1 Introduction
Aluminum nitride-silicon carbide alloys can be prepared with a wide range of physical and electronic properties that are superior to the pure binary components. This is an excellent system for bandgap engineering, as its band gap changes from 6.2 eV (for AlN) down to 2.9 eV (for 6H-SiC), and potentially 2.3 eV if the cubic structure 3C-SiC can be stabilized. The band transition changes from indirect for SiC-rich to direct for AlN-rich alloys (>70 %). Both n and p type conductivities have been reported. AlN-SiC alloys retain the best properties of the pure binary compounds: high electron break down field, high saturated electron drift velocity, and high thermal conductivity Reference Shi, Liu, Liu, Edgar, Payzant, Hayes and Kuball[1] Reference Hunter[2] Reference Hunter[3] Reference Cutler, Miller, Rafaniello, Park, Thomson and Jack[4] Reference Dmitriev[5] Reference Nurmagomedov, Pikhtin, Razbegaev, Safaraliev, Tairov and Tsvetkov[6] Reference Kern, Tanaka and Davis[7] Reference Wongchotigul, Spencer, Chen and Prasad[8].
Much research has already been carried out on the preparation and characterization of AlN-SiC alloy crystals. Both thin films and bulk crystals of AlN-SiC alloys have been prepared over the entire compositional range. Solid solutions of epitaxial (SiC)1−x (AlN) x thin films with a full range of x (from 0 to 1) were successfully grown on 6H-SiC substrate by the sublimation method Reference Safaraliev, Tairov and Tsvetkov[9]. The source materials were polycrystalline SiC-AlN solid solution plates with different AlN concentrations. The resultant polytypic structure was dependent on the AlN concentration: epitaxial (AlN) x (SiC) 1−x films had the 2H polytype (i.e. the wurtzite structure) in the range of x=0.2, while different polytypic structures (3C, 4H, 6H, 2H) were possible when x<0.2. Moreover, the composition strongly affected the morphology and structural quality of (AlN) x (SiC)1−x solid solutions. The surface morphology and quality deteriorated with the AlN concentration, which was probably due to the increasing mismatch between the film and substrate Reference Safaraliev, Tairov and Tsvetkov[9]. Previously we reported on the sublimation growth of AlN-SiC alloy crystals on off-axis Si-face 6H-SiC (0001) substrates from a mixture of AlN and SiC powders Reference Gu, Edgar, Payzant, Meyer, Walker, Sarua and Kuball[10]. A homogeneous AlN-SiC alloy grew two dimensionally with a wurtzite structure and high quality.
In addition to sublimation, other methods have successfully grown AlN-SiC alloy crystals on 6H-SiC (0001) substrates. Dmitriev et al. Reference Dmitriev and Cherenkov[11] produced alloys with AlN concentration up to 10 mol% by liquid phase epitaxy (LPE). Jenkins et al. Reference Jenkins, Irvine, Spencer, Dmitriev and Chen[12] achieved solid solutions of (AlN) x (SiC)1−x over the composition range from x=0.1 to 0.9 by metalorganic chemical vapor deposition (MOCVD). A new approach, sequential supply epitaxy (SSE), was developed by Avramescu et al. Reference Avramescu, Hirayama, Aoyagi and Tanaka[13] to improve the quality of AlN-SiC epilayers. Alternating the supply of precursors in a MOCVD reactor greatly reduced the quality-limiting gas-phase reaction and promoted a 2D-like growth at relatively low temperatures (1200-1300 °C). Most recently gas-source molecular beam epitaxy (GSMBE) was employed by Roucka et al. Reference Roucka, Tolle, Smith, Crozier, Tsong and Kouvetakis[14] to grow SiCAlN thin films of high hardness on Si (111) substrates at low temperatures (550-750 °C). An average hardness of 25 GPa was achieved for the SiCAlN films, using fused silica with the hardness value of 9 GPa as a standard. Rutherford backscattering spectrometry (RBS), Fourier transform infrared spectroscopy (FTIR), cross-sectional transmission electron microscopy (XTEM), and electron energy-loss spectroscopy (EELS) measurements confirmed a 2H wurtzite crystal structure with a near-stoichiometric composition and lattice parameters close to those of 2H-SiC and hexagonal AlN. A novel method - ion beam synthesis - was performed on (0001)-oriented, n-type 6H-SiC wafers by co-implanting N+ and Al+ ions to synthesize (SiC)1−x (AlN) x layers. High substrate temperature (=600 °C) and the sequence of implantation of Al followed by N were favorable to the crystal growth Reference Yankov, Fukarek, Voelskow, Pezoldt and Skorupa[15].
In this paper, we report on the composition and structural properties of AlN-SiC alloy crystals grown by sublimation on SiC substrates. Growth of AlN-SiC alloy crystals on 8° off axis (0001) 6H-SiC substrates was studied in detail. The original composition of the source material was varied to examine its effect on the AlN-SiC alloy crystals grown on 8° off-axis 4H-SiC substrate. This was complemented by thermodynamic calculation to predict the Al to Si ratio in the gas phase to see its relevance to the composition in the solid. The effect of the polytypes and intentional misorientation of the SiC substrates on the AlN-SiC alloy crystals was investigated. Different techniques were performed to characterize the AlN-SiC alloy crystals: optical microscopy and scanning electron microscopy (SEM) for the surface morphology; x-ray diffraction (XRD) for identifying the crystal and lattice constants; transmission electron microscopy (TEM) for the defect identification and high magnification information on the interface and surface properties; electron probe microanalysis (EPMA) for the composition analysis; and x-ray photoelectron spectroscopy (XPS) for both the composition and bonding information.
2 Experimental
2.1 Crystal growth of AlN-SiC alloy on SiC substrate
