INTRODUCTION
Our knowledge of marine biodiversity in the Mediterranean Sea is essentially focused on coastal areas, whereas data relating to deep zones (beyond the continental shelf) remains limited (Fredj & Laubier, Reference Fredj, Laubier, Moraitou-Apostolopoulou and Kiorstsis1985; Myers et al., Reference Myers, Mittermeier, Miettermeier, Da Fonseca Gustavo and Kent2000; Sardà et al., Reference Sardà, Calafat, Mar Flexas, Tselepides, Canals, Espino and Tursi2004; Coll et al., Reference Coll, Piroddi, Steenbeek, Kaschner, Ben Rais Lasram, Aguzzi, Ballesteros, Bianchi, Corbera, Dailianis, Danovaro, Estrada, Froglia, Galil, Gasol, Gertwagen, Gil, Guilhaumon, Kesner-Reyes, Kitsos, Koukouras, Lampadariou, Laxamana, López-Fé de la Cuadra, Lotze, Martin, Mouillot, Oro, Raicevich, Rius-Barile, Ignacio Saiz-Salinas, San Vicente, Somot, Templado, Turon, Vafidis, Villanueva and Voultsiadou2010). Among the anthozoans living on the slope, Dendrobrachiidae Brook, Reference Brook1889, form a scarcely-known group, initially considered as an antipatharian family (Thomson, Reference Thomson1910) until it was definitively classified taxonomically as Octocorallia by Opresko & Bayer (Reference Opresko and Bayer1991), and later Berntson et al. (Reference Berntson, Bayer, McArthur and France2001). This gorgonian family is represented by a single genus (Dendrobrachia), which comprised only three species until the early 1990s: Dendrobrachia fallax Brook, Reference Brook1889; Dendrobrachia multispina Opresko & Bayer, Reference Opresko and Bayer1991; and Dendrobrachia paucispina Opresko & Bayer, Reference Opresko and Bayer1991. These species were initially found at a limited number of locations in the Atlantic (Cape Verde Islands, southern Florida and Ascension Island) (Brook, Reference Brook1889; Thomson, Reference Thomson1910), then more recently in south-western Australia (Opresko & Bayer, Reference Opresko and Bayer1991). In the Mediterranean Sea, Zibrowius & Taviani (Reference Zibrowius, Taviani, Freiwald and Roberts2005) have reported the presence of colonies of Dendrobrachia (initially thought to be D. fallax) at several locations in the Alboran Sea and in southern Sicily at depths between 230 and 632 m. Cruises conducted in 2007 on-board the RV ‘Urania’ confirmed the presence of this octocorallian in the south of Malta. In the same year, samples obtained during cruises conducted in the framework of the TTR (Training Through Research Unesco Programme) also allowed the identification of Dendrobrachia colonies in the Gibraltar Strait. On the basis of these new records and of collections compiled during previous cruises, López-González & Cunha (Reference López-González and Cunha2010) described two new species: Dendrobrachia bonsai and Dendrobrachia sarmentosa.
Between 2008 and 2010, two cruises were conducted with the aim of exploring submarine canyon-heads along the French coasts and for characterizing their habitats and the associated benthic macrofauna and ichthyofauna. Sites harbouring Dendrobrachia bonsai were discovered during these two cruises. This note presents these results, coupled with a discussion on the northern distributional limits of this species in the western Mediterranean basin.
MATERIALS AND METHODS
The data were obtained during two cruises: MEDSEACAN (on the continental coasts of France; 03°10′E, 42°25′N– 07°30′E, 43°00′N) and CORSEACAN (western coast of Corsica; 09°20′E, 43°00′N–09°00′E, 41°25′N). During these cruises, 27 underwater canyons were explored. Although bionomic research had already been conducted on some of these canyons (Reyss, Reference Reyss1970; Bourcier & Zibrowius, Reference Bourcier and Zibrowius1973), most of them are little known or completely unknown, particularly in Corsica. The two field cruises were conducted on-board the RV ‘Minibex’. The ‘Super Achille’ ROV (remotely operated vehicle) and the two-man operated submarine ‘Remora’ were used for explorations up to depths of 700 m. A total of 240 ROV transects and 21 submarine dives were performed. A colony of Dendrobrachia and a fragment of another colony were collected using the ROV, in the month of August 2010. The samples were stored on-board in 70% alcohol for identification and collection.
RESULTS AND DISCUSSION

Fig. 1. Dendrobrachia bonsai: underwater photographs of (A) a colony, Canyon of Porto (−199 m); (B) a colony collected in the Canyon of Porto (−218 m) showing polyps with oocytes.
MATERIAL EXAMINED
Research vessel ‘Minibex’, CORSEACAN cruise, ROV ‘Super Achille’ dive no. POACHP05, Canyon of Porto, 42°19.66′N 8°34.39′E, 300 m depth, rocky wall with isolated colonies of Dendrobrachia, 3 August 2010, fragment of colony (total length (TL) 50 mm).
Research vessel ‘Minibex’, CORSEACAN cruise, ROV ‘Super Achille’ dive no. POACHP09, Canyon of Porto, 42°19.86′N 8°35.43′E, 218 m depth, rocky wall with concretions and dense population of Dendrobrachia, 6 August 2010, one colony (TL 90 mm).
