Introduction
Previously, the genus Psoroma was interpreted widely, and included almost all tripartite Pannariaceae species (Jørgensen & Galloway Reference Jørgensen and Galloway1992), with green algae as major photobionts, and cyanobacteria located in smaller cephalodia. However, many foliose tripartite species were later transferred to the genera Pannaria (see review in Elvebakk & Elix (Reference Elvebakk and Elix2017)) and the newly described Gibbosporina (Elvebakk et al. Reference Elvebakk, Hong, Park, Robertsen and Jørgensen2016). Some squamulose species were transferred to the new genus Joergensenia (Passo et al. Reference Passo, Stenroos and Calvelo2008) and to Psorophorus and Xanthopsoroma (Elvebakk et al. Reference Elvebakk, Robertsen, Park and Hong2010), whereas six bipartite, squamulose species were transferred from Pannaria and Santessoniella to Psoroma (Ekman et al. Reference Ekman, Wedin, Lindblom and Jørgensen2014). After these revisions, the genus Psoroma became much more homogeneous. Its species have squamules, densely distributed or more scattered, and connected by a distinct or indistinct hypothallus/prothallus. In most species, the squamules are brown from melanins and lack secondary compounds that can be detected by TLC, with the presence of pannaric acid and porphyrilic acid or porphyrilic acid methyl ester as the most common exceptions.
The lichen genus Psoroma is mostly terricolous, occasionally corticolous in austral forests, rarely saxicolous, and is concentrated in the Southern Hemisphere, with most of its species distributed in southern South America, south-eastern Australia, and New Zealand (Galloway Reference Galloway2007). Park et al. (Reference Park, Hong and Elvebakk2018) showed that no less than 10 species are known from Antarctica and also listed the four species reaching the Northern Hemisphere. Since then, two rare species have been reported from Alaska and arctic Canada (Elvebakk & Tønsberg Reference Elvebakk and Tønsberg2018; Fryday et al. Reference Fryday, Elvebakk, Anderson and Gagnon2019).
When Jørgensen (Reference Jørgensen2003) reviewed the Pannariaceae flora of the African continent, he included Psoroma asperellum Nyl. and P. fruticulosum P. James & Henssen, both from South Africa. The report of the former from ‘Promontorio Bonæ Spei’ by Nylander (Reference Nylander1863) was later shown to be a misinterpretation of ‘Montis Tabularis’, an old name for Mt Wellington in Tasmania (Galloway Reference Galloway2007). An unpublished record from South Africa of Psoroma hypnorum (Vahl) S.F. Gray has also been posted in the GBIF database. A report from South Africa of the austral species ‘Psoroma sphinctrinum Nyl.’ by van der Byl (Reference van der Byl1931: 9) and cited by Doidge (Reference Doidge1950), possibly refers to material of the tropical genus Gibbosporina.
During studies of Pannariaceae in various herbaria, no African material of Psoroma has been discovered, except the two Esterhuysen collections from BG determined as P. fruticulosum and P. hypnorum, and a third Esterhuysen sample borrowed from BOL. The three Esterhuysen specimens were collected between 1943 and 1951, and a fourth specimen was collected by T. Rämä in 2018. The aim of the present study is therefore to describe the species, but also to search for related species through phylogenetic analyses. This was carried out by comparing the recent collection with other Psoroma species using four phylogenetic markers.
Materials and Methods
Lichen material
Herbarium materials used for this study are housed at BG and BOL, and the species was not found during extensive studies of the Pannariaceae collections in herbaria such as B, BM, C, CANB, O, S, SGO, UPS and W. In microscope sections, iodine reactions were tested by adding IKI to mounts pretreated with KOH (Orange et al. Reference Orange, James and White2001). Perispore structures were studied in water mounts and restricted to spores liberated from the asci. Ascospore morphology was studied in detail by drawing detailed sketches of c. 80 ascospores and copies of all original drawings have been included with the specimens. Several specimens of other species were studied specifically for comparison. Thin-layer chromatography of acetone extracts followed standardized procedures and used solvents A and C (Culberson Reference Culberson1972; Orange et al. Reference Orange, James and White2001). Nomenclature of ascospore structures follows Nordin (Reference Nordin1997).
