Introduction
Rostania Trevis. (Collemataceae, Peltigerales) is a genus of cyanolichens which corresponds to the informal ‘Occultatum’ group in Collema as circumscribed by Degelius (Reference Degelius1954, Reference Degelius1974). Rostania was resurrected by Otálora et al. (Reference Otálora, Aragón, Martínez and Wedin2013a, Reference Otálora, Jørgensen and Wedinb) following major phylogenetic studies of the whole family, and studied further by Košuthová and co-workers, who refined the generic delimitation (Košuthová et al. Reference Košuthová, Westberg, Otálora and Wedin2019). Rostania is, within Collemataceae, characterized by a comparatively small crustose to subfruticulose thallus lacking rhizines. The apothecia are initially immersed, later becoming sessile, with a pale whitish or yellowish to red-brown disc that is concave when young, plane when older but never convex. The spores are muriform with at least five cells, cuboid to oblong or ellipsoid (Košuthová et al. Reference Košuthová, Westberg, Otálora and Wedin2019; Cannon et al. Reference Cannon, Otálora, Košuthová, Wedin, Aptroot, Coppins and Simkin2020). Košuthová et al. (Reference Košuthová, Westberg, Otálora and Wedin2019) excluded three species from Rostania and transferred them to other genera as Collema coccophyllum Nyl., Leptogium paramense (P. M. Jørg. & Palice) A. Košuth. & Wedin, and Scytinium quadrifidum (D. F. Stone & McCune) A. Košuth. & Wedin. The position of Rostania callibotrys (Tuck.) Otálora et al. and R. laevispora (Swinscow & Krog) Otálora et al. in Rostania was uncertain. In the restricted sense, Košuthová et al. (Reference Košuthová, Westberg, Otálora and Wedin2019) accepted three species (R. ceranisca (Nyl.) Otálora et al., R. multipunctata (Degel.) Otálora et al. and R. occultata (Bagl.) Otálora et al.). Rostania ceranisca and R. multipunctata have been well delimited morphologically since Degelius (Reference Degelius1954), where the arctic-alpine R. ceranisca is characterized by its terricolous habit, a lobate thallus with accessory teretiform lobules, and large oblong spores. Rostania multipunctata, a mainly Mediterranean epiphytic species, is distinguished primarily by its large, more or less pulvinate and distinctly lobate thallus. Rostania occultata, a small, morphologically quite variable epiphytic taxon with more or less cuboid spores, is currently divided into two, somewhat vaguely delimited varieties (Degelius Reference Degelius1954; Perlmutter & Rivas Plata Reference Perlmutter and Rivas2018). The genetic and morphological variation in R. occultata and the previously unnoticed variation in photobiont morphology spurred Košuthová et al. (Reference Košuthová, Bergsten, Westberg and Wedin2020) to conduct a species delimitation study of Rostania s. str. using two coalescent-based methods. They concluded that neither of the two varieties of R. occultata corresponded to monophyletic species, but that R. occultata consisted of at least four species in Europe, bringing the number of species in Rostania s. str. to at least six in this region. Here, we will clarify the taxonomy and nomenclature of the R. occultata complex, including the formal description of two new species, and present a key to the currently accepted species of Rostania s. str.
Materials and Methods
The study is based mainly on material collected by the authors in Sweden and complemented by additional herbarium material from the herbaria S and UPS (abbreviations according to Thiers (Reference Thiers2021)), primarily Fennoscandian material. Our own collections are also housed in S and UPS. Specimens were initially studied using a dissecting microscope. Anatomical features were studied using a light microscope on thin sections cut with a razor blade or squash preparations mounted in water. Measurements of mature spores outside of the asci were made in water, under ×100 magnification using oil immersion with a precision of 0.5 μm, or from calibrated digital photographs using NIS-Elements (Nikon, Japan) with a precision of 0.1 μm. All microscopic images were taken on sections mounted in water unless otherwise stated. Spore measurements are presented in the format: (minimum value observed–) range including 80% of the observed values (–maximum value observed), with the mean of all observed values in the centre and italicized, and n being the number of measurements. Chemical reactions were observed using KOH (10% KOH) and Lugol's iodine (1% of I) without pretreatment of KOH. A list of selected representative specimens is cited under each species. Full lists of specimens examined in this study with DNA voucher codes and GenBank Accession numbers for newly generated sequences are given in the Supplementary Material (available online).
Results and Discussion
In our previous study (Košuthová et al. Reference Košuthová, Bergsten, Westberg and Wedin2020), we concluded through species delimitation analyses that the Rostania occultata complex consists of a minimum of four species, which we informally called R. occultata 1, R. occultata 2, R. occultata 3 and R. occultata 4,5,6. We tested and evaluated further potential candidate species within these informal species but found no distinct morphology, substratum ecology or geographical distribution to suggest that these genetically comparatively distinct but poorly sampled candidates should be treated as species. The four accepted species are treated here as Rostania effusa (‘R. occultata 2’), R. occultata (‘R. occultata 4,5,6’), R. pallida (‘R. occultata 1’) and R. populina (‘R. occultata 3’).
Degelius’ concept of dividing Rostania occultata into two varieties (Degelius Reference Degelius1954), which has often been followed in the literature, does not correspond in a clear way to the species presented here. There were apparently few specimens of R. pallida and R. effusa available to Degelius at that time and he appears to have used the name var. juniperinum in the herbarium for some of the material, but later abandoned that taxon and included them in var. occultatum in his monograph. Specimens of R. populina were consistently identified as var. populinum while specimens that we recognize as R. occultata were identified by Degelius either as var. occultatum or var. populinum.
In Sweden, Rostania occultata s. lat. is currently red-listed in the near-threatened (NT) category (SLU Artdatabanken 2020). Our study has not focused on conducting re-inventories of old localities, and we are not able to comment on trends in the abundance or distribution of any of the accepted species. We note, however, that several depend on old-growth, humid forest ecosystems and that recently these have been much affected by forestry activities throughout Fennoscandia and certainly elsewhere. Future red-listing assessments need to consider these four species separately, and appreciate that old reports of the two varieties of Rostania occultata cannot be assigned to these segregated species without studying voucher material. All Rostania species, particularly the two newly described here, are under-collected and should be actively searched for.
Observations regarding some characters
Thallus structure
The morphological thallus structure varies from granular in Rostania effusa (Fig. 1A–D), R. occultata (Fig. 2A & B) and R. pallida (Fig. 3A & B) to small foliose to subfruticulose in R. populina (Fig. 4A & B). The granules in R. effusa and R. pallida are more or less rounded, sometimes coalescing into larger aggregates in R. effusa, and small (up to 100 μm wide) compared to those in R. occultata, which can become up to 200 μm wide and sometimes form minute, lobate squamules often conglomerated in rosette-like structures. Rostania populina is the only species where the thallus is usually formed by lobes up to 0.5 mm long and to 250 μm thick with swollen ends, often forming small rosettes, with the lobes frequently lobulated (Fig. 4A & B).

Fig. 1. Rostania effusa (A, UPS L-611182; B, UPS L-088894; C, UPS L-656297; D, UPS L-929096—holotype; E & F, S F388734). A, habitus – granular thallus with an unusually large number of apothecia (Ap); note the small globose thallus granules. B, habitus – granular thallus dominated by small blastidia-like granules with apothecia (Ap). C, habitus – granular thallus forming small, scattered patches with apothecia (Ap). D, habitus – granular thallus forming scattered patches with apothecia (Ap). E, section of apothecium; note the excipulum thallinum with a simple pseudocortex (Pc) and Nostoc as single cells or 2–4 cells in clusters (N). F, section of apothecium; note the supporting tissue (St) formed by a single layer of rounded to ellipsoid cells and the euthyplechtenchymatous excipulum proprium (Ep). Scales: A–D = 1 mm; E & F = 10 μm. In colour online.
