Introduction
Usnic acid (2,6-diacetyl-7,9-dihydroxy-8,9b-dimethyl-1,3(2H,9bH)-dibenzo-furandione) is a structurally unique, yellow, lipophilic metabolite found selectively in lichens. The biological and pharmacological properties of usnic acid are well documented and comprise antibacterial, antiviral, anti-inflammatory, analgesic, antiprotozoal or insecticidal activities (Galanty et al. Reference Galanty, Paśko and Podolak2019). The compound has been particularly studied for its anticancer potential and the results seem to be very promising, with a marked activity reported both in vitro and in vivo (Araújo et al. Reference Araújo, De Melo, Rabelo, Nunes, Santos, Serafini, Quintans-Junior and Gelain2015). Moreover, usnic acid has been extensively used by the cosmetic industry, as an antiperspirant or an additive in toothpastes (Ingolfsdottir Reference Ingolfsdottir2002). Some recent reports also indicate its photoprotective (Varol et al. Reference Varol, Tay, Candan, Turk and Koparal2015) and wound healing (Pagano et al. Reference Pagano, Ceccarini, Calarco, Scuota, Conte, Primavilla, Ricci and Perioli2019) properties, when applied externally. Taken together, these attributes of usnic acid, its wide distribution in nature in significant amounts of up to 5% (Galanty et al. Reference Galanty, Paśko and Podolak2019), and an easy isolation procedure (Ingolfsdottir et al. Reference Ingolfsdottir, Chung, Skulason, Gissurarson and Vilhelmsdottir1998; Lohezic Le Devehat et al. Reference Lohezic, Tomasi, Elix, Bernard, Rouaud, Uriac and Boustie2007; Galanty et al. Reference Galanty, Koczurkiewicz, Wnuk, Paw, Karnas, Podolak, Węgrzyn, Borusiewicz, Madeja and Czyż2017), make this lichen metabolite an attractive subject of research.
Even though there are many reports describing the extraction of usnic acid from natural sources by different methods (König & Wright Reference König and Wright1999; Yilmaz et al. Reference Yilmaz, Türk, Tay and Kivanc2004; Roach et al. Reference Roach, Musser, Morehouse and Woo2006; Burlando et al. Reference Burlando, Ranzato, Volante, Appendino, Pollastro and Verotta2009; Einarsdottir et al. Reference Einarsdottir, Groeneweg, Björnsdottir, Harðardottir, Omarsdottir, Ingolfsdottir and Ögmundsdottir2010; Honda et al. Reference Honda, Pavan, Coelho, de Andrade Leite, Micheletti, Lopes, Misutsu, Beatriz, Brum and Leite2010; Brovko et al. Reference Brovko, Ivakhnov, Palamarchuk and Boitsova2017; Piska et al. Reference Piska, Galanty, Koczurkiewicz, Żmudzki, Potaczek, Podolak and Pękala2018), they do not provide reliable information on the most effective conditions. This discrepancy and the incompleteness of data concerning the extraction parameters for usnic acid have motivated us to explore this issue in more detail.
Fractional factorial designs are important statistical tools which efficiently allow the influence of several relevant factors to be controlled and varied simultaneously on a planned experiment, in order to obtain the assumed goal in the most effective way. These tools are widely used in monitoring experiments in different research fields, as they can significantly shorten the total time of the experiment, as well as indicate the most effective procedure (Gunst & Mason Reference Gunst and Mason2009). Fractional factorial experiments have so far rarely been used for the optimization of lichen metabolite extraction, with the exception of lepraric acid, erythrin (Parrot et al. Reference Parrot, Peresse, Hitti, Carrie, Grube and Tomasi2015) or diploicine, norstictic and variolaric acids (Bonny et al. Reference Bonny, Hitti, Boustie, Bernard and Tomasi2009).
Thus, the aim of the present study was to optimize the extraction conditions for usnic acid using experiments with a fractional factorial design in order to investigate the influence of a number of parameters on the efficacy of the particular extraction method.
For this purpose, we chose Cladonia arbuscula (Wallr.) Flot. as a lichen material containing usnic acid and compared different types of extractions: heat reflux extraction in a hot water bath, dynamic shaking extraction and ultrasound-assisted extraction. For each type of extraction three parameters were selected for comparative purposes: the time of extraction, the solvent used and the number of extraction repetitions. Then, the influence of these parameters was investigated and the most effective conditions for each method were compared with each other to establish the optimal recovery of usnic acid from the lichen.