Additional material was observed and photographed underwater, but not collected.
DESCRIPTION
The Dendrobrachia samples were examined using the identification key drawn up by López-González & Cunha (Reference López-González and Cunha2010). The entire colony collected in the Canyon of Porto was fragile and ramified in one plane. It possessed non-retractile polyps void of sclerites. The cross-section of its distal branches shows the presence of four axial peaks, plus rows of spines on the largest branches. A few spines were present on the main axis below the first ramification. This colony was characterized by mature distal polyps, with oocytes of approximately 0.7 mm in diameter (Figure 1A). The presence of fertile polyps was observed on a 50-mm fragment collected from another colony in the same canyon, and on in situ photographs taken by the ‘Remora’ ROV (Figure 1B). The presence of sexually mature polyps during summer in Corsica thus confirms the observations of López-González & Cunha (Reference López-González and Cunha2010) in the Mediterranean Sea and Gulf of Gadiz.
BIOGEOGRAPHICAL AND ECOLOGICAL ASPECTS
Despite similar sampling efforts and same investigation means used during the MEDSEACAN and CORSEACAN cruises, no Dendrobrachia colonies were observed in the underwater canyons explored along the French continental coast. In contrast, Dendrobrachia was found on the western coast of Corsica in five canyons situated between the Gulf of St Florent and the Gulf of Ajaccio (Figure 2). These observations corroborate the conclusions of López-González & Cunha (Reference López-González and Cunha2010), asserting that D. bonsai is the most common species of the genus in terms of geographical and depth distribution. These records are also the northernmost observations of the species in the Mediterranean Sea; to date, D. bonsai had only been observed in the Alboran Sea and in the Strait of Sicily (López-González & Cunha, Reference López-González and Cunha2010). Its absence along the coasts of continental France could result from unfavourable environmental conditions, such as high sediment transport rates resulting from direct connections between rivers and canyons (e.g. Rhône, Var) (Savoye & Piper, Reference Savoye and Piper1991; Berne et al., Reference Berne, Loubrieu and équipe1999; Hugot et al., Reference Hugot, Joseph, Savoye and Zaleski2001) and specific hydrodynamic conditions such as downwelling of cold water into certain canyons during the winter months (Durrieu de Madron et al., Reference Durrieu de Madron, Radakovitch, Heussner, Loye-Pilot and Monaco1999; Canals et al., Reference Canals, Puig, Durrieu de Madron, Heussner, Palanques and Fabres2006).
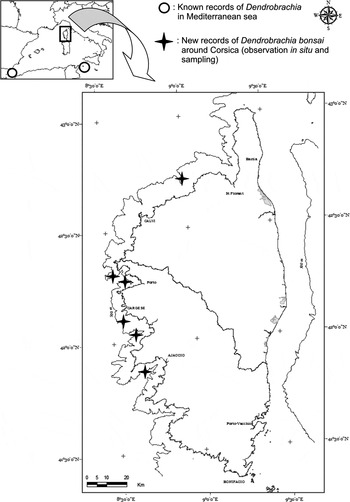
Fig. 2. Distribution of Dendrobrachia bonsai and the location of new records along the Corsican coasts.
Dendrobrachia bonsai colonies were observed between 200 and 500 m depth, mainly along the rocky walls and overhangs of high underwater cliffs. It was also encountered on biogenic concretions (thanatocoenosis of Neopycnodonte- Desmophylllum) and on isolated rocks relatively free of sediment deposits situated at the foot of cliffs. The colonies were scattered (isolated individuals or small groups) between 300 and 500 m depth. Conversely, denser aggregations of colonies occupying several square metres were mainly observed between 200 and 300 m. At this level, the in situ photographs taken by the ROV allowed the estimation for maximum densities of 50 colonies per square metre. The benthic fauna associated with these aggregations predominantly comprises hydroids, gorgonians (Acanthogorgia hirsuta, Bebryce mollis and Muriceides lepida), antipatharians (Leiopathes glaberrima and Paranthipates larix), sponges and brachiopods (Grypheus vitreus) (Figure 3).

Fig. 3. Dendrobrachia bonsai on deep rocky walls. (A) Colonies of D. bonsai associated with other gorgonians (Acanthogorgia hirsuta and Muriceides lepida); (B) a dense population on a rocky wall.
Sightings of Dendrobrachia bonsai along the Corsican coast confirm the affinity of this gorgonian with hard substrates (rock and bioconcretions), at depths ranging from 220 to 600 m previously observed in the Mediterranean Sea (Zibrowius & Taviani, Reference Zibrowius, Taviani, Freiwald and Roberts2005; López-González & Cunha, Reference López-González and Cunha2010). Nevertheless, the depth range where dense Dendrobrachia bonsai aggregations were observed and the associated species tend to indicate that this gorgonian has a particular preference for the upper bathyal stage (between 200 and 300 m) where currents are significant and a limited amount of sediment is deposited on the substrate.
ACKNOWLEDGEMENTS
I would like to thank the Marine Protected Areas Agency (France) for organizing and financing the MEDSEACAN and CORSEACAN cruises, and the COMEX S.A. crews and pilots who took part in them. I also address special thanks to Dr Pablo López-González from the University of Seville.





