Introduction
The ‘species–area relationship’ (SAR) is one of the oldest studied and most widely recognized patterns in ecology, being well established since the 1920s (Arrhenius Reference Arrhenius1921; Gleason Reference Gleason1922). In simple terms, it states that species richness increases with an increase in sampled area size (Connor & McCoy Reference Connor and McCoy1979, Reference Connor, McCoy and Levin2017; Rosenzweig Reference Rosenzweig1995; Lomolino Reference Lomolino2000). Various hypotheses have been suggested to explain the causes of this pattern and its underlying mechanisms (Connor & McCoy Reference Connor and McCoy1979, Reference Connor, McCoy and Levin2017; Scheiner et al. Reference Scheiner, Chiarucci, Fox, Helmus, McGlinn and Willig2011; Moradi et al. Reference Moradi, Fattorini and Oldeland2020). Furthermore, other factors, such as habitat diversity or edge effect, can simultaneously act through increases in area size and themselves modify species richness (Connor & McCoy Reference Connor, McCoy and Levin2017). SARs have been tested for different variations of the concept of ‘area’: 1) islands (Yu et al. Reference Yu, Li, Zhang and Guo2020), 2) contiguous (Dengler et al. Reference Dengler, Matthews, Steinbauer, Wolfrum, Boch, Chiarucci, Conradi, Dembicz, Marcenò and García-Mijangos2020) or fragmented (Hanski et al. Reference Hanski, Zurita, Bellocq and Rybicki2013) habitat patches, 3) ecoregions (Martellos et al. Reference Martellos, d'Agostino, Chiarucci, Nimis and Nascimbene2020), and also 4) administratively defined territories, such as protected areas (Fattorini Reference Fattorini2020). The SAR can also be used as an effective tool in biodiversity conservation, for example to predict the biodiversity loss related to habitat loss or fragmentation (Brooks et al. Reference Brooks, Mittermeier, Mittermeier, Da Fonseca, Rylands, Konstant, Flick, Pilgrim, Oldfield and Magin2002; Halley et al. Reference Halley, Sgardeli and Monokrousos2013; Hanski et al. Reference Hanski, Zurita, Bellocq and Rybicki2013), to test long-term changes in species diversity (Chiarucci et al. Reference Chiarucci, Fattorini, Foggi, Landi, Lazzaro, Podani and Simberloff2017), or to disentangle the combined effect of area size, climate and disturbance on plant species richness (de Bello et al. Reference de Bello, Lepš and Sebastià2007).
SARs have been studied for almost all taxa, for example vascular plants (Krauss et al. Reference Krauss, Klein, Steffan-Dewenter and Tscharntke2004; Powell et al. Reference Powell, Chase and Knight2013; Patiño et al. Reference Patiño, Weigelt, Guilhaumon, Kreft, Triantis, Naranjo-Cigala, Sólymos and Vanderpoorten2014; D'Antraccoli et al. Reference D'Antraccoli, Roma-Marzio, Carta, Landi, Bedini, Chiarucci and Peruzzi2019; Dengler et al. Reference Dengler, Matthews, Steinbauer, Wolfrum, Boch, Chiarucci, Conradi, Dembicz, Marcenò and García-Mijangos2020) and bryophytes (Weibull & Rydin Reference Weibull and Rydin2005; Silva et al. Reference Silva, Sfair, dos Santos and Pôrto2018; Yu et al. Reference Yu, Li, Zhang and Guo2020), but have been poorly investigated in lichens. The positive effect of increasing area size on lichen richness has been highlighted in some papers, but with these mainly considering the area of suitable habitat. For example, in forest habitats, an increase in forested surface area has been shown to correlate with increasing species richness of epiphytic lichens (Marini et al. Reference Marini, Nascimbene and Nimis2011), and the increase in good quality forested habitat has had a positive effect on lichen richness together with the diversity of available substratum types (Lõhmus et al. Reference Lõhmus, Lõhmus and Vellak2007). Alteration of SARs has been investigated in epilithic lichens in relation to increased levels of pollution (Lawrey Reference Lawrey1991). When considering broader areas, such as ecoregions, SARs remain detectable, albeit affected by habitat heterogeneity (Martellos et al. Reference Martellos, d'Agostino, Chiarucci, Nimis and Nascimbene2020). SARs of lichens have also been addressed at local scales, usually in plots in which many taxa were recorded (e.g. Lõhmus et al. Reference Lõhmus, Leppik, Motiejunaite, Suija and Lõhmus2012; Dengler et al. Reference Dengler, Matthews, Steinbauer, Wolfrum, Boch, Chiarucci, Conradi, Dembicz, Marcenò and García-Mijangos2020; Dembicz et al. Reference Dembicz, Dengler, Steinbauer, Matthews, Bartha, Burrascano, Chiarucci, Filibeck, Gillet and Janišová2021). Some studies have considered SARs in lichens as part of investigations into disturbance processes (Lawrey Reference Lawrey1991), but very few studies have examined broader scales with a biogeographical or macroecological focus (Buckley Reference Buckley2005; Lücking et al. Reference Lücking, Rivas, Chaves, Umaña and Sipman2009).