Material and Methods
Lichen material, chemicals and instrumentation
Cladonia arbuscula subsp. squarrosa (Wallr.) Ruoss was collected in July 2015, from northern Poland in dry, non-coastal Scots pine forests; its identity was verified by one of the authors (AG). The voucher specimen was deposited in the herbarium of the Department of Pharmacognosy JU MC (Ref. no. KFg/2015/L3). All samples used during the analysis were prepared from lichen material collected from the one location. The lichen material was dried in the dark at room temperature and cleaned to remove impurities. Before analysis, whole lichen thalli (both upper and lower parts) were ground into small pieces in a mortar to provide uniformity and homogeneity of the tested material. Chloroform, acetone, methanol and water of HPLC grade were obtained from Sigma-Aldrich (Germany) and orthophosphoric acid (98%) from POCh Gliwice (Poland). Usnic acid standard of analytical grade was obtained from Sigma-Aldrich (Germany). A water bath LWT (WSL Poland) and ultrasonic bath Sonic 3 (Polsonic, Poland) were used for heat reflux and ultrasound-assisted extraction, respectively. For dynamic shaking extraction, a laboratory shaker was used (type 3585; ELPAN, Poland).
Usnic acid quantitative determination was performed using a Dionex high-performance liquid chromatography (HPLC) system, with PDA detector, on a C-18 column (5 μm, 250 × 4.6 mm), at 25 °C. The mobile phase consisted of 1% H3PO4 A – methanol B, (A:B 15:85, v/v). Detection was carried out at 240 nm. Quantification of usnic acid was achieved by measuring the peak area with regards to the appropriate standard curve. Details of the analysis were described previously (Studzińska-Sroka et al. Reference Studzińska-Sroka, Tomczak, Malińska, Wrońska, Kleszcz, Galanty, Cielecka-Piontek, Latek and Paluszczak2019).
Sample preparation and extraction
Aliquots of 0.2 g each of the ground lichen thalli were transferred into glass round-bottomed flasks, tightly closed and kept in darkness until needed for further extraction procedures. For a single extraction, 20 ml of solvent was used.
Heat reflux extraction was conducted on a water bath (90 °C). Dynamic shaking and ultrasonic-assisted extractions were conducted at room temperature. Each combination of the experimental parameters (one of nine) was tested in six replicates. For heat reflux and shaking extractions, three different times were applied, namely 15, 30 and 60 min, while ultrasound-assisted extraction times were 10, 20 and 30 min. Acetone, chloroform and methanol were used as solvents. To investigate the influence of the number of extraction repetitions on its efficacy, a single sample was single-, double- or triple-extracted. This was carried out by treating the sample only once with the solvent (1 × 20 ml) or by repeating the extraction twice (2 × 20 ml) or three times (3 × 20 ml).
After extraction, the obtained extracts were transferred into 10 ml volumetric flasks and the usnic acid content was analyzed by high-performance liquid chromatography (HPLC). Before the analysis, extracts were filtered through the 0.45 μm membrane filters into the HPLC 1.5 ml vials.
Experimental design and statistical approach
To optimize the extraction procedure for usnic acid, the three different extraction methods mentioned above were compared. Within each chosen method three parameters were considered: 1) the time of extraction, 2) the solvent used, 3) the number of extraction repetitions from a single sample. In order to investigate the impact of the experimental parameters on the effectiveness of extraction, an experimental planning method was used (Dobrowolska et al. Reference Dobrowolska, Zagrodzki, Woźniakiewicz, Woźniakiewicz, Zwolińska, Winnicka and Paśko2016). This was achieved by using the fractional factorial design of experiments: 33-1 in which 1/3 of the full 33 design was selected, with 9 different combinations of the chosen factors (parameters), resulting in 9 trials. The scheme of factor coding and the plan of the experiment with the original parameters and their levels are shown in Table 1. We purposefully used different numbers of extraction repetitions from a single sample (1, 2, or 3), treating it as one (potentially) significant factor (i.e. independent parameter on three levels) and according to the plan of the experiment. Similarly, we tested three levels of extraction time, using slightly milder conditions (shorter time periods), for ultrasound-assisted extraction because of the risk of degradation of usnic acid, but the essential scheme of the experiment was always preserved. The amount of usnic acid in each of the tested samples was calculated as mg per gram dry weight (mg g−1 d.w.) of the lichen material used, and then the best factorial set was selected for each extraction method.