Phylogenetic analyses
In order to determine the phylogenetic position of the undescribed species from South Africa, the phylogenetic relationships of 12 species of the genera Psoroma, Psorophorus, Xanthoprosoma and Pannaria were reconstructed. Protopannaria pezizoides (G. H. Web.) P. M. Jørg. & S. Ekman was used as an outgroup. The reference materials were selected from those used in a previous study (Park et al. Reference Park, Hong and Elvebakk2018). Four phylogenetic markers, 5.8S-ITS2 rRNA (ITS), the nuclear large subunit rRNA (nucLSU), the mitochondrial small subunit rRNA (mtSSU) and minichromosome maintenance component 7 (Mcm7), were used for phylogenetic reconstruction. Sequence information for ITS, nucLSU and mtSSU of the reference materials was retrieved from a previous study (Park et al. Reference Park, Hong and Elvebakk2018). Sequence information for ITS, nucLSU and mtSSU of the new material was obtained following procedures described by Park et al. (Reference Park, Hong and Elvebakk2018). Mcm7 was amplified using the primers mcm7-709for and mcm7-1348rev (Schmitt et al. Reference Schmitt, Crespo, Divakar, Fankhauser, Herman-Sackett, Kalb, Nelsen, Nelson, Rivas-Plata and Shimp2009). Touchdown PCR amplifications were performed in a T-gradient thermocycler (Biometra, Göttingen, Germany) with the following cycling parameters: 1 min initial denaturation at 95 °C, 6 touchdown cycles of 30 s denaturation at 95 °C, 50 s annealing at 60–56 °C at the ramp of 1° per cycle and 1 min extension at 72 °C, followed by 38 cycles of 45 s denaturation at 94 °C, 50 s annealing at 56 °C, and 1 min extension at 72 °C, with a 5 min final extension at 72 °C. The new sequences, including the holotype of Psoroma capense (cited as NK-1080 in Fig. 5 and Supplementary Material Table S1, available online) and the additional Mcm7 sequences of the samples analyzed previously were deposited in the GenBank database under the accession numbers MT316196 to MT316208 (Supplementary Material Table S1).
Sequence alignments of ITS, nucLSU, mtSSU and Mcm7 were conducted using the software ClustalX (Larkin et al. Reference Larkin, Blackshields, Brown, Chenna, McGettigan, McWilliam, Valentin, Wallace, Wilm and Lopez2007) and manually adjusted. The size variation and ambiguous alignment of the ITS1 domain resulted in it being excluded from the phylogenetic analyses, as were other ambiguously aligned sites. Phylogenetic trees were inferred from each genetic locus and the combined dataset by maximum parsimony (MP), maximum likelihood (ML), and Bayesian analyses. MP trees were obtained using the Tree-Bisection-Regrafting (TBR) algorithm of MEGA X (Kumar et al. Reference Kumar, Stecher, Li, Knyaz and Tamura2018) with search level 5 in which the initial trees were obtained by the random addition of sequences (1000 replicates). ML trees were constructed using MEGA X based on the GTR + I + G evolutionary model (Lanave et al. Reference Lanave, Preparata, Sacone and Serio1984), the search options of best tree topology finding by branch swapping of NNIs and SPRs, and random addition of sequences (1000 replicates). Aligned sites with less than 95% coverage by alignment gaps, missing data, or ambiguous bases were excluded. The Bayesian tree was generated using a search approach by MrBayes ver. 3.2. (Ronquist et al. Reference Ronquist, Teslenko, van der Mark, Ayres, Darling, Höhna, Larget, Liu, Suchard and Huelsenbeck2012) with the GTR + I + G model. Two parallel Markov chain Monte Carlo (MCMC) runs were performed for 1 000 000 cycles, each with one cold and three heated chains and the temperature parameter set to 0.1; trees were sampled every 100 generations. A consensus tree was calculated after discarding the first 25% of trees as burn-in.
