Introduction
Dry grassland habitats often host diverse lichen communities (Biurrun et al. Reference Biurrun, Pielech, Dembicz, Gillet, Kozub, Marcenò, Reitalu, Van Meerbeek, Guarino and Chytrý2021) as components of ‘biological crusts’. These crusts are complex assemblages of organisms including mosses, liverworts, cyanobacteria, algae, lichens, fungi and bacteria that grow on and within the uppermost layers of the soil (Eldridge Reference Eldridge2000). Terricolous species of the genera Aspicilia, Circinaria and Megaspora (Megasporaceae) are often present in the biological crusts of arid regions. Although vagrant Circinaria species (Sohrabi et al. Reference Sohrabi, Stenroos, Myllys, Søchting, Ahti and Hyvönen2013) are attractive elements of desert habitats, only a small number of crustose aspicilioid species of this genus attached to soil were previously known (Rosentreter Reference Rosentreter, Glenn, Harris, Dirig and Cole1998; Sohrabi et al. Reference Sohrabi, Owe-Larsson, Nordin and Walter2010; McCune & Di Meglio Reference McCune and Di2021).
The taxonomy of aspicilioid lichens at the genus level is very complicated. For instance, the use of conidia length, which was crucial for the separation of Aspicilia s. str. and Circinaria (Nordin et al. Reference Nordin, Savic and Tibell2010), has recently been criticized (McCune & Di Meglio Reference McCune and Di2021). There has also been some discussion about the genus Circinaria, which, in most species, is characterized by large, broadly ellipsoid to globose spores, 2–4 per ascus, and the presence of aspicilin in several species. The genus is either considered within the large clade of Aspicilia s. lat. (McCune & Di Meglio Reference McCune and Di2021), or partially split into smaller monophyletic clades treated as genera (e.g. Kondratyuk et al. Reference Kondratyuk, Gromakova, Khodosovtsev, Kim, Kondratiuk and Hur2015) although these lack statistical support. We follow the concept of Nordin et al. (Reference Nordin, Savic and Tibell2010) of Circinaria as a monophyletic clade containing the type species.
Although the lichens, lichenicolous fungi and lichen communities of the large dune areas of the Lower Dnipro valley (Kherson Region, Ukraine) have been studied in detail previously (e.g. Khodosovtsev et al. Reference Khodosovtsev, Boiko, Nadyeina and Khodosovtseva2011, Reference Khodosovtsev, Darmostuk, Suija and Ordynets2018), participants of the 15th international research expedition (known as a ‘Field Workshop’) of the Eurasian Dry Grassland Group (EDGG) (Moysiyenko et al. Reference Moysiyenko, Vynokurov, Shyriaieva, Skobel, Babitskyi, Bednarska, Bezsmertna, Chusova, Dengler and Guarino2022) discovered on sandy soil, an unknown sterile, aspicilioid crustose lichen with rhizomorphs. As it did not match any previously described species, we describe it here as new to science, with detailed information on the morphology, chemistry, ecology and phylogeny based on nrITS, nrLSU and mtSSU sequences.
Materials and Methods
Taxon sampling and morphological studies
Specimens were collected in sandy habitats of the Lower Dnipro sand dunes (Ukraine) during an international research expedition organized by the Eurasian Dry Grassland Group (EDGG; https://edgg.org) in June 2021 (Moysiyenko et al. Reference Moysiyenko, Vynokurov, Shyriaieva, Skobel, Babitskyi, Bednarska, Bezsmertna, Chusova, Dengler and Guarino2022). Specimens were examined with Optica-1 and MICROMED–2 microscopes using standard microscopy techniques. Microscopic examination was carried out on material mounted in water and 10% KOH (K) (Smith et al. Reference Smith, Аptroot, Coppins, Fletcher, Gilbert, James and Wolseley2009). Photographs were taken with a Levenhuk C510 camera. Measurements were made in water with a precision of 0.5 μm for microscopical structures and 5 μm for anatomical layers of thalli. Dimensions are given as (min–) x̄–SD – x̄+SD (–max) (n), where x̄ is the mean, SD is the standard deviation and n is the number of measurements taken. All examined specimens are deposited in the herbaria of Kherson State University, Ukraine (KHER) and the W. Szafer Institute of Botany, Polish Academy of Sciences (KRAM L). Vascular plants, bryophytes and lichens were recorded with the standardized ‘biodiversity plot’ sampling of EDGG (Dengler et al. Reference Dengler, Boch, Filibeck, Chiarucci, Dembicz, Guarino, Henneberg, Janišová, Marcenò and Naqinezhad2016a). Presence of species is given for substratum grain sizes of 0.0001, 0.001, 0.01, 0.1 and 1 m2, whereas percentage cover is indicated for 10 m2. Nomenclature of vascular plants follows the Euro+Med Plantbase (Euro+Med 2006) at the species level.