In the present paper, we aim to fit traditional SAR models using data for three main substratum-related guilds of lichens, namely epiphytic, epilithic and epigaeic, obtained from exhaustive floristic inventories carried out in protected areas across Italy, and to test different functions to find which are the best in describing SARs for the different guilds. This has never been carried out before, to the best of our knowledge. A better understanding of the role of area size on lichen richness can be useful to inform area-based conservation (Maxwell et al. Reference Maxwell, Cazalis, Dudley, Hoffmann, Rodrigues, Stolton, Visconti, Woodley, Kingston and Lewis2020; Hoffmann Reference Hoffmann2022), and to highlight further knowledge gaps in the study of SARs for lichens, for example the comparison between poorly investigated versus well-studied areas, or between well-preserved natural areas versus areas located in anthropized landscapes.
Materials and Methods
Lichen inventories
Italy is an environmentally heterogeneous country, ranging from the Alpine chain to the centre of the Mediterranean Sea, in which lichenological studies have been widely carried out over the last four decades (Nimis Reference Nimis1993, Reference Nimis2016; Nimis & Martellos Reference Nimis and Martellos2022). We retrieved 44 sources reporting exhaustive lichen inventories carried out mainly in well-defined protected areas of various sizes within this context, over the last 25 years. Most inventories were retrieved from published papers, each dealing with a single protected area; for the area of the Ticino River, we merged the Piedmont and the Lombardy Ticino Natural Parks, since the multiple data sources referring to this area often lacked precise locality details for the species (G. Gheza, unpublished data). The distribution of the 44 areas is shown in Fig. 1 and their metadata is provided in Supplementary Material File S1 (available online).
Figure 1. A map of Italy showing the areas considered in this study. The numbers refer to the area identification code (ID) used in Supplementary Material File S1 (available online). Sites with an area > 50 km2 are shown in blue/shaded. In colour online.
For each area, we extracted separate lists of the three guilds (i.e. species growing on the three main substratum types colonized by lichens: bark and wood (epiphytic), rock and bryophytes on rock (epilithic), and soil, bryophytes on soil and plant debris (epigaeic)). Species lists were not available for all substrata within each protected area, which resulted in 40 lists of epiphytic species, 37 of epilithic and 37 of epigaeic species.
Data analysis
Polygons of most of the areas were retrieved from regional or national databases. When the relevant polygon was not available, the area was digitized by hand using QGIS 3.28 (QGIS Development Team 2022) based on the information available in the relevant paper.
All the subsequent analyses were performed using R v. 4.2.2 (R Core Team 2022). The area of each polygon (km2) was calculated using the ‘st_area’ function in the sf package (Pebesma Reference Pebesma2018). In cases where the area calculated using the available or digitized polygon differed from that declared in the paper, we retained the latter, presuming this to be the more accurate calculation of the true area surveyed.