Taxonomy
Psoroma capense Elvebakk, S. G. Hong & Rämä sp. nov.
MycoBank No.: MB 836049
Superficially similar to Psoroma tenue var. tenue Henssen but with ascending thallus squamules, regularly short-ellipsoid to ovoid spores, and lacking TLC-detectable secondary compounds.
Type: South Africa, Western Cape, Witzenberg municipality, Hex River Mountains/Hexrivierberge, Matroosberg, Spekrivierskloof, 33°21′13″S, 19°37′42″E, 1310 m, S-exposed slope 50 m NW of a small dam in the river, 1‒2 m high rock outcrop located 30 m NE of the river channel, on soil in a vertical rock cavity, apothecia occurring in an area of c. 3 × 5 cm, 18 March 2018, T. Rämä 1-2018 (BOL 59675—holotype). GenBank Accession nos.: MT316196, MT316197, MT316208.

Fig. 1. Psoroma capense. A, holotype. B, Esterhuysen 19747 (BOL). Images by M. Karlstad. Scales: A = 5 mm; B = 1 mm. In colour online.

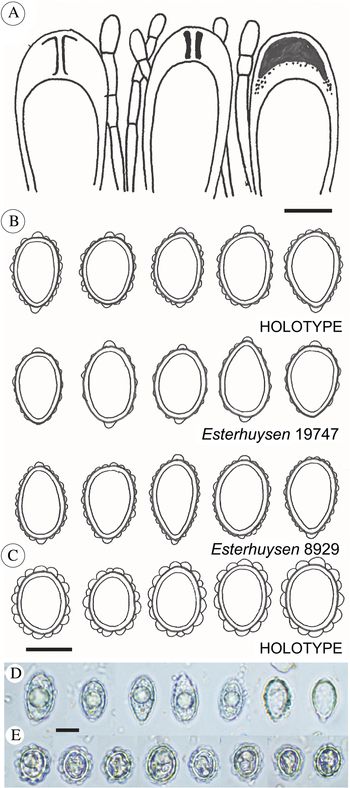
Fig. 2. Psoroma capense and P. esterhuyseniae. A, ascus structures of P. capense. B, ascospore sketches of P. capense (from three collections). C, ascospore sketches of P. esterhuyseniae. D, ascospore micrographs of P. capense, from the holotype. E, ascospore micrographs of P. esterhuyseniae, from the holotype. Scales: A–C = 10 μm; D & E = 15 μm. In colour online.

Fig. 3. Psoroma capense. A, habitat of the holotype, in the crevice immediately to the right of the backpack. B, the holotype specimen prior to being collected. In colour online.
Thallus squamulose, tripartite, terricolous, forming 3–5 cm wide patches. Chloromorph squamules 150–250 μm thick, starting as small, 0.1–0.3 mm wide, circular and appressed squamules peripherally, developing into a dense mat of irregularly lobate and mostly ascending squamules, 0.5–2 mm tall. Upper surface pale chestnut brown, darker at apices, glabrous and weakly glossy. Upper cortex 30–50 μm thick, sclerenchymatic, upper third dark brown, pale brown below, paraplectenchymatic, lumina mostly isodiametric, 6–12 μm wide, walls 2–3.5 μm thick. Chlorobiont layer c. 60–100 μm thick, of cf. Trebouxia cells, globose to irregularly globose, 8–20 μm diam., with papillose chloroplasts. Medulla 80–130 μm thick; lower cortex absent. Prothallus/hypothallus indistinct, but visible in peripheral parts as a pale, byssoid network.
Cephalodia common, blackish, forming coralloid cushions in between or on chlorobiont squamules, 0.5–2.5 mm wide, cortex as in the chlorobiont squamules. Cyanobiont Nostoc, small-celled, greenish blue, obtusely angular, 3–6 × 3–7 μm, arranged in indistinct glomeruli, 30–50 μm wide, and without visible chain structures.
