Published online by Cambridge University Press: 05 July 2018
Bendadaite, ideally Fe2+Fe23+ (AsO4)2(OH)2·4H2O, is a new member of the arthurite group. It was found as a weathering product of arsenopyrite on a single hand specimen from the phosphate pegmatite Bendada, central Portugal (type locality). Co-type locality is the granite pegmatite of Lavra do Almerindo (Almerindo mine), Linópolis, Divino das Laranjeiras county, Minas Gerais, Brazil. Further localities are the Veta Negra mine, Copiapó province, Chile; Oumlil-East, Bou Azzer district, Morocco; and Pira Inferida yard, Fenugu Sibiri mine, Gonnosfanadiga, Medio Campidano Province, Sardinia, Italy.
Type bendadaite occurs as blackish green to dark brownish tufts (<0.1 mm long) and flattened radiating aggregates, in intimate association with an intermediate member of the scorodite–mansfieldite series. It is monoclinic, space group P21/c, with a = 10.239(3) Å, b = 9.713(2) Å, c = 5.552(2) Å, β = 94.11(2)°, V = 550.7(2) Å3, Z = 2. Electron-microprobe analysis yielded (wt.%): CaO 0.04, MnO 0.03, CuO 0.06, ZnO 0.04, Fe2O3 (total) 43.92, Al2O3 1.15, SnO2 0.10, As2O5 43.27, P2O5 1.86, SO3 0.03. The empirical formula is (Fe2+0.52Fe3+0.32☐0.16)Σ1.00(Fe3+1.89Al0.11)Σ2.00(As1.87P0.13)Σ2.00O8(OH)2.00·4H2O based on 2(As,P) and assuming ideal 8O, 2(OH), 4H2O and complete occupancy of the ferric iron site by Fe3+ and Al. Optically, bendadaite is biaxial, positive, 2Vest. = 85±4°, 2Vcalc. = 88°, with α 1.734(3), β 1.759(3), γ 1.787(4). Pleochroism is medium strong: X pale reddish brown, Y yellowish brown, Z dark yellowish brown; absorption Z > Y > X, optical dispersion weak, r > v. Optical axis plane is parallel to (010), with X approximately parallel to a and Z nearly parallel to c. Bendadaite has vitreous to sub-adamantine luster, is translucent and non-fluorescent. It is brittle, shows irregular fracture and a good cleavage parallel to {010}. Dmeas. 3.15±0.10 g/cm3, Dcalc. 3.193 g/cm3 (for the empirical formula). The five strongest powder diffraction lines [d in Å (I)(hkl)] are 10.22 (10)(100), 7.036 (8)(110), 4.250 (5)(111), 2.865 (4)(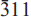 ), 4.833 (3)(020,011). The d spacings are very similar to those of its Zn analogue, ojuelaite. The crystal structure of bendadaite was solved and refined using a crystal from the co-type locality with the composition (Fe2+0.95☐0.05)Σ1.00(Fe3+1.80Al0.20)Σ2.00(As1.48P0.52)Σ2.00O8(OH)2·4H2O (R = 1.6%), and confirms an arthurite-type atomic arrangement.
), 4.833 (3)(020,011). The d spacings are very similar to those of its Zn analogue, ojuelaite. The crystal structure of bendadaite was solved and refined using a crystal from the co-type locality with the composition (Fe2+0.95☐0.05)Σ1.00(Fe3+1.80Al0.20)Σ2.00(As1.48P0.52)Σ2.00O8(OH)2·4H2O (R = 1.6%), and confirms an arthurite-type atomic arrangement.