Table 1. Optimization of usnic acid extraction conditions, from Cladonia arbuscula, using experiments with a fractional factorial design: parameter values and coded sets of experimental conditions.

HRE = heat reflux extraction; SE = shaking extraction; UAE = ultrasound assisted extraction
First, the outlying values of usnic acid concentration, if they appeared in a particular factor (parameter) combination, were removed as outliers. For this purpose, we used the software ‘Extreme Outlier’ (MP System Co., Chrzanów, Poland), with an algorithm implemented by Shoemaker and based on a robust technique proposed by Tukey (Shoemaker Reference Shoemaker2018). Altogether we found 10 outliers out of 162 results (6%). Then, the results obtained for each type of extraction were compared using an ANOVA test with a Tukey post-hoc test (using the package STATISTICA v.13; StatSoft, Tulsa, USA) to identify the best (i.e. biggest) two extraction results. The same procedure was applied to ultimately identify the best type of extraction and the best combination of parameters from the six results (two best scores for each tested extraction type) previously obtained. Differences with P < 0.05 were considered to be statistically significant.
Results and Discussion
Quantitative analyses of usnic acid content in various lichen species have been performed using several different extraction methods. The results of these studies are difficult to compare because the analytical conditions vary substantially, often depending on the research goal, and giving inaccurate information on the real content of usnic acid in the given lichen material. Thus, the aim of our study was to explore what would be the most effective, optimal extraction conditions for obtaining usnic acid for its further use in pharmacological studies. For this purpose, we compared three different extraction methods, and within each of these methods the influence of the selected three parameters on the extraction efficacy was investigated. A fractional factorial design was applied for the first time to study usnic acid extraction optimization. The results obtained are shown in Table 2.
Table 2. Optimization of usnic acid extraction conditions, from Cladonia arbuscula, using experiments with a fractional factorial design: scores for all sets of usnic acid extraction parameters (see Table 1 for parameter values and experimental conditions for each set).
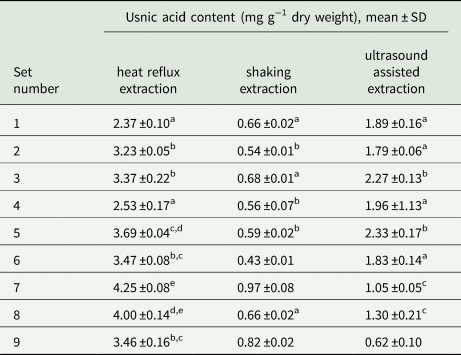
When comparing the results, values with different letters (in superscript) differ significantly for columns only.
For both tested conventional extractions (heat reflux, shaking) the same set provided the best scores, that is 60 minutes acetone extraction, but the results for heat reflux extraction were four times higher (4.25 ±0.08 mg g−1 d.w.) than shaking extraction (0.97 ±0.08 mg g−1 d.w.). Such a large variation is probably a consequence of the different temperature conditions for both extractions. This is an additional important observation, emphasizing the influence of temperature on usnic acid extraction. The highest amount of extracted usnic acid was observed for the sets with a single extraction, which means that the samples obtained by different numbers of repetitions did not differ statistically. This result was surprising as it is a common practice, in many pharmaceutical or ecological studies, to carry out two or three consecutive repetitions of the extraction process.
For ultrasound-assisted extraction the best score was achieved for the set with two repetitions of extraction, 20 minutes each, with acetone as a solvent (2.33 ±0.17 mg g−1 d.w.); however, the effect did not differ significantly from the set with a single, 10 minute extraction using the same solvent (2.27 ±0.13 mg g−1 d.w.). Notably, the amount of usnic acid obtained by ultrasound-assisted extraction was about two times lower (2.33 ±0.17 mg g−1 d.w.) when compared to heat reflux extraction (4.25 ±0.08 mg g−1 d.w.), P < 0.05. The two highest results of each method were finally compared, proving that the optimal conditions were for heat reflux extraction with one repetition and acetone as the solvent (P < 0.05).