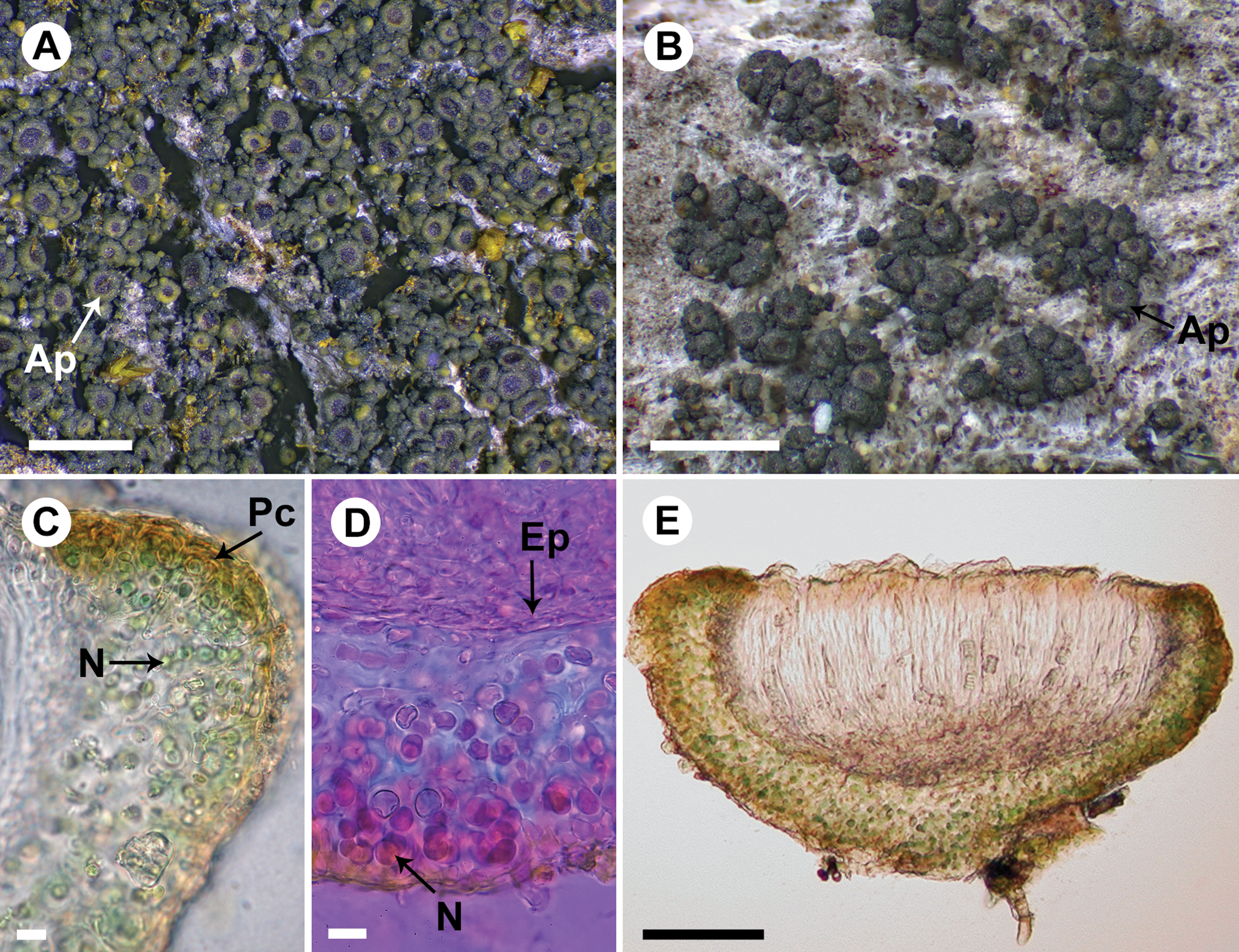
Fig. 2. Rostania occultata (A, UPS L-003461; B, S F388790; C & E, UPS L-872297; D, UPS L-123018). A, habitus – granular thallus composed of globose granules and apothecia dominating the thallus (Ap). B, habitus – thallus composed of granules and small squamules and apothecia (Ap). C, section of apothecium; note the excipulum thallinum with a simple pseudocortex (Pc) and Nostoc cells in short chains (N). D, section of apothecium mounted in Phloxin; note the euthyplechtenchymatous excipulum proprium (Ep) below the hymenium and Nostoc cells (N) with short coiled chains. E, section of apothecium. Scales: A & B = 1 mm; C & D = 10 μm; E = 50 μm. In colour online.

Fig. 3. Rostania pallida (A, UPS L-984886; B, S F388771—holotype; C, S F388769; D, UPS L-880093). A, habitus – granular thallus with apothecia (Ap) with the typical pale whitish to brownish disc. B, habitus – granular thallus dominated by the apothecia (Ap) with a pale disc; note the excipulum proprium which is visible at the surface as a whitish ring around the disc. C, section of apothecium margin with an excipulum thallinum which has a pseudocortex (Pc) with rounded cells. D, section of apothecium; note the excipulum thallinum with a pseudocortex (Pc) with rounded cells. Scales: A & B = 1 mm; C = 10 μm; D = 20 μm. In colour online.
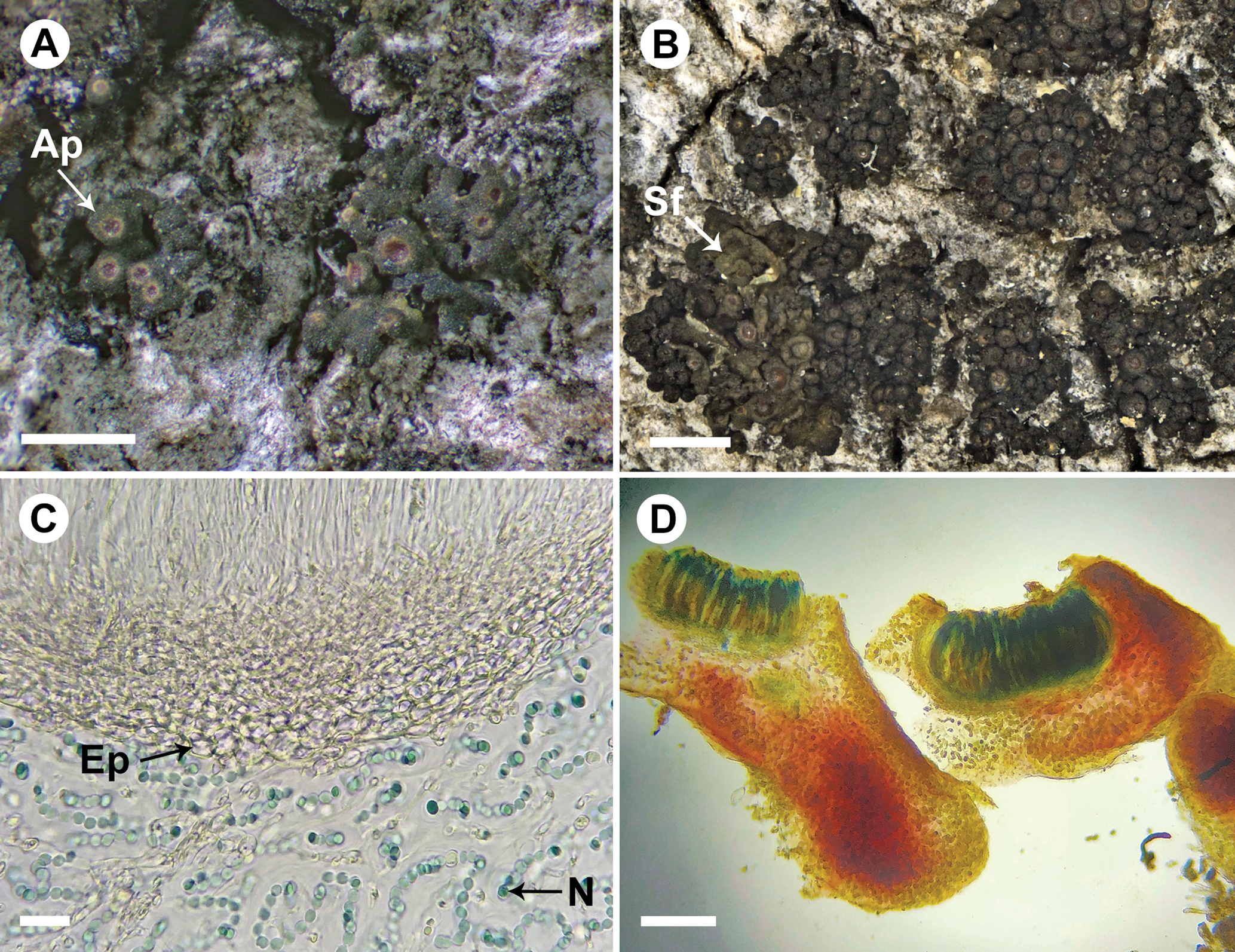
Fig. 4. Rostania populina (A, UPS L-125813; B, S F322805; C & D, UPS L-530093). A, habitus – distinctly lobate, subfruticulose thallus with apothecia (Ap); note the distinct excipulum proprium visible as a whitish ring around the disc. B, habitus – indistinct lobes covered by apothecia, growing together here with the similar species Scytinium fragrans (Sf); note the colour difference and the larger, rounded lobes in the latter species. C, section of apothecium; note the euparaplechtenchymatous excipulum proprium (Ep) formed by large, ellipsoid to round cells below the hymenium and Nostoc cells in long chains (N). D, apothecium section treated with Lugol's solution; note the nearly innate habitus of the apothecia and the I+ magenta to purple reaction of the thallus matrix. Scales: A & B = 1 mm; C = 20 μm; D = 100 μm. In colour online.
