Published online by Cambridge University Press: 14 March 2018
An ideal solution model has been used to calculate the effect of the substitutions 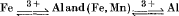 on the stability fields of the Al2SiO5 minerals. The results show that divariant assemblages of andalusite+sillimanite and sillimanite + kyanite solid solutions can coexist over ranges of 0·1 to 0·4 kbar at 527° C, but that kyanite+andalusite would be stable over a much narrower range. On adding (Fe, Mn)3+ to the system, the andalusite/sillimanite curve can be considered to rotate about the invariant point, first eliminating the sillimanite field, and then penetrating the kyanite field. Wide zones of viridine+sillimanite and viridine+kyanite are thus formed.
on the stability fields of the Al2SiO5 minerals. The results show that divariant assemblages of andalusite+sillimanite and sillimanite + kyanite solid solutions can coexist over ranges of 0·1 to 0·4 kbar at 527° C, but that kyanite+andalusite would be stable over a much narrower range. On adding (Fe, Mn)3+ to the system, the andalusite/sillimanite curve can be considered to rotate about the invariant point, first eliminating the sillimanite field, and then penetrating the kyanite field. Wide zones of viridine+sillimanite and viridine+kyanite are thus formed.
In view of the ease with which epitaxial nucleation occurs in the Al2SiO5 system, it is considered that extensive metastable growth of andalusite, sillimanite, and kyanite is improbable in nature. Metastable persistence is likely to occur in dry systems, or in the presence of an ‘armour’ of the stable phase, but reaction of millimetre-sized crystals should be completed in geologically short times when aqueous or silicate liquids are present. The combination of the effects of metastable persistence and solid solution seems adequate to account for essentially all the examples of kyanite + sillimanite and kyanite + andalusite, and for many sillimanite +andalusite assemblages. In addition, if small supersaturations develop during metamorphism, simultaneous growth of andalusite + sillimanite could occur over a restricted temperature range.