We compared three commonly used SAR models: 1) the Gleason model (Gleason Reference Gleason1922), where S (number of species) is a function of LogA (area), 2) the Arrhenius power function and 3) the linear model. All models were fitted using the sars package (Matthews et al. Reference Matthews, Triantis, Whittaker and Guilhaumon2019), using ‘sar_loga’, ‘sar_pow’ and ‘sar_linear’ for the Gleason (LogA), Arrenhius (Power) and linear (Linear) models respectively. The three models were compared using the Akaike information criterion corrected for small sample sizes (AICc).
Results
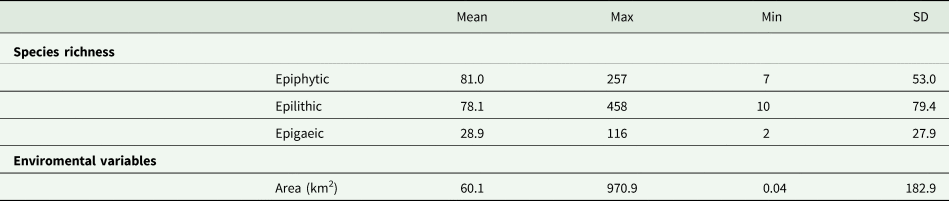
The 44 protected areas investigated have an average area size of 60.1 km2, the smallest area measuring 0.04 km2 and the largest 970.9 km2. In the lichen inventories there were reported an average of 81.0 epiphytic (min. 7, max. 257), 78 epilithic (min. 10, max. 458) and 28 epigaeic (min. 2, max. 116) species (Table 1).
Table 1. Species richness and area of the sites in Italy where the 44 lichen inventories were made, that were used in the analysis. SD = Standard deviation.
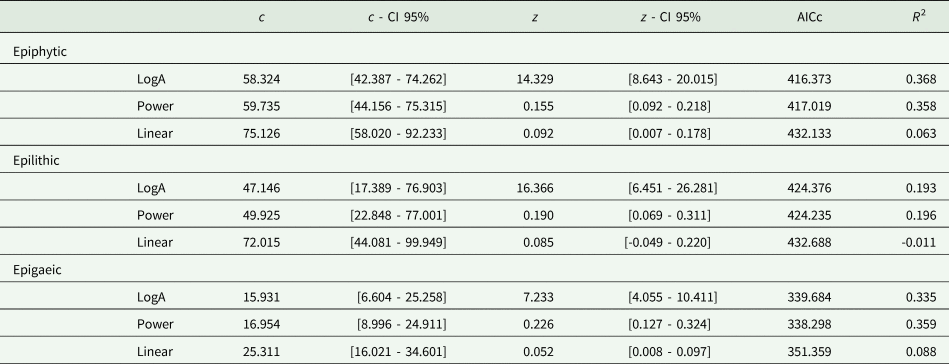
A SAR based on the LogA model was the best fit in the case of the epiphytic guild, while in the epilithic and epigaeic species the Power model fitted better (Fig. 2; Table 2). In all three cases the Linear model resulted in a poor fit. Given the small differences in AICc, all subsequent comparisons were carried out using the Power model, which was the best performing model in two out of the three cases. The amount of variance in species richness explained by the SAR models was constantly low (< 36%), indicating that area size significantly affected lichen species richness but that other factors probably contributed to a higher amount of species richness variation for the three lichen guilds. Using the Power model, the epiphytic guild presented the highest number of species found per unit area (1 km2), as indicated by the c value of the SAR (59.7 species), followed by the epilithic (49.9 species) and epigaeic (16.9 species) guilds. Epigaeic lichens had the higher slope values (0.23), followed by epilithic (0.19) and epiphytic (0.15) species.
