On the Effect of Continued Small Additions of Poisonous Substances on the Velocity of Gaseous Catalytic Reaction in Closed Vessels
Published online by Cambridge University Press: 24 October 2008
Extract
The Langmuir-Frenkel theory of surface action has been shown to involve a simple relation between the partial pressure of the catalyst poison, and the reduction in reaction velocity which results. Very interesting observations have been made by Beebe showing that the heat of adsorption of carbon monoxide on a reduced copper catalyst falls considerably and reaches a limiting value as the surface approaches saturation. Pease and Stewart have shown that the presence of less than one percent, of the total carbon monoxide that a given copper catalyst can adsorb will reduce the reaction velocity of the combination of hydrogen and ethylene at the surface of the copper by 88 per cent.
- Type
- Research Article
- Information
- Mathematical Proceedings of the Cambridge Philosophical Society , Volume 23 , Issue 7 , July 1927 , pp. 832 - 837
- Copyright
- Copyright © Cambridge Philosophical Society 1927
References
* Constable, , Proc. Camb. Phil. Soc., vol. 23, pp. 172–182 (1926), “On the behaviour of the centres of activity of saturated surfaces during the initial stages of unimolecular reactions”;CrossRefGoogle Scholarpp. 593–606 (1926), “Surface adsorption and the velocity of chemical action at gas solid interfaces.”Google Scholar
† J. Phys. Chem., vol. 30, pp. 1538–1544 (1926).Google Scholar
‡ J. Amer. Chem. Soc., vol. 47, p. 1235 (1925).CrossRefGoogle Scholar
§ Becker, , Phys. Rev., vol. 28, pp. 341–361, has shown by direct experiment with caesium films on tungsten that the work necessary to remove an atom of caesium decreases as the fraction of the surface covered increases; but that the work done in removing a caesium ion increases under similar conditions.CrossRefGoogle Scholar
* Nature, vol. 117, p. 230 (1926). The symbols are defined here.CrossRefGoogle Scholar
* Larmor, , Proc. Lit. Phil. Soc. Manchester, vol. 52 (No. 10), p. 28 (1908), has pointed out the possibilities arising bom this principle in connection with gas reactions.Google Scholar
* Note that 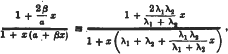 , and is always less than unity provided that
, and is always less than unity provided that
i.e.
which is always satisfied by a molecule which has a positive mean life on the centres of activity. The variation in ξ with x has been neglected, bat each variation would only intensify the effect already predicted. The variation is absent with saturated centres, and it seems probable that the ‘centres of activity’ in many cases are always covered by adsorbed molecules.
- 3
- Cited by