Apothecia common, substipitate, 1–3.5 mm wide; disc dark chestnut brown, weakly concave; thalline excipulum 1–3 mm wide when viewed from above, irregularly crenulated, occasionally with small verrucose or scale-like thalline outgrowths but generally non-squamulose, lower half of the sides thickly covered by a dense, thin and white tomentum, sometimes eroded. Epithecium c. 20–25 μm thick, sclerenchymatic, pale brown, upper third hyaline. Hymenium 100–120 μm thick, colourless, but strongly IKI+ blue. Asci clavate, 70–80 × 15 μm, 8-spored, with an internal apical structure shown as a distinct tube in moderate concentration IKI. Proper ascospores hyaline, non-septate, short-ellipsoid to ovoid, 15–21 × 9–13 μm. Perispores of the same shape, 16–24 × 10–16 μm, with large, distinct verrucae, mostly with distinct, nodulose apical extensions, up to 2.5 × 3 μm. Paraphyses septate, simple to sparingly branched, c. 2.5 μm wide, apices slightly swollen. Hypothecium pale brownish, 0–50 μm thick, IKI−. An algal layer, 60–100 μm thick, is distributed uniformly below the hypothecium.
Pycnidia scattered, black and verrucose, 150–200 μm wide, ostiole fissure-like, c. 50 μm long, spermatia 1.5–2 × 0.5 μm, mostly curved.
Chemistry
Brownish melanins present, of a chestnut-coloured type, appearing similar to those of P. tenue Henssen. No TLC-detectable components found.
Etymology
Named after its occurrence in the Western Cape Region.
Distribution and ecology
Only known as three collections from the Western Cape Province of South Africa, found at moist sites at high altitudes in cool, mostly south-facing sites, often near water.
Additional specimens examined (paratypes)
South Africa: Western Cape Province: Worcester Div., Mt Waaihoek (= ‘Waaihoekpiek’), 5000 ft, damp southern cliffs above ravine, 1943, E. Esterhuysen 8929 (BOL 155421); Tulbagh Div., Sneeuwgat Peak (= ‘Sneeugatpiek’), 6000 ft, on mossy, sandy bank on cliffs, near seasonal watercourse, 1951, E. Esterhuysen 19747 (BOL 155420; BG L-71578; LD not seen).
Psoroma esterhuyseniae Elvebakk sp. nov.
MycoBank No.: MB 836050
Similar to Psoroma hypnorum but ascospores subglobose to short-ellipsoid without apical perispore extensions, and tomentum of the lower parts of thalline excipuli less prominent.
Type: South Africa, Western Cape, Hexerivier Mts (= Hexrivierberge), mountain ridge peak, 4500 ft, damp cliffs, S side, 11 November 1943, E. Esterhuysen 9419 (BG L-71579—holotype).

Fig. 4. Psoroma esterhuyseniae (holotype). Image by M. Karlstad. Scale bar = 5 mm. In colour online.
Thallus squamulose, tripartite, terricolous, 3–5 cm wide. Chloromorph squamules c. 150 μm thick, 0.1–0.3 mm wide, horizontal to weakly ascending, irregularly lobate, 1–2 mm tall. Upper surface chestnut brown, glabrous and glossy. Upper cortex c. 30 μm thick, sclerenchymatic, upper third dark brown, pale brown below, paraplectenchymatic, lumina mostly isodiametric, 6–12 μm wide, walls 2–3.5 μm thick. Chlorobiont layer c. 50 μm thick, of cf. Trebouxia cells, globose to irregularly globose, 7–15 μm diam., with angular chloroplasts. Medulla 60–100 μm thick; lower cortex absent. Prothallus/hypothallus indistinct, but visible in peripheral parts as a pale, byssoid network.
Cephalodia rare, pale, forming a coarse coralloid cushion in between or on chlorobiont squamules, c. 1 mm wide, cortex as in the chlorobiont squamules. Cyanobiont Nostoc, cells greenish blue, obtusely angular, 3–6 × 3–7 μm, arranged in indistinct glomeruli, 30–50 μm wide, and without visible chain structures.