Colour of the thallus and apothecium disc
The thallus colour in most Rostania species has the typical greenish to brownish tones found in Collema s. lat. Specimens of R. occultata and R. populina are sometimes almost black. In Rostania pallida, however, the thallus is usually pale grey, which separates it from the other species. Sometimes it becomes darker with brownish and greenish tones. The apothecium disc in R. pallida is almost always pale, whitish, greyish or yellowish, rarely pale brown (Fig. 3A & B). The remaining species have the red-brown apothecium disc (Figs 1C, 2A & 4A) that is commonly associated with Collema s. str., for example in C. nigrescens.
Iodine reactions in the thallus
In the majority of the specimens of Rostania populina (8, including the type, of 11 specimens examined) we have observed a slow but distinct I+ magenta to purple reaction to Lugol's solution in the thallus (Fig. 4D). This reaction was discussed by Degelius (Reference Degelius1954: p 247) and is apparently due to the presence of glycogen in the gelatinous matrix derived from the photobiont (see Degelius (Reference Degelius1954) and references therein). The reaction is common throughout Collemataceae and it is also present in R. multipunctata and R. ceranisca, as well as in Scytinium fragrans, a species similar to R. populina. The reaction is not constant in R. populina but we have never observed it in the other species of the R. occultata complex. However, in R. occultata s. str., as well as in the species described here as R. effusa and R. pallida, one can often find single Nostoc cells (these are probably heterocysts) surrounded by an I+ purple envelope, c. 1 μm thick. These cells occur scattered in the thallus and excipulum thallinum of the apothecia but the number of such cells varies and they are not always found.
Photobionts
Košuthová et al. (Reference Košuthová, Bergsten, Westberg and Wedin2020) found a correlation between thallus morphology and the Nostoc morphotypes; species with granular thalli have Nostoc that produce either single cells or cells in small clusters (Fig. 1E), whereas species with lobate thalli have Nostoc with cells in short to long chains (Figs 2D & 4C). We have not investigated whether these Nostoc-morphotypes constitute distinct species, or if they are the result of the different medullary structure that the granular and lobate thalli produce, but we plan to return to this issue in a future study.
Cortical structures of the excipulum thallinum
Degelius (Reference Degelius1954: pp. 46–47) recognized three different types of cortical structure in Collemataceae: a true cortex (or eucortex), which is not present in any Rostania species, a ‘typical’ (to follow the terminology of Degelius (Reference Degelius1954)) pseudocortex, and what Degelius called a ‘primitive’ pseudocortex. The eucortex is characterized by one (rarely more) continuous layer of uniform cells, sharply delimited towards the inner part of the thallus. The ‘typical’ pseudocortex is formed by two or more continuous layers of isodiametric (globose or polygonal) or somewhat oblong cells indistinctly separated from the inner part. A ‘primitive’ pseudocortex is a simple structure that does not have a continuous layer of cells. We refer to this here as a ‘simple’ pseudocortex, to avoid implying that this is something primitive from an evolutionary viewpoint. These distinctions are primarily relevant in the excipulum thallinum because cortical structures are usually poorly developed in Rostania thalli. Rostania effusa (Fig. 1E), R. occultata (Fig. 2C) and R. populina all have a very simple pseudocortex. Rostania pallida (Fig. 3C) has, at least in the apothecium margin, a more distinct and well-developed cellular pseudocortex with a continuous layer of isodiametric cells that fits Degelius’ description of a typical pseudocortex. In R. effusa, a more well-developed structure can sometimes occur on the lower side of the apothecium to form a supporting tissue with a single layer of rounded to ellipsoid cells (Fig. 1F).
Excipulum proprium
Degelius (Reference Degelius1954; see also Swinscow & Krog (Reference Swinscow and Krog1986)) described three types of excipulum proprium in Collema s. lat.: euthyplechtenchymatous (Fig. 2D), subparaplechtenchymatous and euparaplechtenchymatous (Fig. 4C). The first is characterized by elongated, narrow cells, the second has comparatively short but still elongated cells and the third has isodiametric (globose to polygonal) cells. The differences are not clear-cut and variation occurs to some degree throughout the excipulum in an individual apothecium as the cells change shape and size depending on the position relative to the hymenium. Nevertheless, the structure of the excipulum is useful to distinguish between species; in particular, the euparaplechtenchymatous structure with large cells in Rostania populina (Fig. 4C) is a distinctive feature compared to the other three species in the R. occultata complex (e.g. Fig. 2D).
Ascospores
The spores in the Rostania occultata species complex are often stated to be more or less cuboid (Degelius Reference Degelius1954; Jørgensen Reference Jørgensen2007; Košuthová et al. Reference Košuthová, Westberg, Otálora and Wedin2019), but in reality they vary from ellipsoid-oblong to oblong with truncated ends, or almost cuboid (Košuthová et al. Reference Košuthová, Bergsten, Westberg and Wedin2020). They are often not easy to classify since immature spores of all species are first globose and then become ellipsoid to more edged. Mature spores in R. pallida (Fig. 5A & B) are ellipsoid, often somewhat constricted at the centre. In the other species, mature spores are usually oblong with truncated ends or cuboid (Fig. 5C & D). When we observed spores in KOH, immature or otherwise untypical spores often appeared more typical.