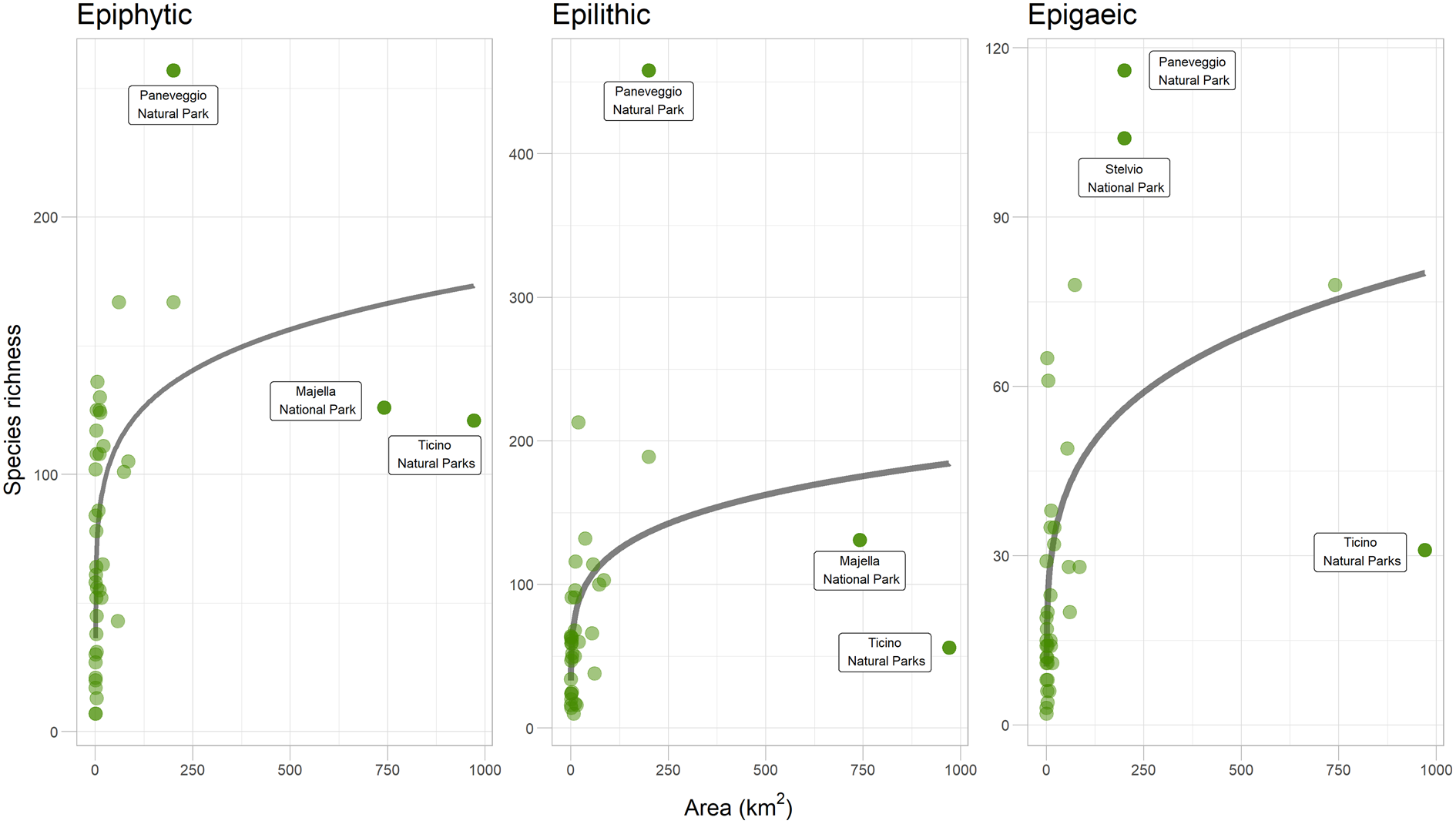
Figure 2. Species–area relationships (SAR) using the Power model for the three lichen guilds from inventories from the 44 protected areas from across Italy. Outliers discussed in the text are indicated. In colour online.