The sublimation growth of AlN-SiC alloy crystals was conducted in a resistively-heated graphite furnace. The growth chamber consists of a graphite retort surrounding a crucible to contain the highly reactive source vapors. The graphite heating element was designed to provide an axial temperature gradient of 5-10 °C/cm between the source material and crystal growth region, which was the driving force for the sublimation growth. The distance between the source and the substrate was kept constant at ~1 cm. Ultra high purity nitrogen continuously flowed through the system at a constant pressure of ~810 Torr. The source AlN-SiC mixture powder was obtained by mixing ultra high purity AlN powder with SiC powder.
The effects of three process parameters on the properties of the AlN-SiC layers on SiC substrates were investigated: the substrate polytype and misorientation; temperature; and the composition of the original source. AlN-SiC alloy crystals were grown on different polytypes of SiC substrates at 1850 °C for 24 h. The seed crystals were Si-face (0001) 6H-SiC substrates intentionally misoriented 8° (± 0.5°) off axis toward (1
2.2 Characterization
The surface morphology was examined by optical microscopy (low magnification) and scanning electron microscopy (SEM, high magnification). The lattice constants and crystal identification were measured by x-ray diffraction (XRD) with Cu-Kα radiation, using a PANalytical X'Pert PRO MPD diffractometer with an incident-beam parabolic mirror, 0.04 rad incident and diffracted beam Soller slits, and an X'Celerator real-time multiple-strip detector. A JEOL 4000EX transmission electron microscope (TEM) with a point resolution of 1.7 Å was used to examine the alloy crystals, the crystal/substrate interface properties, and defects. EDX for the composition analysis was realized in a Philip CM200 TEM operated at 200 kV. All TEM specimens were prepared by mechanical polishing followed by argon ion milling.
In the previous work Reference Gu, Edgar, Payzant, Meyer, Walker, Sarua and Kuball[10], scanning Auger microprobe (SAM) was executed to measure the surface elemental composition, and the depth profile of each component. However, the AlN-SiC alloy crystals have a serious charging problem in SAM. In addition, our experience indicates that SAM overestimates the oxygen concentration. Oxygen in the UHV system is adsorbed on the sample surface, leading to a higher concentration of oxygen on the surface than in the bulk. Therefore electron probe microanalysis (EPMA) was performed instead using a focused beam of high energy electrons (10 keV) for elemental analysis. EPMA is less surface sensitive than XPS or AES, and provides more accurate measurement on the bulk sample composition. Before measurements, samples were coated with carbon for conductivity, therefore the carbon concentration was determined by difference. The silicon x-ray had sufficient energy to penetrate the carbon film without absorption. A near 1:1 ratio of Si:C was detected in the SiC substrate, suggesting the accuracy of the EPMA analysis.
The composition and bonding information were obtained ex situ by x-ray photoelectron spectroscopy (XPS) on a PHI quantum 2000 x-ray photoelectron spectrometer using a focused monochromatic aluminum K x-ray beam. Ar ions were employed to sputter the adsorbents off the sample surface. In XPS, the relative concentration of different elements is determined by calculating the peak intensity, using elemental sensitivity factor, spectrometer transmission function, and correction for emission angle. However, XPS is only sensitive to 5-10 monolayers of the surface, therefore the bulk composition measured by XPS may differ from that by other surface sensitive techniques.
2.3 Thermodynamic calculation
A thermodynamic calculation was performed to predict the distribution of each element between the source material and the gas phase. Since the alloy crystals are formed from the vapor phase, this provides a theoretical means for estimating changes in the crystal composition with temperature.
The sublimation growth of AlN occurs by the reaction
while in the sublimation growth of SiC the following reactions can occur:
Reaction (3) is the most important for SiC sublimation, as confirmed by the calculation of Gibbs free energy of reaction for each reaction using the Gibbs free energy of formation from JANAF thermochemical tables. Al, N2, Si, SiC, Si2C, SiC2, and possibly SiN and Si2N are present in the gas phase, while SiC, AlN, C, possibly Si3N4 may be present in the solid phase. If assuming that the vapor phase over AlN and SiC consists of Al(g), Si(g), SiC(g), Si2C(g), SiC2(g), N2(g), SiN(g) and Si2N(g), and no AlN molecule, and the N2 pressure of 810 torr, the mole fraction of each species can be calculated from the JANAF data. The original mole ratio of AlN to SiC in the source material was changed from 2:1 to 3:1 and 5:1.
3 Results
The thermodynamic calculation confirmed that Si, Si2C, Si2N and SiC2 are the main Si-containing species in the gas phase, and the importance of Si2C, SiC and SiC2 increased dramatically (one order of magnitude for Si2C, 2 orders of magnitude for SiC, and 2-3 orders of magnitude for SiC2) with temperature. Figure 1 displays the predicted change of mole ratio of Al (in AlN) to Si (in Si, Si2C, Si2N, SiC2, SiC, and SiN) in the gas phase with temperature from 1800 °C to 2000 °C. It is evident that the mole ratio of Al:Si increases with temperature. After reaching a maximum (at ~1910-1920 °C), it decreases gradually. The mole ratio of Al to Si in the gas phase was different from the original composition ratio of Al:Si in the source material; the gas phase contained a higher Al:Si ratio under most conditions.