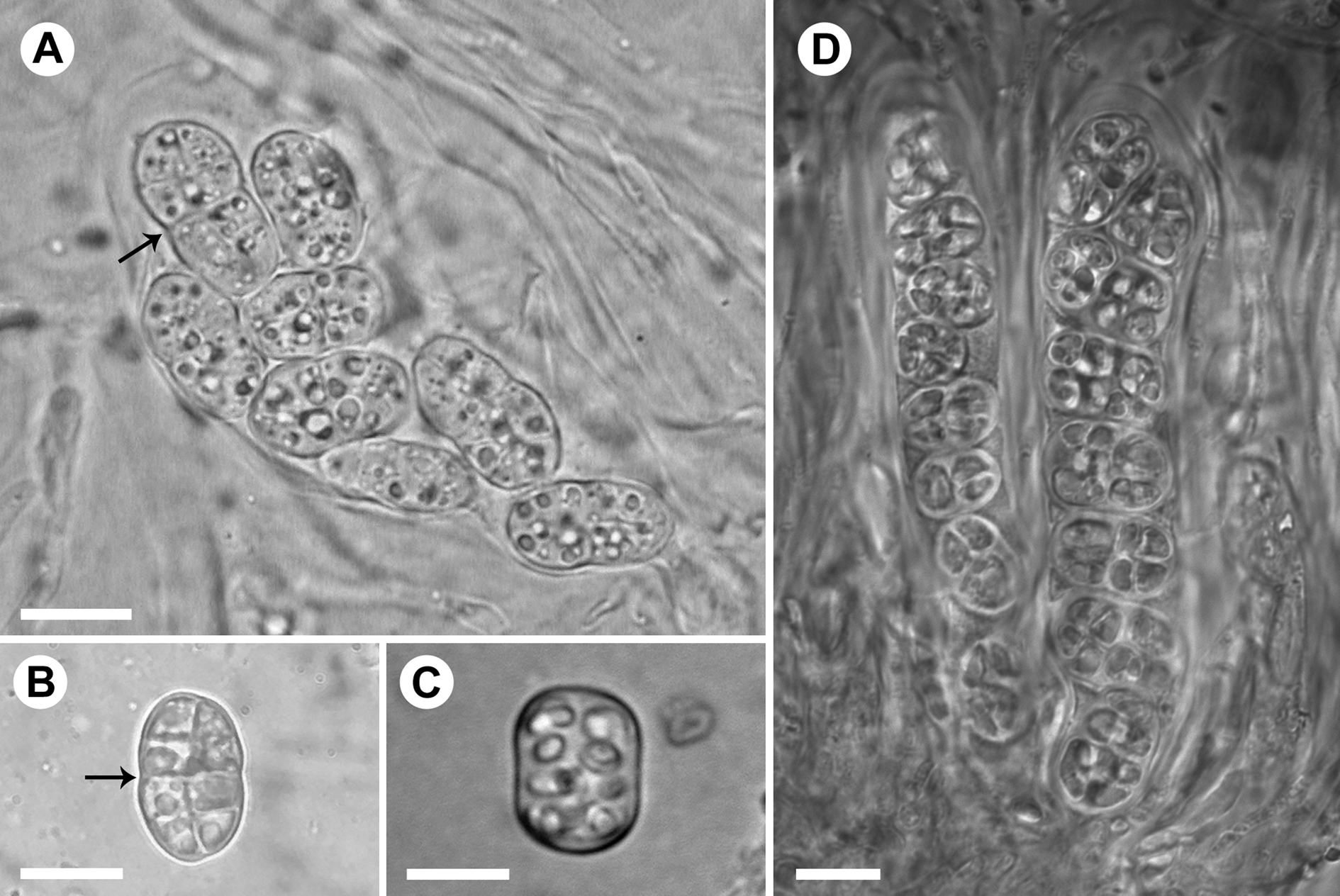
Fig. 5. Asci and spores in the Rostania occultata species complex. A, R. pallida (S F388719) – ascus with ellipsoid immature spores; note constriction at the centre (arrow). B, R. pallida (UPS L-980332) – ellipsoid mature spore; note constriction at the centre (arrow). C, R. occultata (UPS L-872297) – oblong mature spore with truncated ends. D, R. populina (S F332481) – ascus with oblong spores with truncated ends (right) and ascus with oblong immature spores (left). Scales: A–D = 10 μm.
Key to Rostania s. str.
For an updated key to the genera in the Collemataceae, see Cannon et al. (Reference Cannon, Otálora, Košuthová, Wedin, Aptroot, Coppins and Simkin2020).
1 Terricolous in arctic-alpine areas; thallus lobate with accessory teretiform lobules, forming dense, intricate cushions; spores 20–40 μm long………R. ceranisca
Corticolous; thallus granular, squamulose or foliose with flattened lobes; spores < 20 μm long………2
2(1) Thallus distinctly foliose, up to c. 3 cm wide………R. multipunctata
Thallus an effuse, granular to subsquamulose crust, or with small foliose to subfruticulose lobes and then thallus up to c. 2 mm wide………3
3(2) Apothecium disc whitish, yellowish or rarely with a pale brown tinge (Fig. 3A & B); mature spores ellipsoid, often constricted at the centre (Fig. 5A & B); excipulum thallinum with a distinctly cellular pseudocortex (Fig. 3C)………R. pallida
Apothecium disc red-brown; mature spores cuboid to oblong with truncated ends (Fig. 5C & D); excipulum thallinum with a simple pseudocortex without clear cellular structure (Figs 1E & 2C)………4
4(3) Thallus with distinct, up to 0.5 mm long lobes raised from the substratum; excipulum proprium below the hymenium euparaplectenchymatous with rounded cells up to 15 μm wide (Fig. 4C); Nostoc mainly in distinct, long chains (up to 20 cells)………R. populina
Thallus granular or rarely with minute squamules up to 0.2 mm long; excipulum proprium below the hymenium euthyplectenchymatous with mostly narrow, elongated or sometimes rounded cells up to 8 μm wide (Fig. 2D); Nostoc single or in clusters, or in shorter, often indistinct chains (up to 10 cells)………5
5(4) Thallus an effuse to crowded crust of small granules, c. 30–75(–100) μm diam.; apothecia mostly sparse; Nostoc in single cells or 2–4 cells in small clusters (Fig. 1)………R. effusa
Thallus granular to subsquamulose, of scattered to crowded granules or forming small cushions, rarely forming small, lobate squamules c. 120–170(–200) μm diam. (Fig. 2B); apothecia often abundant and dominating the thallus; Nostoc cells in short chains or when coiled, up to 10 cells long (Fig. 2C & D)………R. occultata
Taxonomy
Rostania effusa A. Košuth., M. Westb. & Wedin sp. nov.
MycoBank No.: MB 842249
Similar to Rostania pallida but differs in the dark green to dark brown, granular thallus, apothecia with reddish brown discs and distinctly cuboid mature spores. Differs from R. occultata in the much smaller, up to 100 μm wide thallus granules, and in having a Nostoc photobiont that is single-celled or with 2–4 cells in small clusters.
Type: Sweden, Härjedalen, Lillhärdal par., Bäreggen, N of Mt Galberget, c. 5.4 km SW of Lillhärdal church, on Populus tremula, elev. 529 m, 61.82408°N, 13.98514°E, 11 September 2018, Westberg HH032 (UPS L-929096—holotype; US, PRA—isotypes). DNA voucher (holotype) AL507, GenBank Accession nos: MT705311 (mtSSU), MT906284 (Mcm7), MT906220 (RPB2 part 5–7), MT906257 (RPB2 part 7–11).
(Fig. 1)
Thallus effuse, of more or less globose granules scattered to contiguous or coalescing and forming a granular crust (Fig. 1A–D); granules c. 30–75(–100) μm wide, dark green to dark brown, ecorticate or with a very indistinct pseudocortex; medulla densely packed with reticulately branched hyphae forming a net around the photobiont. Photobiont Nostoc with cells (up to 8 μm diam.) singular or in small clusters (2–4 cells usually visible) (Fig. 1E).
