Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Siidra, Oleg I.
Nazarchuk, Evgeny V.
Zaitsev, Anatoly N.
Polekhovsky, Yury S.
Wenzel, Thomas
and
Spratt, John
2019.
Dokuchaevite, Cu8O2(VO4)3Cl3, a new mineral with remarkably diverse Cu2+ mixed-ligand coordination environments.
Mineralogical Magazine,
Vol. 83,
Issue. 5,
p.
749.
Zhang, Wanwan
He, Zhangzhen
Xie, Yaxin
Cui, Meiyan
Zhang, Suyun
Chen, Sihuai
Zhao, Zhiying
Zhang, Mengsi
and
Huang, Xiaoying
2020.
Molybdate–Tellurite Compounds with Capped-Kagomé Spin–Lattices.
Inorganic Chemistry,
Vol. 59,
Issue. 4,
p.
2299.
Britvin, S. N.
Pekov, I. V.
Yapaskurt, V. O.
Koshlyakova, N. N.
Göttlicher, J.
Krivovichev, S. V.
Turchkova, A. G.
and
Sidorov, E. G.
2020.
Polyoxometalate chemistry at volcanoes: discovery of a novel class of polyoxocuprate nanoclusters in fumarolic minerals.
Scientific Reports,
Vol. 10,
Issue. 1,
Pekov, Igor V.
Lykova, Inna
Koshlyakova, Natalia N.
Belakovskiy, Dmitry I.
Vigasina, Marina F.
Turchkova, Anna G.
Britvin, Sergey N.
Sidorov, Evgeny G.
and
Scheidl, Katharina S.
2020.
A new mineral species zincobradaczekite, NaCuCuZn2(AsO4)3, and a new isomorphous series bradaczekite–zincobradaczekite in the alluaudite group.
Physics and Chemistry of Minerals,
Vol. 47,
Issue. 8,
Pekov, Igor V.
Zubkova, Natalia V.
Yapaskurt, Vasiliy O.
Polekhovsky, Yury S.
Britvin, Sergey N.
Turchkova, Anna G.
Sidorov, Evgeny G.
and
Pushcharovsky, Dmitry Yu.
2020.
Kainotropite, Cu4Fe3+O2(V2O7)(VO4), a new mineral with a complex vanadate anion from fumarolic exhalations of the Tolbachik volcano, Kamchatka, Russia.
The Canadian Mineralogist,
Vol. 58,
Issue. 2,
p.
155.
Borisov, Artem S.
Siidra, Oleg I.
Kovrugin, Vadim M.
Golov, Andrey A.
Depmeier, Wulf
Nazarchuk, Evgeny V.
and
Holzheid, Astrid
2021.
Expanding the family of mineral-like anhydrous alkali copper sulfate framework structures: new phases, topological analysis and evaluation of ion migration potentialities.
Journal of Applied Crystallography,
Vol. 54,
Issue. 1,
p.
237.
Siidra, Oleg I.
Nekrasova, Diana O.
Charkin, Dmitry O.
Zaitsev, Anatoly N.
Borisov, Artem S.
Colmont, Marie
Mentré, Olivier
and
Spiridonova, Darya V.
2021.
Anhydrous alkali copper sulfates – a promising playground for new Cu2+ oxide complexes: new Rb-analogues of fumarolic minerals..
Mineralogical Magazine,
Vol. 85,
Issue. 6,
p.
831.
Zhitova, E. S.
Anikin, L. P.
Sergeeva, A. V.
Ismagilova, R. M.
Rashidov, V. A.
Chubarov, V. M.
and
Kupchinenko, A. N.
2021.
Volborthite Occurrence at the Alaid Volcano (Atlasov Island, Kuril Islands, Russia).
Geology of Ore Deposits,
Vol. 63,
Issue. 7,
p.
735.
Siidra, Oleg I.
Borisov, Artem S.
Charkin, Dmitri O.
Depmeier, Wulf
and
Platonova, Natalia V.
2021.
Evolution of fumarolic anhydrous copper sulfate minerals during successive hydration/dehydration.
Mineralogical Magazine,
Vol. 85,
Issue. 2,
p.
262.
Kornyakov, Ilya V.
Vladimirova, Victoria A.
Siidra, Oleg I.
and
Krivovichev, Sergey V.
2021.
Expanding the Averievite Family, (MX)Cu5O2(T5+O4)2 (T5+ = P, V; M = K, Rb, Cs, Cu; X = Cl, Br): Synthesis and Single-Crystal X-ray Diffraction Study.
Molecules,
Vol. 26,
Issue. 7,
p.
1833.
Pekov, Igor V.
Zubkova, Natalia V.
Zolotarev, Andrey A.
Yapaskurt, Vasiliy O.
Krivovichev, Sergey V.
Belakovskiy, Dmitry I.
Lykova, Inna
Vigasina, Marina F.
Kasatkin, Anatoly V.
Sidorov, Evgeny G.
and
Pushcharovsky, Dmitry Yu.
2021.