Apothecia common, substipitate, 1–3 mm wide; disc dark chestnut brown, weakly concave; thalline excipulum 2–3 mm wide when viewed from above, irregularly crenulated, squamulose, external parts glabrous or occasionally with a tomentum-like mycelium in lower parts. Epithecium c. 20–25 μm thick, sclerenchymatic, pale brown, upper third hyaline. Hymenium 100–120 μm thick, colourless, but strongly IKI+ blue. Asci clavate, 70–80 × 15 μm, 8-spored, with an internal apical structure shown as a distinct tube in moderate concentration IKI. Proper ascospores hyaline, non-septate, subglobose to short-ellipsoid, 15–19 × 11–15 μm. Perispores of the same shape, 18–23 × 14–17 μm, with up to 2.5 × 3 μm wide, distinct verrucae, appearing inflated, and without apical perispore extensions. Paraphyses septate, simple to sparingly branched, c. 2.5 μm wide, apices slightly swollen. Hypothecium pale brownish, 40–50 μm thick, IKI−. An algal layer, 60–100 μm thick, is distributed uniformly below the hypothecium.
Pycnidia not seen.
Chemistry
Brownish melanins present, appearing similar to those of P. hypnorum. No TLC-detectable components found.
Etymology
Named after the South African botanist Elsie Elizabeth Esterhuysen (1912–2003), who collected three of the four samples of Psoroma known from South Africa.
Distribution and ecology
Known only from the holotype collected from damp cliffs in Western Cape, South Africa.
Results
Molecular analysis and phylogeny
The phylogeny based on the concatenated multi-locus dataset of ITS, nucLSU, mtSSU and Mcm7 indicates that Psoroma capense forms a well-supported monophyletic group (referred to here as the Psoroma hypnorum lineage) with P. antarcticum Hong & Elvebakk, P. buchananii (Knight) Nyl., P. fruticulosum, P. hypnorum and P. paleaceum (Fr.) Timdal & Tønsberg (Fig. 5). The monophyletic group was consistently recovered by MP, ML and Bayesian methods, and also based on single-locus analyses (data not shown). The group was clearly separated from the Psoroma tenue lineage, including P. cinnamomeum Malme and P. tenue, and from the genera Psorophorus, Xanthopsoroma and Pannaria. The phylogenetic position of P. capense within the Psoroma hypnorum lineage was not clearly resolved and the relationship was poorly supported by bootstrap and posterior probability. Psoroma capense was grouped with P. buchananii, P. fruticulosum and P. paleaceum in the ML tree based on the combined dataset (Fig. 5), but the relationship was not always recovered by MP, ML, and Bayesian methods with single-locus datasets. Branch lengths from the common ancestor of the group leading to terminal taxa were generally very short and statistical support for bifurcation was generally very low. Sequence similarity of the combined dataset between P. capense and the other species of the Psoroma hypnorum lineage ranged between 97 and 98%, which is close to similarity values among the other species of the group.

Fig. 5. Bayesian tree based on concatenated sequences of ITS, nucLSU, mtSSU and Mcm7. Black thick branches indicate those that were conserved in maximum likelihood (ML) and maximum parsimony (MP). Grey thick branches indicate those that were conserved in ML or MP. Asterisks indicate that branches were conserved but not supported by high bootstrap values. Bayesian posterior probabilities (PP ≥ 0.90) and bootstrap values in ML and MP trees (≥ 80%) are indicated above or below the nearest branches (PP/ML/MP). The geographical origins of the specimens are shown after the voucher number. KGI = King George Island, Antarctica; P. tenue l. = P. tenue lineage.
Discussion
In recent phylograms, the genus Psoroma has either appeared as polyphyletic (Ekman et al. Reference Ekman, Wedin, Lindblom and Jørgensen2014) or paraphyletic (Park et al. Reference Park, Hong and Elvebakk2018; the present study), with species of the P. hypnorum and P. tenue groups forming separate lineages. The possible recognition of these two lineages as separate genera has not been proposed due to insufficient taxon sampling. The P. hypnorum and P. tenue lineages both clearly have evolutionary histories featuring adaptations to cold climates, probably initiated in or near Antarctica, where glaciation occurred at c. 34 Ma (Pollard & DeConto Reference Pollard and DeConto2020). Data from thermophilous Psoroma species from austral forests should be incorporated in future phylogenies, since they are potential members of older lineages needed in analyses to define the genus.