Apothecia often sparse or absent, perithecial when young, rarely expanding when mature, up to 0.3 mm wide; disc flat, usually distinctly red-brown, up to 0.15 mm diam. with excipulum proprium normally not visible in surface view; excipulum thallinum thin, persistent, up to c. 75 μm wide with hyphal arrangement similar to that of the thallus and with a simple pseudocortex (Fig. 1E); a basal supporting tissue (Henssen Reference Henssen, Keuck, Renner, Vobis and Reynolds1981) sometimes present, 6.5–8 μm thick, consisting of one layer of continuous isodiametric polygonal cells (Fig. 1F); excipulum proprium thin (up to 10 μm wide), euthyplectenchymatous, composed of hyphae with elongated, rectangular cells (up to 15.5 × 3 μm), cells below the hymenium and in the parathecial crown shorter (up to 10 × 3 μm); hypothecium colourless, I+ blue, but reaction is often weak, up to 20 μm thick; hymenium I+ blue, c. 85–125 μm tall, in the upper part with a reddish to brownish colour; paraphyses simple, sometimes anastomosing, up to 1.5 μm wide in mid-hymenium, tips usually 3(–5) μm wide, cylindrical to clavate; asci (n = 6) sub-clavate to sub-cylindrical, c. 70–110 × 12–30 μm; ascospores cubic to oblong with truncated ends when mature (immature spores globose to ellipsoid), muriform with up to 12 visible cells, (8.7–)10.3–13.2–15(–16.3) × (8–)10–10.9–12.5(–14.5) μm (n = 95).
Pycnidia not seen.
Etymology
Effusa (‘effuse’) refers to the structure of the thallus, composed of granules spreading, without a distinct margin, into an effuse crust (Fig. 1).
Ecology and distribution
This species occupies a wide range of substrata but in Fennoscandia is most common on Populus tremula, Salix caprea and Sorbus aucuparia. It is also known from Acer platanoides, Alnus incana, Betula spp., Fraxinus excelsior, Picea abies and Salix fragilis. It has been found in many different habitats, mostly in humid and rather open situations such as along forest margins near streams, lakes or bogs, but also on solitary trees in gardens and avenues, in open coniferous forests and in subalpine birch forests. Rostania effusa is probably the most common and widespread of the four species in the R. occultata complex in the Fennoscandian boreal zone but scattered finds have also been made further south. Outside of Fennoscandia it is so far known only from single collections from Russia, Scotland and Slovenia.
Notes
Rostania effusa is the species called ‘R. occultata 2’ in Košuthová et al. (Reference Košuthová, Bergsten, Westberg and Wedin2020). It often has a distinct appearance with very small granules, which often appear blastidiate or are similar to the goniocysts that can be found in the Micarea prasina group. It can be mistaken for Rostania pallida, which also has a minutely granular thallus. However, R. effusa is often sterile and the apothecia, when produced, are very small and often quite indistinct, with a brownish to reddish disc which distinguishes it from the pale, whitish to yellowish, rarely pale brown disc in R. pallida. The spores are cubic when mature compared to the oblong to ellipsoid mature spores in R. pallida. When the thalline granules are sparsely produced it can be very similar to R. occultata, but the granules are always smaller in R. effusa than in R. occultata. Furthermore, the Nostoc forms singular cells or small clusters in R. effusa and not long, coiled chains as in R. occultata.
Exsiccates
Finland: Regio kuusamoënsis: Salla, 1 July 1937, Laurila (Räsänen, Lich. Fenn. Exs. 626; UPS L-534969, S F64160). Tavastia borealis: Saarijärvi, 23 Aug. 1943, Koskinen (Räsänen, Lich. Fenn. Exs. 948; UPS L-535423, S F64158).—Sweden: Södermanland: Stora Malm par. 15 Sept. 1914, Malme (Malme, Lich. Suec. Exs. 468; UPS L-109060, in mixture with R. occultata, S F147920). Värmland: Södra Finnskoga par. 27 July 1979, Sundell (Vězda, Lich. Sel. Exs. 2005; UPS L-004538, S F147910).
Selected specimens examined
Finland: Regio kuusamoënsis: Kuusamo, 1963, Ahti 18020 (UPS L-609250). Savonia borealis: Nilsiä, 1959, Henssen 4549 (UPS L-003498). Tavastia borealis: Mahlu [Saarijärvi], 1943, Koskinen (S F64158); Saarijärvi, 1944, Koskinen 56 (UPS L-079700).—Norway: Finnmark: Sør-Varanger, 1957, Th. M. Fries (UPS L-003494). Nordland: Vega, 1976, Degelius V-1520 (UPS L-079696).—Russia: Komi Republic: 2004, Hermansson & Pystina 13548a (UPS L-162655).—Slovenia: Notranjsko-kraska: Snežnik-Javorniki, 1997, Prügger & Surina (GZU 56-2003, DNA voucher AL485).—Sweden: Dalarna: Idre par., 2002, Hermansson 11912 (UPS L-656297); Svärdsjö par., 2012, Westberg (S F304739, DNA voucher AL256); Söderbärke par., 1997, Hermansson 8055 (UPS L-088894). Gästrikland: Järbo par., 2006, Delin (UPS L-163158). Hälsingland: Järvsö par., 2017, Košuthová et al. 103 (S F388772, DNA voucher AL308). Härjedalen: Lillhärdal par., 2018, Westberg HH053 (UPS L-929106, DNA voucher AL506). Jämtland: Krokom par., 2017, Jonsson FU6545 (S F388734, DNA vouchers AL270 and AL269, GenBank Accession number MW724307); Åre par., 1913, Du Rietz (UPS L-611182, L-616784). Lule Lappmark: Jokkmokk par., 1993, Nordin 3335 (UPS L-058678); Liden par., 2017, Westberg URL339 (UPS L-880300). Närke: Kvistbro par., Berglund (S F403779). Pite Lappmark: Arjeplog par., Westberg PL021, PL035 (S L876859, DNA voucher AL510; S F388749, DNA voucher AL316). Småland: Hult par., 2020, Westberg (UPS L-963723, S F403831, DNA voucher AL618, GenBank Accession number MW724312). Södermanland: Stora Malm par., 1913, Malme (S L18353). Uppland: Åland par., 2017, Westberg, Ekman, Zamora (UPS L-834451, DNA voucher AL244). Värmland: Bjurtjärn par., 2017, Berglund 37 (S F388782, DNA voucher AL480). Västerbotten: Skellefteå par., 1984, Muhr (UPS L-596495). Åsele Lappmark: Dorotea par., 1939, Du Rietz (UPS L-947502, L-947504).—Great Britain: Scotland: Highland, 2017, Coppins 25250 (S F321109, DNA voucher AL481).
Rostania occultata (Bagl.) Otálora, P. M. Jørg. & Wedin
Fungal Diversity 64, 289 (2013).—Collema occultatum Bagl. Comm. Soc. Crittog. Ital. 1 (Fasc. 1), 23 (1861); type: [Italy, Liguria:] ‘Ad castanearum truncos in Alpen. Ligustiae prope Serravalle’. Pietro Ferrari (UPS L-074630—lectotype (Degelius Reference Degelius1954: p. 248, 250), S F148057—isolectotype).
Collema coccophylloides Hepp ex. Müll. Arg. Mém. Soc. Phys. Hist. Nat. Genève 16(2), 426 (1862); type: Switzerland, Genève, ‘tres-rare, sur un Populus pyramidalis, avant d'arriver au Bois de la Batie, en venant du vieuz pont sur l'Arve’, Hepp 1860, in hb. Müll. Arg. (G00278941—syntype).
Rostania quadrata (J. Lahm ex Körb.) Trevis., Rc. Ist. Lomb., Milano, ser. 2 13, 75 (1880).—Collema quadratum J. Lahm ex Körb., Parerga Lichenol. (Breslau) 5, 411 (1865); type: Germany, Am Fuße des Burgberges (Ob Crailsheim an Maßholder), 1859, Kemmler (S F148064—lectotype, designated here, MBT 10004691; UPS L-986294, L-986289—isolectotypes).
Thallus granular to sometimes indistinctly subsquamulose, of widely scattered granules or conglomerates of granules forming small cushions (Fig. 2A & B); granules globose, dark green to almost black, 120–170(–200) μm diam., granular cushions up to c. 2 mm wide, sometimes of minute lobes that can form minute rosette-like thalli, lobes to c. 0.2 mm long, rosettes to c. 1 mm wide; medulla composed of a network of reticulately branched hyphae with thin walls, at the surface forming a simple pseudocortex as the end cells swell to a rounded, up to 5 μm wide cell or with the hyphae looping around the outermost photobiont cells, interior densely filled with the photobiont. Photobiont Nostoc in distinct but mostly coiled chains of up to 10 cells (Fig. 2C & D), individual cells 4–7 μm diam.