Table 2. Parameters and model fitting of the three species–area relationship (SAR) models for the three lichen guilds from inventories of 44 protected areas from across Italy. For each SAR model, the c value, representing the intercept, and the z value, representing the slope of the fitting line, are reported in terms of the number of species. For both c and z, the lower and upper confidence intervals (CI) are also given. The last two columns show the model evaluation data as corrected Akaike's information criterion (AICc) and R 2.
Discussion
Our results indicate that area size has a significant effect on species richness for all three guilds of lichens, albeit with different patterns. In fact, we found support for a positive correlation between species richness and area size, thus confirming the general validity of SARs in lichens, with major differences according to the substratum guild. In particular, epigaeic lichens were revealed to have the lowest species richness at the unit area (1 km2) and the highest increase in species richness with increasing area size. On the other hand, species richness of epiphytic and epilithic lichens showed high values at the unit area and a lower increase with increasing area size. These findings are consistent with the suggestion that availability of the different substratum types could be expected to vary with the increase in area size, for example depending on the geographical zone. However, larger areas are more likely to include a higher habitat heterogeneity (Scheiner et al. Reference Scheiner, Chiarucci, Fox, Helmus, McGlinn and Willig2011; Connor & McCoy Reference Connor, McCoy and Levin2017; Drira et al. Reference Drira, Lasram, Jenhani, Shin and Guilhaumon2019; Martellos et al. Reference Martellos, d'Agostino, Chiarucci, Nimis and Nascimbene2020; Moradi et al. Reference Moradi, Fattorini and Oldeland2020), which leads to a greater diversity in climate, substrata and microhabitats available for lichen colonization, than small areas. Larger areas could also be expected to include wider elevational spans, and lichen assemblages are known to vary along altitudinal gradients (Bruun et al. Reference Bruun, Moen, Virtanen, Grytnes, Oksanen and Angerbjörn2006; Grytnes et al. Reference Grytnes, Heegaard and Ihlen2006; Bässler et al. Reference Bässler, Cadotte, Beudert, Heibl, Blaschke, Bradtka, Langbehn, Werth and Müller2016; Di Nuzzo et al. Reference Di Nuzzo, Vallese, Benesperi, Giordani, Chiarucci, Di Cecco, Di Martino, Di Musciano, Gheza and Lelli2021; Vallese et al. Reference Vallese, Di Musciano, Muggia, Giordani, Francesconi, Benesperi, Chiarucci, Di Cecco, Di Martino and Di Nuzzo2022), thus promoting species richness as broader gradients are considered. Interestingly, the form of a SAR can be directly influenced by elevation: at increasing elevations, plant species richness decreases due to an increase of bare rock (Moradi et al. Reference Moradi, Fattorini and Oldeland2020). This could imply a decrease in epiphytic lichens but, on the other hand, it could increase the diversity of epilithic lichens owing to the greater substratum availability, and also of epigaeic species that can easily colonize the thin bare soil layer often developed in rock crevices.
Our analysis highlighted the presence of a small number of remarkable outliers in the dataset. The Paneveggio-Pale di San Martino Natural Park hosts a far higher richness for all three guilds than expected, given its area, confirming its claimed role as a ‘lichen diversity hotspot’. This is explained by the high environmental heterogeneity but also by its long history of lichenological exploration (Nascimbene et al. Reference Nascimbene, Gheza, Bilovitz, Francesconi, Hafellner, Mayrhofer, Salvadori, Vallese and Nimis2022). A similar pattern is highlighted, even if only for epigaeic species, for the Trentino sector of the Stelvio National Park (Nascimbene et al. Reference Nascimbene, Thor and Nimis2012). However, two outliers show a far lower richness than expected given their areas, probably for two different reasons. The Majella National Park (Gheza et al. Reference Gheza, Di Nuzzo, Vallese, Benesperi, Bianchi, Di Cecco, Di Martino, Giordani, Hafellner and Mayrhofer2021) is probably under-investigated, whereas the area of the Ticino Natural Parks lies within the western Po Valley, a territory that is largely a plain and severely impacted by human activities. Indeed most of its extent is covered by urbanized and agricultural lands, which has led to a depletion of its lichen biota (Nimis Reference Nimis1993). These results are consistent with the analysis by Martellos et al. (Reference Martellos, d'Agostino, Chiarucci, Nimis and Nascimbene2020), who tested SARs with lichens in the ecoregions of Italy, finding that the Montane and Subalpine ecoregions (the most represented in the Paneveggio-Pale di San Martino and Stelvio Parks) are positive outliers, whereas the Padanian ecoregion (in which most of the Ticino River area is located) represents a negative outlier. The case of Majella Park also highlights the limitations of not-so-exhaustive inventories when investigating SARs, which requires as comprehensive data as possible, especially when considering territories with a high environmental heterogeneity; good quality data from extensive fieldwork are therefore crucial to reliably test SAR.