Figure 1. Calculated mole ratio of Al:Si in the gas phase during the sublimation growth of AlN-SiC alloy crystals when the mole ratio of Al:Si in the source material is 2:1, 3:1 and 5:1.
Figure 2 shows optical micrographs of an AlN-SiC alloy crystal grown on 8° off-axis 6H-SiC substrate at 1860 °C for 24 h. The resultant AlN-SiC alloy crystal was colorless and transparent. The film was cracked, probably due to the large stress introduced during the cooling process from sublimation temperature to room temperature, which was caused by the thermal expansion mismatch between the SiC substrate and the AlN-SiC alloys. EPMA measurements confirmed that all four constituents, Si, Al, C, and N, were present in every region investigated, without any indication of phase separation of SiC and AlN or any segregation of individual elements.

Figure 2a Optical micrograph of AlN-SiC alloy crystal grown on 8° off-axis 6H-SiC substrate at 1860 °C for 24 h (the smallest grid size is 5 mm).

Figure 2b. Optical micrograph of AlN-SiC alloy crystal grown on 8° off-axis 6H-SiC substrate at 1860 °C for 24 h (magnification: 200X).
Figure 3 displays a SEM image of an AlN-SiC alloy crystal grown on 8° ff-axis 6H-SiC substrate at 1860 °C for 20 h. The AlN-SiC alloy was initially deposited in an island growth mode which was evident from the hexagonal pyramids with flat tops. The hexagonal grains then merged together to form a continuous layer.

Figure 3a. SEM of AlN-SiC alloy crystal grown on 8° off-axis 6H-SiC substrate at 1860 °C for 20 h.

Figure 3b. SEM of AlN-SiC alloy crystal grown on 8° off-axis 6H-SiC substrate at 1860 °C for 20 h.
AlN-SiC alloy crystals grown on off-axis SiC substrates were basically similar in morphology under optical microscope (as in Figure 2), independent of the ifferent polytypes (6H or 4H) and miscut angles (8° or 3.68°). hey only differed slightly in the width of terraces and heights of steps. The growth developed layer by layer, with individual hexagonal grains around the ample edges. The steps originally present at the miscut SiC substrates provided the initial nucleation sites, leading to a three-dimensional nucleation of islands, followed by a two-dimensional layer-by-layer growth due to the island coalescence. In contrast, AlN-SiC alloy crystals formed on on-axis 6H-SiC substrate were rough with individual hexagonal islands all over the surface (demonstrated in Figure 4). Therefore, growth on on-axis SiC substrate was not further investigated.