Apothecia common, scattered to aggregated, sometimes covering the small thallus cushions, perithecial when young; disc expanding to up to 0.14 mm diam., dark red-brown, occasionally poorly pigmented and then pale yellow-brown (Fig. 2E) with excipulum proprium indistinct or visible as a pale, diffusely delimited, often incomplete ring surrounding the disc and with a thin, persistent excipulum thallinum; excipulum thallinum similar to the thallus, a simple pseudocortex (Fig. 2C); excipulum proprium I−, euthyplechtenchymatous, usually thin below the hymenium, up to c. 10 μm thick, rather compact with small ellipsoid cells, up to c. 8 × 3 μm (Fig. 2D) but mostly smaller, next to the hymenium thin, to c. 10 μm thick but often less, with more narrow and elongated, often more or less rectangular cells, the uppermost cells becoming shorter and rounded, not or slightly expanding at the top to form an up to 15 μm wide parathecial crown; hypothecium colourless, I+ blue but the reaction is often weak and sometimes not apparent, variable in thickness, often thin, to c. 15 μm thick but can be up to 50 μm thick; hymenium I+ blue, 80–140 μm tall, upper part with a red-brown colour; paraphyses to 2 μm wide, tips clavate, to 5 μm wide; asci (n = 3) subclavate to subcylindrical, c. 60–110 × 8–15 μm; ascospores cubic-oblong with truncated ends when mature (immature spores globose to ellipsoid), muriform with (4–)8–12 cells in optical view, (11–)13–14.6–16(–19) × (9.5–)10–11.0–12(–14.5) μm (n = 54).
Pycnidia not seen.
Ecology and distribution
In the northern part of its range in Sweden, Rostania occultata often grows on Populus tremula, but otherwise it is known from a wide range of deciduous trees, such as Ulmus, Fraxinus, Acer and Malus. It occurs in a variety of habitats and has been collected in moist deciduous forests, mixed forests and in spruce-dominated forests. It is often found in open situations, for example along gravel roads or on solitary trees around farms. In dense forests it can grow on Populus tremula high up on the trunk near the canopy. Rostania occultata is distributed throughout southern Sweden, extending to the province of Uppland in the north. It is widely distributed throughout Europe and we have seen specimens from Austria, Bosnia and Herzegovina, Germany, Greece, Italy, Scotland, Spain and Switzerland.
Nomenclatural notes on Collema coccophylloides Hepp ex. Müll. Arg
We have studied the original specimen that Degelius (Reference Degelius1954: p. 246 and p. 250) mentions, but we likewise refrain from lectotypifying on this rather poor sample.
Nomenclatural notes on Collema quadratum J. Lahm ex Körb
In the original description, a number of syntypes (see Supplementary Material, available online) were included, collected by Lahm, Nitschke and Kemmler. Körber distributed material collected by Kemmler in 1860, in the exsiccate Lich. Sel. Germ. 269, but the duplicate we have studied in S (F148065) is rather poorly developed. As lectotype we designate the sample in S, F148064, which is a rich and well-developed sample from the same locality as distributed in the exsiccate, but collected by Kemmler a year earlier (in 1859). Kemmler clearly collected this species on several occasions at Crailsheim.
Notes
Rostania occultata was informally called ‘R. occultata 4,5,6’ in our previous species delimitation study (Košuthová et al. Reference Košuthová, Bergsten, Westberg and Wedin2020), which suggested that it may be composed of three lineages that could represent species. We have, however, not been able to find any distinguishing characters or geographical distribution patterns that could support a further separation and we currently view this clade as a variable and widely distributed species. Rostania occultata can be confused with both R. effusa and R. populina. Compared to R. effusa it usually has larger and fewer granules and often has a distinctly different aspect to its habitus, that is somewhat Collema s. str.-like with a green thallus and red-brown apothecia, compared to the small and often brownish and/or blastidiate appearance in R. effusa. Well-developed specimens with a more squamulose habit can be difficult to separate from small and poorly developed specimens of R. populina. Rostania occultata often has a less distinct excipulum proprium in external view, but anatomically there are several differences: R. populina has a distinct excipulum proprium which is euparaplechtenchymatous in section (Fig. 4C) and a comparatively loose medulla with scattered, long Nostoc chains (Fig. 4C). A small number of specimens of R. occultata, mainly from continental Europe, are somewhat intermediate between R. occultata and R. populina (e.g. the type material of Collema quadratum) having a subparaplechtenchymatous excipulum proprium below the hymenium and more distinct Nostoc chains. The medulla, however, appears less loose and the photobiont densely fills the thallus interior and, on the whole, the appearance is rather different from R. populina. In addition, we have never observed a purple iodine reaction of the gelatinous matrix of the thallus interior similar to that present in most specimens of R. populina (Fig. 4D).
Exsiccates
France: Grand-Est: Vosges, Harmand (Claudel, Claudel & Harmand, Lich. Gall. 304) (UPS L-987571). Occitanie: Aveyron. Marc (Krypt. Exs. (Vindob.) 1542) (UPS L-677687, S F148059); Aveyron. Marc (Zahlbruckner, Lich. Rar. Exs. 77) (UPS L-987569).—Germany: Baden-Württemberg: Crailsheim, Kemmler (Zwack-Holzhausen, Lich. Exs. 412) (UPS L-987570, S F148061—syntypes of Collema quadratum); Crailsheim, 1861, Kemmler (Körber, Lich. Sel. Germ. 269) (S F148065—syntype of Collema quadratum).—Sweden: Södermanland: Stora Malm par., 15 Sept. 1914, Malme (Malme, Lich. Suec. Exs. 468) (UPS L-109060, in mixture with Rostania effusa).
Selected specimens examined
Austria: Kärnten: Karawanken, 1985, Hafellner 13607 (GZU P-85). Niederösterreich: 1917, Suza (UPS L-973902). Steiermark: Hochschwab-Gruppe, 1993, Poelt (GZU 1-93, DNA voucher AL488).—Bosnia and Herzegovina: Republika Srpska: 2008, Bilovitz 3819 (GZU 58-2012, DNA voucher AL528).—France: Alpes Cote d'Azur: 2012, Jonsson (S F403821, DNA voucher AL661, GenBank Accession number MW724317). Pays de la Loire: 1907, (S F148063).—Germany: Baden-Württemberg: Kemmler (S F148052—syntype of Collema quadratum). Niedersachsen: 1930, Erichsen (UPS L-973901). Nordrhein-Westfalen: Lahm (UPS L-973903, S F148068—syntypes of Collema quadratum).—Greece: Crete: nomos Chania, 2005, Llop 506060303 (S F233720, DNA voucher AL264).—Italy: Toscana: passo del'Abetone, 2002, Tretiach (TSB 36983).—Spain: Asturias: 2018, Westberg (UPS L-933974, DNA voucher AL502).—Sweden: Bohuslän: Ljung par., 1940, Degelius (UPS L-079759); Marstrand par., 1920, Magnusson 5186 (UPS L-003461). Dalsland: Dalskog par., 1944, Degelius (UPS L-079762). Halland: Släp par., 1932, Magnusson 13782 (UPS L-003470, S F18349). Närke: Svennevad par., 1872, Björkman (UPS L-003475). Skåne: Torekov par., 2008, Hultengren 2365 (UPS L-704977, DNA voucher AL508). Småland: Sävsjö par., 2017, Isaksson (S F388790, DNA voucher AL294). Södermanland: Tumbo par., 2002, Nordin 5407 (UPS L-120396, DNA voucher MWE87). Uppland: Funbo par., 1955, Santesson 11127 (UPS L-123018); Vänge par., Ekman & Westberg 352 (UPS L-872297, DNA voucher AL367). Västergötland: Västerlövsta par., 2020, Westberg (UPS L-963072). Värmland: Färnebo par., 2010, Odelvik 10528 (S F178903, DNA voucher AL262).—Switzerland: Bern: Berner Alps, 2006, Hafellner 77470 (GZU 39-2011, DNA voucher AL487). Jura: 1996, Groner 13988 (hb. SwissLichens WSL 6793, DNA voucher AL607, GenBank Accession number MW724311). Zürich: 1883, Hegetschweiler (S F148060).—Great Britain: Scotland: Argyll and Bute, 1949, Degelius (UPS L-081088).