Dioskouriite, CaCu4Cl6(OH)4∙4H2O: A New Mineral Description, Crystal Chemistry and Polytypism.
Minerals,
Vol. 11,
Issue. 1,
p.
90.
Kiriukhina, G. V.
Yakubovich, O. V.
Dimitrova, O. V.
and
Volkov, A. S.
2022.
New Microporous Copper Diphosphate Chloride in a Series of Homeotypic Compounds: Hydrothermal Synthesis, Crystal Structure, and Crystal Chemistry.
Crystallography Reports,
Vol. 67,
Issue. 4,
p.
545.
Shablinskii, Andrey P.
Avdontceva, Margarita S.
Vergasova, Lidiya P.
Filatov, Stanislav K.
Avdontseva, Evgenia Yu.
Povolotskiy, Alexey V.
Moskaleva, Svetlana V.
Kargopoltsev, Anatoly A.
Britvin, Sergey N.
and
Shorets, Olga U.
2022.
Medvedevite, KMn2+V5+2O6Cl⋅2H2O, a new fumarolic mineral from the Tolbachik fissure eruption 2012–2013, Kamchatka Peninsula, Russia.
Mineralogical Magazine,
Vol. 86,
Issue. 3,
p.
478.
Ginga, Victoria A.
Siidra, Oleg I.
Breitner, Franziska
Jesche, Anton
and
Tsirlin, Alexander A.
2022.
Chemical Vapor Transport Synthesis of Cu(VO)2(AsO4)2 With Two Distinct Spin-1/2 Magnetic Ions.
Inorganic Chemistry,
Vol. 61,
Issue. 42,
p.
16539.
Gavryliv, Liubomyr
Ponomar, Vitalii
Bermanec, Marko
and
Putiš, Marián
2022.
The Taxonomy of Mineral Occurrence Rarity and Endemicity.
The Canadian Mineralogist,
Vol. 60,
Issue. 5,
p.
731.
Ginga, Victoria A.
Siidra, Oleg I.
Firsova, Vera A.
Charkin, Dmitri O.
and
Ugolkov, Valery L.
2022.
Phase evolution and temperature-dependent behavior of averievite, Cu5O2(VO4)2(CuCl) and yaroshevskite, Cu9O2(VO4)4Cl2.
Physics and Chemistry of Minerals,
Vol. 49,
Issue. 9,
Yakubovich, Olga V.
Kiriukhina, Galina V.
Simonov, Sergey V.
Volkov, Anatoly S.
and
Dimitrova, Olga V.
2023.
(Na,Li)3(Cl,OH)[Cu3OAl(PO4)3]: a first salt-inclusion aluminophosphate oxocuprate with a new type of crystal structure.
Acta Crystallographica Section B Structural Science, Crystal Engineering and Materials,
Vol. 79,
Issue. 1,
p.
24.
Kiriukhina, Galina
Yakubovich, Olga
Verchenko, Polina
Volkov, Anatoly
Shvanskaya, Larisa
Dimitrova, Olga
and
Simonov, Sergey
2023.
Mineral-like Synthetic Compounds Stabilized under Hydrothermal Conditions: X-ray Diffraction Study and Comparative Crystal Chemistry.
Minerals,
Vol. 14,
Issue. 1,
p.
46.
Zhang, Xu
Meng, Liao
Yang, Chengchang
Bo, Tao
Chen, Jiazhuang
Guo, Yan
Yu, Jipan
Wang, Lin
Hu, Kongqiu
Shi, Weiqun
Zhang, Meng
and
Mei, Lei
2024.
Insight into γ-Irradiation-Induced Structure Evolution of Uranium(IV) Germanates as Crystalline Nuclear Waste Forms.
Inorganic Chemistry,
Vol. 63,
Issue. 45,
p.
21719.
Ginga, Victoria A.
Siidra, Oleg I.
Tsirlin, Alexander A.
and
Setzer, Annette
2024.
Assembling the Puzzle of Coparsite Polymorphism: Synthesis, Thermal Expansion, and Quantum Magnetism of α- and β-Cu4O2(VO4)Cl.
Inorganic Chemistry,
Vol. 63,
Issue. 52,
p.
24573.
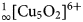 ${}_\infty ^1 [{\rm Cu_5O_2}]^{6 +} $ oxocentred bands in aleutite is novel and has not been described before in minerals and synthetic materials. The structural architecture of the
${}_\infty ^1 [{\rm Cu_5O_2}]^{6 +} $ oxocentred bands in aleutite is novel and has not been described before in minerals and synthetic materials. The structural architecture of the 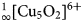 ${}_\infty ^1 [{\rm Cu_5O_2}]^{6 +} $ band in aleutite can be derived from a kagome network and represents a one-dimensional slice from it. In addition, aleutite is an interesting and complex example of a natural salt-inclusion phase.
${}_\infty ^1 [{\rm Cu_5O_2}]^{6 +} $ band in aleutite can be derived from a kagome network and represents a one-dimensional slice from it. In addition, aleutite is an interesting and complex example of a natural salt-inclusion phase.