The c value (i.e. the number of species per km2) seems to depend, among other factors, on the overall number of species within the guild considered (Triantis et al. Reference Triantis, Guilhaumon and Whittaker2012; Fattorini et al. Reference Fattorini, Borges, Dapporto and Strona2017). The whole lichen biota of Italy is composed of a low number of epigaeic (326) species and an intermediate number of epiphytic (663) species, while epilithic species represent the highest number (1352). This is partially consistent with our results, as the c value is lowest for the epigaeic (17) guild, while a different pattern is found for epilithic (44) and epiphytic (58) species. This difference between the epilithic and epiphytic guilds could be partially explained by the area effectively available for each guild. For example, in regions where forests were prevalent, the surface/substratum area available for epiphytic colonization was higher than that available for epilithic species, which are restricted to rocks not covered by vegetation. By contrast, except for high altitude zones, at least some trees that can harbour epiphytic species are always present in the areas considered. The lower number of epigaeic lichens could be due to this effect, and also to their overall lower diversity, which is probably driven by multiple factors, including higher competition with vascular plants and/or their higher sensitivity to environmental alteration and habitat loss (Scheidegger & Clerc Reference Scheidegger, Clerc, Dietrich, Frei, Groner, Keller, Roth, Stofer and Vust2002).
However, in complex landscapes it will be difficult to disentangle the effects of area size from those of other environmental variables, such as climatic or habitat heterogeneity. Finer-scale data would be required to test this relationship fully, for example by comparing different areas selected ad hoc to include both size and environmental gradients in a balanced design. Furthermore, precise data on microclimate would also be required, since lichens are greatly influenced by this factor (Di Nuzzo et al. Reference Di Nuzzo, Benesperi, Nascimbene, Papini, Malaspina, Incerti and Giordani2022).
Implications for conservation and future perspectives
Our results show that larger areas host more lichen species across all three substratum guilds, making larger protected areas more likely to display a higher species richness. Habitat heterogeneity and geographical context are expected to play a role in this, but area size itself seems quite crucial in the pattern. This supports the idea that mitigation of the main current threats to biodiversity conservation (i.e. habitat loss and global change) could be improved in the case of lichens by protecting larger areas, in a framework of area-based conservation. Larger areas are also more likely to include a higher number of so-called ‘microrefugia’, sites with locally favourable conditions that are placed outside the main range of a species or that are surrounded by unfavourable habitats, the preservation of which is considered one of the best strategies to mitigate the effects of climate change on sensitive lichens (Ellis Reference Ellis2020; Greiser et al. Reference Greiser, Ehrlén, Luoto, Meineri, Merinero, Willman and Hylander2021; Porada et al. Reference Porada, Bader, Berdugo, Colesie, Ellis, Giordani, Herzschuh, Ma, Launiainen and Nascimbene2023), even with recognized limitations (Di Nuzzo et al. Reference Di Nuzzo, Benesperi, Nascimbene, Papini, Malaspina, Incerti and Giordani2022). To date, there is contrasting evidence about the effectiveness of already established protected areas in lichen conservation (Martínez et al. Reference Martínez, Carreño, Escudero and Rubio2006; Rubio-Salcedo et al. Reference Rubio-Salcedo, Martínez, Carreño and Escudero2013), even though in some cases protected areas have been recognized as lichen diversity hotspots (Nascimbene et al. Reference Nascimbene, Gheza, Bilovitz, Francesconi, Hafellner, Mayrhofer, Salvadori, Vallese and Nimis2022) or refugia for fragmented species at the border of their distributional range (Gheza et al. Reference Gheza, Di Nuzzo, Vallese, Benesperi, Bianchi, Di Cecco, Di Martino, Giordani, Hafellner and Mayrhofer2021). To verify this, however, a comparison of SAR patterns with non-protected areas, that sometimes can be included in area-based conservation frameworks (Hoffmann Reference Hoffmann2022), should also be made, to examine whether the protection regime could influence SAR.
