Published online by Cambridge University Press: 05 July 2018
A new occurrence of barysilite, Pb8Mn(Si2O7)3, at the polymetallic Garpenberg Norra Zn-Pb deposit, Hedemora, Dalarna, Sweden, is described. The mineral, which forms colourless, transparent grains, is characterized by X-ray diffraction and electron-microprobe analyses. The assemblage includes tephroite, zincian jacobsite, manganoan diopside and others. The crystal structure of a barysilite crystal from Garpenberg Norra was redetermined using single-crystal X-ray diffraction data (Mo-Kα, CCD area detector) and has been refined in space group R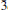 c with a = 9.804(1), c = 38.416(8)Å, V = 3197.8(8)Å3, to R1 = 2.32% for 1025 ‘observed’ reflections with Fo >4σ(Fo). A previous, low-precision structure determination (Lajzérowicz, 1965; R = 20%) is confirmed but improved considerably. The structure contains one distorted MnO6 polyhedron with six equivalent Mn–O bonds (2.224 Å), one Si2O7 disilicate unit with an Si–O–Si angle of 120.9°, and two non-equivalent Pb sites. The Pb1 site has a highly irregular, one-sided coordination with six O ligands, indicating a stereoactive 6s2 lone-electron pair on the Pb2+ ion, whereas the [6+3]-coordinated Pb2 site is fairly regular, with Pb–O distances of 2.540 (3×), 2.674 (3×) and 3.098 (3×) Å. The Pb2 site contains ~10% of Ca (+Ba) replacing Pb, corresponding to the structural formula Pb16(Pb,Ca)22Mn(Si2O7)3. This is the first direct proof that not only the M site in barysilite-type Pb8M(Si2O7)3 compounds can be replaced by divalent cations.
c with a = 9.804(1), c = 38.416(8)Å, V = 3197.8(8)Å3, to R1 = 2.32% for 1025 ‘observed’ reflections with Fo >4σ(Fo). A previous, low-precision structure determination (Lajzérowicz, 1965; R = 20%) is confirmed but improved considerably. The structure contains one distorted MnO6 polyhedron with six equivalent Mn–O bonds (2.224 Å), one Si2O7 disilicate unit with an Si–O–Si angle of 120.9°, and two non-equivalent Pb sites. The Pb1 site has a highly irregular, one-sided coordination with six O ligands, indicating a stereoactive 6s2 lone-electron pair on the Pb2+ ion, whereas the [6+3]-coordinated Pb2 site is fairly regular, with Pb–O distances of 2.540 (3×), 2.674 (3×) and 3.098 (3×) Å. The Pb2 site contains ~10% of Ca (+Ba) replacing Pb, corresponding to the structural formula Pb16(Pb,Ca)22Mn(Si2O7)3. This is the first direct proof that not only the M site in barysilite-type Pb8M(Si2O7)3 compounds can be replaced by divalent cations.