From its gross morphology alone, Psoroma capense resembles P. tenue. The latter species is distributed in Antarctica and subantarctic areas, but also in the Northern Hemisphere by a taxon considered to represent a separate variety (Henssen & Renner Reference Henssen and Renner1981; Jørgensen Reference Jørgensen2004b), a concept which has recently been challenged by Marthinsen et al. (Reference Marthinsen, Rui and Timdal2019). Psoroma capense and P. tenue share a related melanin colour and strongly subsessile apothecia with crenate-lobate margins, but without the excipulum squamules typical of P. hypnorum. However, P. capense differs from P. tenue by ascending thallus squamules, short-ellipsoid to ovoid ascospores and a lack of TLC-detectable compounds.
Psoroma esterhuyseniae resembles P. hypnorum, although the characteristic regular tomentum on the apothecia of P. hypnorum (see Elvebakk & Tønsberg Reference Elvebakk and Tønsberg2018) is lacking; replaced by some mycelium-like cover in only the least exposed apothecia. The few cephalodia seen in P. esterhuyseniae are regularly coarsely coralloid, whereas they are irregular in P. hypnorum. The ascospores of P. esterhuyseniae are very different from those of both P. hypnorum and P. capense (Fig. 2C), in being subglobose to short-ellipsoid, and very rarely ovoid. The apical perispore extensions present in both these species are absent in P. esterhuyseniae.
Phylogenetically, P. capense is very distinct from P. tenue, and is instead positioned within the Psoroma hypnorum lineage based on a concatenated dataset of ITS, nucLSU, mtSSU and Mcm7 sequences. This is very well supported by all phylogenetic methods and by all datasets examined in the present study. Within the P. hypnorum lineage, P. capense is in a poorly supported sister group position to a clade including P. paleaceum, P. fruticulosum and P. buchananii. None of these have any resemblance to P. capense. The former has characteristic long scales along apothecium margins (Elvebakk & Tønsberg Reference Elvebakk and Tønsberg2018) and the two latter species were previously considered to form a subgroup within Psoroma by Henssen et al. (Reference Henssen1983), a conclusion confirmed by our ongoing studies, as well as by the present phylogram. The Esterhuysen 19947 specimen (erroneously cited by Jørgensen (Reference Jørgensen2003) as Esterhuysen 9419) was determined as P. fruticulosum because of its ‘erect, isidioid lobules, which are partly flattened’ (Jørgensen Reference Jørgensen2003), a character resembling P. capense. However, both P. fruticulosum and P. buchananii have conspicuous black pycnidia, prominent apothecia almost appearing stipitate, and spores deviating from those of the remaining Psoroma species.
Subantarctic islands of the Indian Ocean are the Psoroma sites closest to the distribution area of P. capense and P. esterhuyseniae. These areas house endemic species such as Psoroma absconditum Øvstedal and P. xanthorioides (P. M. Jørg.) P. M. Jørg., and represent the major distribution area of P. dichroum (Hooker f. & Taylor) P. M. Jørg. (Jørgensen Reference Jørgensen2000, Reference Jørgensen2004c; Øvstedal & Gremmen Reference Øvstedal and Gremmen2008; Ekman et al. Reference Ekman, Wedin, Lindblom and Jørgensen2014). Psoroma absconditum is the most similar to the South African species; however, it is not well understood since it was not compared to other members of the P. hypnorum lineage, but instead to the very different species P. asperellum Nyl. (Øvstedal & Gremmen Reference Øvstedal and Gremmen2008). Psoroma esterhuyseniae has shorter spores and apothecia with squamulose margins compared to P. absconditum, the latter named after its sunken apothecia, partly hidden by squamules.