Rostania pallida A. Košuth., M. Westb. & Wedin sp. nov.
MycoBank No.: MB 842248
Similar to Rostania effusa but differs in the ellipsoid (not cuboid when mature) spores, the grey, granular thallus, and abundant apothecia with pale, white to yellowish discs and a cellular pseudocortex with cells up to 15 μm wide.
Type: Sweden, Hälsingland, Färila par., 3.5 km SW of Kårböle church, just outside Grötvallsskogen Nature Reserve, E of the forest road, wet spruce forest with Equisetum, on Salix caprea, alt. 250 m., 61.966389°N, 15.257778°E, 15 July 2017, Alica Košuthová, Martin Dinga, Maros Dinga, Mats Wedin 117 (S F388771—holotype; UPS L-987704, US—isotypes). DNA voucher (holotype) AL309, GenBank Accession nos.: MT705303 (mtSSU), MT906250 (RPB2 partial).
Collema byssinum β juniperinum Sommerf., Suppl. Fl. lapp. (Oslo), 118 (1826); type: Norway, ‘Norvegia septentr.: Saltdalen, Nordlandiae, in juniperis, 1822, Sommerfelt’ (UPS L-003492—syntype; O L-014065, not seen—syntype).
Thallus effuse, granular; granules scattered to contiguous or coalescing and forming small, up to 1.5 mm wide cushion-like structures, spreading over the substratum to form widespread patches, pale grey (usually) or brownish grey or sometimes greenish grey; individual granules c. 35–100 μm wide (mostly below 70 μm) (Fig. 3A), ecorticate or with a very indistinct pseudocortex; medulla of densely packed, reticulately branched hyphae forming a net around the photobiont. Photobiont Nostoc forming singular cells or in small clusters (2–3(–4) cells usually visible).
Apothecia common and usually abundant, globose, perithecial when young; disc when mature smooth, flat, whitish, yellowish or rarely with a pale brown tinge, up to 0.25(–0.35) mm diam. (mostly between c. 0.12–0.17 mm) in fully developed apothecia (Fig. 3A & B) with excipulum proprium visible as a distinctly paler (whitish) ring around the disc in surface view (Fig. 3B); excipulum thallinum usually thin, up to c. 25–30 μm wide, with a distinct up to 20 μm thick cellular pseudocortex best visible towards the lower surface (Fig. 3C & D), composed of 1–2(–3) cell layers, individual cells rounded to ellipsoid, thin-walled, cell lumina up to 15 μm wide; excipulum proprium I−, below the hymenium euthyplectenchymatous forming a dense, up to 10 μm thick layer with narrow hyphae with elongated, rectangular cells similar to those beside the hymenium that become thicker and rounded towards the surface, finally forming an up to 30 μm thick layer in the parathecial crown; hypothecium colourless, I+ blue but the reaction is often weak, c. 15–50 μm thick; hymenium I+ blue, c. 85–100 μm tall, colourless throughout or upper 5–20 μm yellowish; paraphyses simple, sometimes anastomosing, mostly c. 1–1.5 μm wide in mid-hymenium, with cylindrical to clavate, up to 3 μm wide tips, some paraphyses becoming considerably thicker towards the surface and having tips up to 6 μm wide; asci subclavate to subcylindrical, c. 60–90 × 15–28 μm (n = 9); ascospores ellipsoid, often slightly constricted at the centre (Fig. 5B), muriform with 8–12 visible cells (11–)13–14.3–15.5(–20) × (7–)8–9.1–10(–11) μm (n = 80).
Pycnidia not seen.
Etymology
Pallida (‘pale’) refers to the pale colour of the thallus and especially the apothecial discs.
Ecology and distribution
The vast majority of collections in Fennoscandia have been made on Salix caprea and Sorbus aucuparia with only a small number of collections made on other substrata (i.e. Juniperus communis, Salix pentandra and on a Betula stump). It appears to be most common in humid localities, for example along streams and in swampy spruce forests, but also grows in subalpine birch forests up to the tree limit. In very humid localities, it may also occur on thin twigs of Picea abies. It has the most pronounced northern distribution of the species in the Rostania occultata complex and occurs throughout the boreal zone and the subalpine birch forests in Fennoscandia. The most southern locality known in Sweden lies in the province of Värmland. Otherwise, it is known from Russia (Komi Republic), where it also grows on Populus tremula, and from a single find in the USA (Alaska) on Alnus sp. Other material belonging to the Rostania occultata complex reported from Alaska by McCune et al. (Reference McCune, Arup, Breuss, Di Meglio, Di Meglio, Esslinger, Miadlikowska, Miller, Rosentreter and Schultz2020) was not studied.
Notes
This species was called ‘Rostania occultata 1’ by Košuthová et al. (Reference Košuthová, Bergsten, Westberg and Wedin2020) and is usually easily recognized by its small and granular, grey thallus and abundant apothecia with pale discs. However, the colour varies somewhat and the thallus can be very similar to R. effusa which sometimes lacks the otherwise typical distinctly red-brown disc. The ellipsoid spores being slightly constricted at the centre is a unique character for R. pallida (Fig. 5A & B). The presence of distinct cuboid spores, or oblong spores with truncated ends, should rule out R. pallida (Fig. 5A–D). However, spore shape can unfortunately be difficult to observe since mature spores are often lacking. In apothecial sections, the well-developed cellular pseudocortex with large, isodiametric cells (Fig. 3C & D) is another character distinguishing R. pallida.
Exsiccates
Finland: Tavastia australis: Hollola, 1874, Lang (Norrlin, Herb. Lich. Fenn. 153) (UPS L-984886); Jämsä, 13 Apr. 1938, Koskinen (Räsänen, Lich. Fenn. Exs. 627) (UPS L-534970, S F64162); Saarijärvi, 21 Aug. 1947, Koskinen (Räsänen, Lichenoth. Fenn. 332) (UPS L-175740, S F64156).
Selected specimens examined
Finland: Karelia borealis: Juuka, 1954, Degelius (UPS L-079701). Savonia borealis: Kuopio, 1954, Degelius (UPS L-079702). Tavastia australis: Evo [Hämeenlinna], 1873, Norrlin (UPS L-003499, S F64155).—Norway: Finnmark: Sør-Varanger, 1864, Fries (UPS L-003495). Nordland: Grane, 2013, Tønsberg 44463 (BG L100120, DNA voucher AL491); Rana, 2013, Tønsberg 43237 (BG L97889, DNA voucher AL490).—Russia: Komi Republic: 2007, Hermansson 15468 (UPS L-933975, DNA voucher AL503).—Sweden: Dalarna: Särna par., 2015, Hermansson 19593 (UPS L-767676, DNA voucher AL377). Hälsingland: Ramsjö par., 2004, Berglund (S F317571). Härjedalen: Ljungdalen par., 2004, Odelvik 04811 (S F57382, DNA voucher AL263). Jämtland: Krokom par., 2017, Jonsson FU6541 (S F388719, DNA vouchers AL268 and AL267, GenBank Accession number MW724306); Lit par., 2017, Košuthová 173 (S F388769, DNA voucher AL302); Kall par., 2020, Westberg JMT 127, 158 (UPS L-980331, L-980332). Lule Lappmark: Gällivare par., 2010, Nordin 7094 (UPS L-520806, DNA voucher AL266). Lycksele Lappmark: Tärna par., 1967, Sundell 5897 (UPS L-012109). Medelpad: Sättna par., Jonsson (S F403820). Pite Lappmark: Arjeplog par., 2017, Westberg & Hedenäs PL460 (UPS L-974037). Torne Lappmark: Jukkasjärvi par., 1827, Sylvén (S F148023). Värmland: Dalby par., 2020, Ekman 5755 (UPS L-989198). Ångermanland: Björna par., 1991, Hermansson 2767, 2766 (UPS L-125630, L-093928); Tåsjö par., 2017, Westberg ULF073 (UPS L-880093, DNA voucher AL242). Åsele Lappmark: Vilhelmina par., 1995, Löfgren 95-334 (UPS L-147303).—USA: Alaska: Kenai Peninsula Borough, 2015, Tønsberg 45685 (BG L101159, DNA voucher AL409).