DNA extraction, PCR amplification and DNA sequencing
Genomic DNA was extracted from a small number of clean lobes using the QIAamp DNA Investigator Kit (Qiagen, Hilden, Germany) following the manufacturer's instructions. We amplified the mtDNA small subunit (mtSSU) using the primer pair mrSSU1 and mrSSU3R (Zoller et al. Reference Zoller, Scheidegger and Sperisen1999), as well as nrITS (ITS1 + 5.8S + ITS2) and the nuclear large subunit rDNA (nrLSU) with the primers ITS1F and LR5 (Vilgalys & Hester Reference Vilgalys and Hester1990; Gardes & Bruns Reference Gardes and Bruns1993). Polymerase chain reactions (PCR) were performed in a volume of 25 μl comprising 1 μl of DNA template, 0.2 μl of AmpliTaq 360 DNA polymerase (Applied Biosystems, California, USA), 2.5 μl of 10× AmpliTaq 360 PCR Buffer, 2.5 μl 25mM MgCl2, 1 μl of each primer (10 μM), 2 μl GeneAmp dNTPs (10 mM; Applied Biosystems, California, USA), 0.2 μl bovine serum albumin (BSA; New England Biolabs, Massachusetts, USA), with sterile distilled water added to attain the final volume. PCR amplifications were performed using the thermocycling conditions of Rodriguez-Flakus & Printzen (Reference Rodriguez-Flakus and Printzen2014). PCR products were visualized by running 3 μl of the PCR product on 1% agarose gels.
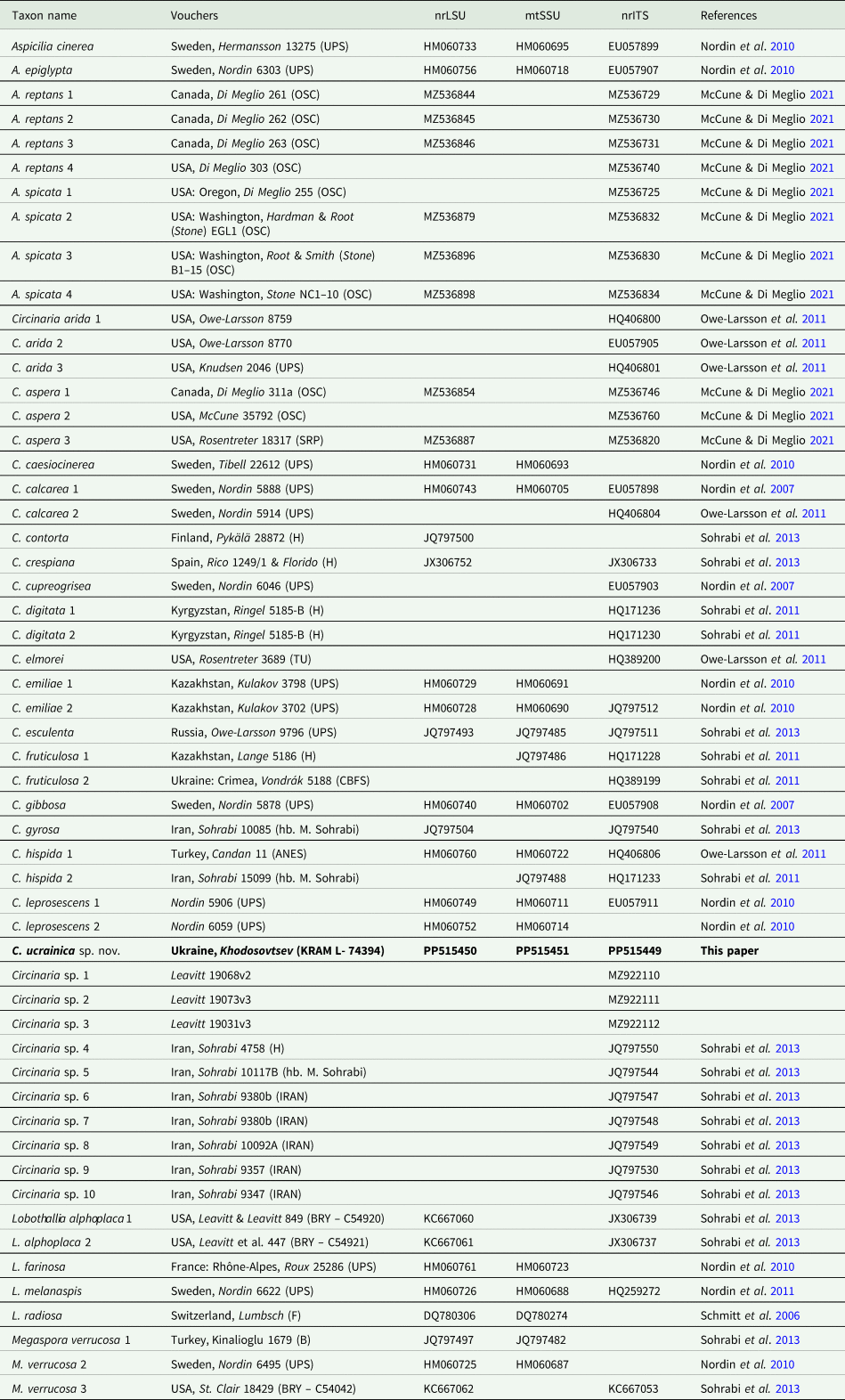
The newly generated sequences were checked, assembled and edited manually using Geneious Pro v. 8.0. (Biomatters, Auckland, New Zealand) and deposited in GenBank. Accession numbers are provided in Table 1.