Figure 4. Optical micrograph of AlN-SiC alloy crystal grown on on-axis 6H-SiC substrate at 1850 °C for 24 h (magnification: 200X).
The lattice constants for one AlN-SiC alloy were accurately measured by XRD. The alloy crystal grown on 8° off-axis 6H-SiC substrate at 1985 °C for 24 h (with the original mole composition Al:Si as 3:1) was ground to powder so as to obtain the complete XRD spectra. Figure 5 shows the XRD spectra of the ternary AlN-SiC alloy crystal and a binary AlN crystal. The diffraction peak of the AlN-SiC alloy crystal was shifted toward AlN, since AlN was the main component. The XRD patterns confirm that as expected, the SiC from the substrate was the 6H polytype. The lattice constants a and c measured for 6H-SiC were 3.083(9) Å, and 15.04(2) Å, which were somewhat altered from the values of a and c for a ground 6H-SiC substrate without AlN-SiC alloy layer, measured on the same instrument as 3.0818(7) Å and 15.111(4) Å. The AlN-SiC alloy had the wurtzite crystal structure. The lattice constants a and c calculated for the grown AlN-SiC alloy crystal were 3.098(7) Å and 4.996(9) Å, respectively. The appearance of SiO2 peaks was probably artifacts introduced by the grinding process.

Φιγυρε 5. θ-2θ XRD patterns for AlN-SiC alloy crystal grown on 8° off-axis 6H-SiC substrate at 1985 °C for 24 h.
Figure 6 shows the XPS spectra for the AlN-SiC alloy crystal grown on 8° off-axis 6H-SiC substrate at 1865 °C for 24 h. The measurement indicated the presence of Al, N, Si, C and O elements on the surface. Al 2p, N 1s peaks of the alloy crystal were compared with those of a pure AlN substrate, and Si 2p, C 1s peaks were compared with those of pure SiC substrate. Both Al 2p and N 1s peaks shifted to a slightly higher binding energy in the AlN-SiC alloy crystal in comparison to those in pure AlN substrate. However, C 1s peak shifted to a lower binding energy in the alloy crystal, whereas Si 2p peak shifted to a much higher binding energy than that in pure SiC substrate. A higher binding energy was generally associated with a higher positive oxidation state. The shift of Si 2p, C 1s, Al 2p, and N 1s peaks as compared with bulk SiC and AlN confirmed no phase segregation of AlN and SiC, and the formation of AlN-SiC alloy crystals.

Figure 6. XPS spectra for AlN-SiC alloy crystal grown on 8° off-axis 6H-SiC substrate at 1865 °C for 24 h.
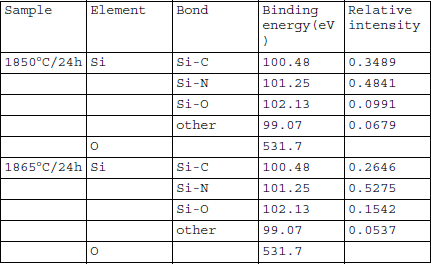
Figure 7 shows the Si 2p XPS spectra for the AlN-SiC alloy crystal grown on 8° off-axis 6H-SiC substrate at 1850 °C for 24 h. The Si 2p spectrum was decomposed into four components at 99.07, 100.48, 101.25, and 102.13 eV. The employed decomposition parameters were as follows: the full width at half maximum (FWHM) was 1.34 eV, and the broadening function, 70% Gaussian. The 100.48 eV peak can be ascribed to Si-C, the 101.25 eV peak to Si-N, and the 102.13 eV to Si-O Reference Peng, Song, Le and Hu[16]. The peak fitting results are listed in Table 1. In the AlN-SiC alloy crystal, Si was not only bonded to C, but also to N to a large extent. This provides further confirmation that AlN-SiC crystal was actually an alloy without phase separation of SiC and AlN. However, it is difficult to apply the same procedure to the Al 2p, C 1s and N 1s spectra to obtain quantitative results, because of the large number of overlapping peaks.