The study of the SAR itself can also be used as a powerful tool in biodiversity conservation, to set baseline targets for conservation based on area and/or species richness. In the latter case, however, these need to be set according to the local situation and studied considering alternate model frameworks (Desmet & Cowling Reference Desmet and Cowling2004; Metcalfe et al. Reference Metcalfe, Delavenne, Garcia, Foveau, Dauvin, Coggan, Vaz, Harrop and Smith2013; Drira et al. Reference Drira, Lasram, Jenhani, Shin and Guilhaumon2019). Obviously, the selection of potential protected areas cannot be based solely on their area size, since other factors must be taken into account (e.g. habitat diversity and heterogeneity, or elevational ranges), and species richness is not the only valid criterion with which to assess the conservation value of a site. Furthermore, the validity of the SAR is also dependent on scale (Dolnik & Breuer Reference Dolnik and Breuer2008; Chiarucci et al. Reference Chiarucci, Bacaro, Filibeck, Landi, Maccherini and Scoppola2012; Powell et al. Reference Powell, Chase and Knight2013) and sampling effort (Azovsky Reference Azovsky2011; Metcalfe et al. Reference Metcalfe, Delavenne, Garcia, Foveau, Dauvin, Coggan, Vaz, Harrop and Smith2013), and this could also apply when considering lichens.
To better understand the processes underlying the SAR, making a transition from a taxonomically descriptive and pattern-based approach towards a more predictive and generalizable process-based ecological approach, could make functional traits a valuable tool (Ellis et al. Reference Ellis, Asplund, Benesperi, Branquinho, Di Nuzzo, Hurtado, Martínez, Matos, Nascimbene and Pinho2021; Hulshof & Umaña Reference Hulshof and Umaña2023). An increase in traits variation with increasing area size has been demonstrated for lichens, although this is dependent not only on the area size but also on scale and environmental factors (Giordani et al. Reference Giordani, Malaspina, Benesperi, Incerti and Nascimbene2019).
The present work can be considered as a starting point towards a better knowledge of the multiple issues related to SARs applied to the study of lichen diversity patterns, and which need to be addressed to achieve a better understanding of the possible applications to conservation.
Finally, the present work highlights the importance of exhaustive species inventories, realized at different scales, to address ecological and conservation issues. Such endeavours are challenging, yet crucial for providing knowledge on the ecology and distribution of lichen species, and also for detecting diversity hotspots (Nascimbene et al. Reference Nascimbene, Gheza, Hafellner, Mayrhofer, Muggia, Obermayer, Thor and Nimis2021, Reference Nascimbene, Gheza, Bilovitz, Francesconi, Hafellner, Mayrhofer, Salvadori, Vallese and Nimis2022; Vondrák et al. Reference Vondrák, Svoboda, Malicek, Palice, Kocourkova, Knudsen, Mayrhofer, Thues, Schultz and Kodnar2022). High quality floristic research should therefore be recognized as a key tool to support more applied tasks in lichenology.
Author Contributions
Gabriele Gheza and Luca Di Nuzzo contributed equally to this paper.
Author ORCID
Paolo Giordani, 0000-0003-0087-7315.
Supplementary Material
The Supplementary Material for this article can be found at https://doi.org/10.1017/S0024282923000488.