Fresh material of P. capense appeared to have a yellowish brown melanin colour where the pigments were not strongly concentrated, which in combination with the chlorobiont cells gave the lichen a peculiar ‘grass green’ colour, even in a dried specimen two years after collection. A similar colour, contrasting with most other Psoroma species, has been observed in fresh specimens of the New Zealand species P. cyanosorediatum P. M. Jørg. (A. Elvebakk, unpublished data), which has very different, long and narrowly ellipsoid ascospores according to Jørgensen (Reference Jørgensen2004a). Among the rather few Psoroma species described with short ascospores, P. antarcticum Elvebakk & S. G. Hong, P. saccharatum Scutari & Calvelo and P. pannarioides Henssen lack other similarities with the two new species from South Africa (Henssen Reference Henssen1983; Scutari & Calvelo Reference Scutari and Calvelo1995; Park et al. Reference Park, Hong and Elvebakk2018).
All the specimens of the new species were collected from moist and S-facing sites at altitudes between 1300 and 1800 m at Sneeugatpiek, Waaihoekpiek, and Hexrivierberge in the Western Cape Province, only 140–200 km NNE of Cape Town. A search for the species by TR in Spekrivierskloof, on 18 March and 17 November in 2018, revealed no additional findings. At higher altitudes in this area the habitats were drier, and the species might be truly rare here due to a scarcity of moist, suitable habitats. There is a clear need to search in the Western Cape for more populations of these species, which appear as Red List candidates, and to determine if more species are present in South African mountains. South Africa features extreme speciation in many groups of organisms, for example in the plant genus Erica which has evolved no less than 690 endemic species in the Cape Region during the last 15 million years (Pirie et al. Reference Pirie, Olivier, de Kuppler A, Gehrke, Le Maitre, Kandziora and Bellstedt2016).
Chlorobiont acquisition is an important evolutionary feature in lichens but has not yet been studied in Pannariaceae. Previously, Myrmecia was the most commonly identified chlorobiont in Psoroma, but recent studies instead identify it as Trebouxia (Park et al. Reference Park, Kim, Noh, Elvebakk and Hong2016; Muggia et al. Reference Muggia, Leavitt and Barreno2018). In Psoroma esterhuyseniae the chlorobiont has cells with angular chloroplasts, whereas they are differently shaped and papillose in P. capense.
In conclusion, there are currently no candidates closely related to P. capense and P. esterhuyseniae, from phylogenetic analysis or by comparison of taxonomic characters. In this context, it should be added that a high proportion of specimens collected throughout the distribution area of Psoroma represent misunderstood or undescribed species (A. Elvebakk & S. G. Hong, unpublished data). For these reasons, it is difficult to hypothesize on the migration history of the ancestors of these two species into Africa, where they are the only known members of the genus Psoroma. The bipolar element within the genus is most easily explained by migrations along American mountain chains during the Pleistocene; several Psoroma species occur in the Central Andes (Jørgensen & Palice Reference Jørgensen and Palice2010) where rapid diversification has taken place during this period in Lobariaceae lichens, as shown by Widhelm et al. (Reference Widhelm, Grewe, Huang, Mercado-Díaz, Gofinet, Lücking, Moncada, Mason-Gamer and Lumbsch2019). The dramatic cooling during the Pleistocene probably represented a scenario of expansion of the cold-adapted groups within Psoroma, and our hypothesis is that P. esterhuyseniae and P. capense obtained their isolated and shared geographical positions as a result of one or two long-distance dispersal events during this period.
Acknowledgements
Curators of the herbaria mentioned are acknowledged for making herbarium material available for study, including T. Trinder-Smith at BOL during 2018. An anonymous referee and Associate Editor T. Randlane provided comments which greatly improved the manuscript. Photographs were taken by M. Karlstad at UiT – the Arctic University of Norway. This study was partly supported by the Korea Polar Research Institute (Grant PE 20170) and Ocean Medicines project (H2020-MSCA-RISE; Grant ID 690944).
Author ORCIDs
Arve Elvebakk, 0002-7682-3797; Teppo Rämä, 0001-8111-8075.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0024282920000377.