Figure 7. High resolution photoemission spectra of the Si 2p region for AlN-SiC alloy crystal grown on 8° off-axis 6H-SiC substrate at 1850 °C for 24 h (with the background subtracted).
Table 1 Binding energies of fitted features of Si 2p XPS peaks of two AlN-SiC alloy crystals grown on 8° off-axis 6H-SiC substrate at different temperatures.
The lattice image of AlN-SiC alloy crystal (which was grown on 8° off-axis 6H-SiC substrate at 1985 °C for 24 h) at <11

Figure 8. Lattice image and its corresponding diffraction pattern of AlN-SiC alloy at <11
The origin and paths of defects were examined by TEM. Figure 9 demonstrates the dislocation density in the initial growth was on the order of 1010 cm−2. The threading dislocations bent over as the alloy layer became thicker, and propagated horizontally (as shown by Figure 10). The defect density was reduced significantly at the surface region to less than 108 cm−2. In a second sample repeated at the same growth condition, the dislocations propagated in a zigzag manner from the beginning. The dislocation density at the alloy surface was less than 106 cm−2, which exceeded the examination limit of cross-section sampling. It was 3 orders of magnitude lower than the dislocation density at the early stage of growth of 109 cm−2.

Figure 9a. TEM image for AlN-SiC alloy crystal grown on 8° off-axis 6H-SiC substrate at 1985 °C for 24 h (AlN-SiC alloy crystal surface).

Figure 9b. TEM image for AlN-SiC alloy crystal grown on 8° off-axis 6H-SiC substrate at 1985 °C for 24 h (cross-sectional image).

Figure 10. TEM image for AlN-SiC alloy crystal grown on 8° off-axis 6H-SiC substrate at 1985 °C for 24 h, showing the bending of the threading dislocations at specific positions and propagating horizontally.
The composition of the alloy crystal should depend on the vapor phase composition, and the diffusion rate of each vapor species. Diffusion rates are generally inversely proportional to the molecular weight of species involved. Therefore, Al and Si should diffuse at rates with the same order of magnitude, with the diffusion rates of Si2C, Si2N and SiC2 much lower. Consequently, the overall diffusion rate of Al-containing species (only Al) should be higher than that of Si-containing species (Si, Si2C, Si2N, and SiC2). An approximately uniform composition was achieved from the interface to the crystal surface, as confirmed by both XPS and EPMA. When the source composition AlN:SiC was 3:1 (growth was repeated for three times under the same growth conditions of 1850 °C and 24 h), XPS measurements demonstrated non-stoichiometric ratios of Al:N and Si:C. The N concentration was always slightly higher than the Al concentration (5-15%). The ratio of Al:Si was higher than 3 (3.6-4.4), comparable to 4.0 in the gas phase as predicted by JANAF calculation in Figure 1. In comparison, the N concentration measured by EPMA was also higher than Al (10-14%), however, the ratio of Al:Si was lower than 3 (1.8-2.4), independent of the wide range of growth temperature from 1860 °C to 1990 °C. The Si concentration by EPMA differed significantly from that by XPS, with EPMA giving a much higher Si content. When the mole ratio of Al:Si in the source material was 5:1 and 2:1, the ratio of Al:Si in the alloy crystals changed with the growth temperature, and it was different from the original composition, which agreed well with the results calculated by JANAF data.
4. Conclusions
AlN-SiC alloy crystals were grown on as-received 4H- and 6H-SiC substrates with different misorientation angles by sublimation-recondensation in a resistively-heated graphite furnace. On-axis SiC substrate was proven not suitable for the seeded growth of AlN-SiC alloy crystals. Different polytypes (6H or 4H) and miscut (8° or 3.68°) had negligible effect on the crystal morphology. The AlN-SiC alloy crystal had non-stoichiometric ratios of Al:N and Si:C. Homogeneous wurtzite structured compounds of AlN and SiC were obtained, with a decreasing dislocation density away from the crystal/substrate interface. The mole ratio of Al to Si in the final alloy crystals changed with the growth temperature, and differed from the original source composition, which was consistent with the thermodynamic predictions.
Acknowledgments
We are thankful to the research sponsored in part by the Assistant Secretary for Energy Efficiency and Renewable Energy, Office of FreedomCar and Vehicle Technologies, as part of the High Temperature Materials Laboratory User Program, Oak Ridge National Laboratory, managed by UT-Battelle, LLC, for the U.S. Department of Energy under contract number DE-AC05-00OR22725. The XPS work was performed at the EMSL, a national scientific user facility sponsored by DOE's Office of Biological and Environmental Research and located at Pacific Northwest National Laboratory, operated for DOE by Battelle. Support from the National Science Foundation, grant number DMR-0408874, is gratefully appreciated.