The methods so far commonly used by various authors for usnic acid extraction have been mainly conventional techniques, with heat reflux (König & Wright Reference König and Wright1999; Piska et al. Reference Piska, Galanty, Koczurkiewicz, Żmudzki, Potaczek, Podolak and Pękala2018), Soxhlet (Einarsdottir et al. Reference Einarsdottir, Groeneweg, Björnsdottir, Harðardottir, Omarsdottir, Ingolfsdottir and Ögmundsdottir2010; Honda et al. Reference Honda, Pavan, Coelho, de Andrade Leite, Micheletti, Lopes, Misutsu, Beatriz, Brum and Leite2010) or shaking (Yilmaz et al. Reference Yilmaz, Türk, Tay and Kivanc2004; Roach et al. Reference Roach, Musser, Morehouse and Woo2006) extractions, while a green technology approach was represented by ultrasound-assisted (Burlando et al. Reference Burlando, Ranzato, Volante, Appendino, Pollastro and Verotta2009; Brovko et al. Reference Brovko, Ivakhnov, Palamarchuk and Boitsova2017) or supercritical fluid (Brovko et al. Reference Brovko, Ivakhnov, Palamarchuk and Boitsova2017) extractions. It is interesting to note that these methods are most often used when analytical aspects of usnic acid or its pharmacological activity are of interest, while in the majority of ecologically directed studies, a simple, room-temperature maceration is a common approach (Nybakken et al. Reference Nybakken, Helmersen, Gauslaa and Selås2010; Asplund et al. Reference Asplund, Siegenthaler and Gauslaa2017). As the extraction times applied by different authors varied to a large extent, from several minutes to hours, in our study we opted for 15, 30 and 60 minutes for heat reflux and shaking extractions, and 10, 20 and 30 minutes for ultrasound-assisted extraction. The latter choice was based on our previous preliminary investigation, indicating that exposure to sonication exceeding 30 minutes results in usnic acid degradation (data not shown). As far as the solvents are concerned, data from the literature indicate that hexane, chloroform, acetone or methanol were used most often (Yilmaz et al. Reference Yilmaz, Türk, Tay and Kivanc2004; Bomfim et al. Reference Bomfim, Araújo, Cuadros-Orellana, Melo, Quintans-Júnior and Cavalcanti2009; Behera et al. Reference Behera, Mahadik and Morey2012), even though the lipophilic nature of usnic acid together with some experimental data do not support the use of methanol (Stark et al. Reference Stark, Walter and Owens1950; Podterob Reference Podterob2008). The results of our study also confirmed that methanol is not suitable for effective usnic acid extraction.
The results of our study, indicating a significant advantage of heat reflux extraction over the other two tested methods, do not question the reliability of previously published studies on the content of usnic acid in different lichen species. The discrepancy in usnic acid levels determined in our study and those obtained by other authors may result from many factors, including the differences in preparation of lichen material (degree of grinding) but also the geographical site of its collection. For example, samples of pulverized C. arbuscula from Finland, extracted by homogenization with acetone at room temperature, were found to contain 2.1% of usnic acid (Nybakken & Julkunen-Tiitto Reference Nybakken and Julkunen-Tiitto2006), while in a consecutive study by the same authors on ‘pieces of C. arbuscula’ collected in Norway, extracted with acetone at room temperature (2 × 20 min), only 0.3% of usnic acid was reported, which is an order of magnitude lower (Nybakken et al. Reference Nybakken, Helmersen, Gauslaa and Selås2010). Moreover, in a study by Falk et al. (Reference Falk, Green and Barboza2008) on different samples of C. arbuscula collected in Alaska, usnic acid levels ranged from 0.29 to 1.1%; the lichen material was ground in a mortar and extracted by ultrasound-assisted extraction with acetone, followed by 24 hours maceration at room temperature. In our previous unpublished experiments, a direct comparison of usnic acid yield from pulverized and ground C. arbuscula indicated significant differences with higher yields from the latter (data not shown).
Conclusions
With the support of fractional factorial design, we have shown that the optimal approach for the most effective extraction of usnic acid from lichen material is a single 60 minute heat reflux method with acetone. This may help substantially in designing the best procedure for the acquisition of usnic acid from lichen material, not only for pharmaceutical research purposes but also for further applications in the cosmetic industry. A simpler, but experimentally optimized, procedure should also be designed in the future for the purposes of ecological experiments. The use of a comparable extraction protocol would undoubtedly help to evaluate the results obtained by various authors within each area of expertise.
However, further studies are needed to examine other factors that might influence the effectiveness of usnic acid extraction, namely the degree of grinding of lichen thalli or the temperature during the process.
Acknowledgements
This work was supported by grants from the Polish Ministry of Science and Higher Education, project N42/DBS/000045.
Author ORCIDs
Agnieszka Galanty, 0000-0001-5636-8646.