Rostania populina (Th. Fr.) A. Košuth., M. Westb. & Wedin comb. nov.
MycoBank No.: MB 842250
Collema verruciforme b. populinum Th. Fr., Lich. Arct., 279 (1860).—Collema occultatum var. populinum (Th. Fr.) Degel., Symb. Bot. Upsal. 13(2), 245 (1954).—Rostania occultata var. populina (Th. Fr.) Perlmutter & Rivas Plata, Opuscula Philolichenum 17, 320 (2018); type: Norway, Nordland, Saltdalen, Sommerfelt (UPS L-003493—holotype; O L-14066—isotype).
Thallus (Fig. 4A & B) foliose to subfruticulose, dark green to nearly black, fertile thalli 0.5 to c. 2 mm wide, consisting of small ascending to erect lobes, loosely attached to the substratum, lobes often distinct, up to 0.5 mm long and to 250 μm thick; lobes entire or somewhat lobulated, often with swollen ends, usually a few arranged in irregular rosettes, sometimes indistinct and crowded and forming pulvinate structures; medulla composed of a loose network of reticulately branched hyphae, at the surface forming a thin simple pseudocortex. Photobiont Nostoc in distinct, long chains (up to 20 cells) (Fig. 4C), cells globose or somewhat ellipsoid, 5 × 5(–6) μm wide, chains rather widely scattered in the central parts of the thallus and becoming more dense towards the surface, embedded in a gelatinous matrix which is colourless in the central parts and yellow to brownish yellow near the surface. In most specimens this matrix reacts distinctly I+ magenta to purple (Fig. 4D).
Apothecia common, stipitate to nearly innate (Fig. 4D); disc flat, dark red-brown, up to 0.22 mm diam. with the excipulum proprium usually visible as a distinct, yellowish white and rather sharply delimited ring surrounding the disc and the excipulum thallinum persistent, smooth to uneven or sometimes minutely lobate in young apothecia still semi-immersed in the lobes; excipulum thallinum similar to that of the thallus; excipulum proprium I−, below the hymenium to 30 μm thick, euparaplechtenchymatous, with large, ellipsoid to round cells, variable in size, 3–10 × 7–15 μm (Fig. 4C), towards the hymenium the cells often become more elongate to almost rectangular, up to 10 μm thick next to the hymenium, and towards the surface the cells become smaller and rounded, near the surface usually expanding to form an up to 50 μm wide parathecial crown; hypothecium colourless, I+ blue but the reaction is often weak, up to 20 μm thick; hymenium I+ blue, 105–180 μm tall, upper part with a red-brown colour; paraphyses 1.5–2 μm wide, tips clavate, to 4 μm wide; asci (n = 8) 70–120 × 15–25 μm; ascospores cubic-oblong with truncated ends when mature (immature spores globose to ellipsoid), muriform with (4–)8–12 cells in optical view, (12–)15–16.7–19(–20) × 10–11.8–13(–13.5) μm (n = 33).
Pycnidia rare, immersed in the thallus; conidia oblong, 3.5–4 × 1.5 μm.
Ecology and distribution
This species is so far known only to grow on trunks of Populus tremula. It appears to prefer somewhat open habitats and has been recorded in small openings or in slopes in mixed forests, along edge-zones in spruce-dominated forests, for example at the margin of bogs, and in Populus successions after forest fires. We currently know this species with certainty only from northern Europe. In Sweden it seems to have a limited distribution and is so far known from 15–20 localities in the northern boreo-nemoral to southern boreal zone in the provinces of Gästrikland, Jämtland, Närke, Södermanland, Uppland and Västmanland. In Norway, so far we know it only from the type locality in Nordland and in Russia from one locality near Kurkijoki, north of Lake Ladoga. Material reported from North America as R. occultata var. populina by Perlmutter & Rivas Plata (2018) and McCune et al. (Reference McCune, Arup, Breuss, Di Meglio, Di Meglio, Esslinger, Miadlikowska, Miller, Rosentreter and Schultz2020) was not studied.
Notes
Rostania populina was called ‘R. occultata 3’ in Košuthová et al. (Reference Košuthová, Bergsten, Westberg and Wedin2020). The large size and the lobate thallus make this a distinct species in the R. occultata complex but poorly developed thalli can be difficult to separate from R. occultata. Typically, the apothecia in R. populina have a more distinct excipulum proprium in surface view and in section the loose medulla with scattered, long Nostoc chains in combination with a euparaplechtenchymatous excipulum proprium makes it easy to identify. Well-developed specimens can possibly be confused with Scytinium fragrans, which is normally considerably larger, with larger, ellipsoid spores.
Exsiccata examined
Russia: Karelia: Kurkijoki, 1935, Räsänen (Räsänen, Lich. Fenn. Exs. 267; UPS L-533271, S F64161, mixed with Scytinium fragrans).
Selected specimens examined
Sweden: Gästrikland: Hedesunda par., 2001, Odelvik 01269 (S L42490, DNA voucher AL261). Jämtland: Brunflo par., 2002, Jonsson 4523 (S F322805); Lit par., 2017, Košuthová et al. 174 (S F332481, DNA voucher AL300); Mattmar par., 2017, Košuthová 137 (S F388773, DNA voucher AL304). Närke: Götlunda par., 1872, Blomberg (UPS L-200588). Södermanland: Julita par., 1888, Malme (S F147914). Uppland: Vänge par., 1947, Degelius, Hedlund & Ahlner (UPS L-530093); Åland par., 2014, Knutsson (UPS L-689811, DNA voucher AL257). Västmanland: Enåker par., 1991, Hermansson 2614 (UPS L-125813).
Acknowledgements
We gratefully acknowledge Ulrika Nordin, Fredrik Jonsson, Lars Arvidsson, Janolof Hermansson and Jana Steinová for their kind help during fieldwork. We further thank Toni Berglund, Robin Isaksson, Göran Odelvik and Brian Coppins for providing samples. The County Administrative Board of Uppsala län provided the collecting permit for Fiby urskog Nature Reserve. The staff of the herbaria AMNH, BG, G, GZU, H, O, TSB and UPS kindly provided loans. We are grateful to the staff at S (Annelie Jörgensen, Marcus Arnerup, Johannes Lundberg, Dennis Strid and Göran Odelvik) who kindly assisted with administering our loans and databasing the specimens. We thank the staff at the Department of Bioinformatics and Genetics at the Swedish Museum of Natural History, in particular Bodil Cronholm for her skilful laboratory assistance. Two anonymous reviewers provided very valuable feedback, which greatly improved the manuscript. Finally, we received generous funding from the Swedish Taxonomy Initiative (Svenska Artprojektet) administered by the Swedish Species Information Centre (ArtDatabanken; grants 2016-207 4.3 and 2019.4.3-48) and the Swedish Research Council (Vetenskapsrådet; grant 2016-03589).
Author ORCIDs
Alica Košuthová, 0000-0001-5991-7444; Mats Wedin, 0000-0002-8295-5198; Martin Westberg, 0000-0002-8346-0322.
Supplementary Material
To view Supplementary Material for this article, please visit https://doi.org/10.1017/S0024282921000487








