]-coordinated Si sites occupied solely by Si, with <Si—0> = 1.621 Å. The topology of the TS block is as in Group II of the Ti-disilicates (Ti = 2 a.p.f.u. per minimal cell) in the structure hierarchy of Sokolova (2006). There are six peripheral (P) sites, four [8–12]-coordinated Ba-dominant AP sites, giving ideally 3 Ba p.f.u., and two [10]-coordinated Na-dominant BP sites, giving ideally 1 Na p.f.u. There are two I blocks: the I1 block is a layer of Ba atoms (two AP sites); the I2 block is a layer of Ba (two AP sites) and Na atoms (two BP sites). Along c, there are two types of linkage of TS blocks: (1) TS blocks link via AP cations which constitute the I1 block, and (2) TS blocks link via common vertices of MH octahedra (as in astrophyllite-group minerals) and AP and BP cations which constitute the I2 block. Cámaraite is the only mineral of Group II with two types of linkage of TS blocks and two types of I blocks in its structure. The relation of cámaraite to the Group-II minerals is discussed.
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 2009
Footnotes
Permanent address: CNR — Istituto di Geoscienze e Georisorse, Unita di Pavia, Via Ferrata 1, I-27100 Pavia, Italy
References
- 18
- Cited by