Table 1. Voucher information and GenBank Accession numbers of sequences of Circinaria and related species included in the phylogenetic analyses (Fig. 1).
Phylogenetic analyses and taxon selection
All generated sequences were checked by BLAST (Altschul et al. Reference Altschul, Gish, Miller, Myers and Lipman1990) to verify potential contamination by unrelated fungi. BLAST searches of the obtained sequences revealed the highest similarity with Circinaria species (Circinaria aff. arida for the nrITS and nrLSU regions, and C. caesiocinerea (Nyl. ex Malbr.) A. Nordin et al. for mtSSU). Alignments were generated for each region using MAFFT (Katoh & Standley Reference Katoh and Standley2013) as implemented on the GUIDANCE2 web server (Penn et al. Reference Penn, Privman, Ashkenazy, Landan, Graur and Pupko2010). GUIDANCE2 assigns a confidence score to each ambiguous nucleotide site in the alignment and later removes regions of uncertain columns. We used the default cut-off score of 0.93 in all single gene alignments. The subsequent analyses were performed using the CIPRES Science Gateway (http://www.phylo.org/portal2/) (Miller et al. Reference Miller, Pfeiffer and Schwartz2010). PartitionFinder 2 was used to select the best partition for our data and substitution models (Lanfear et al. Reference Lanfear, Frandsen, Wright, Senfeld and Calcott2016). A single substitution model was selected for each region (SYM + G for ITS1 and ITS2, K80 for 5.8S, TRN + I + G for nrLSU, HKY + I for mtSSU) under a greedy search algorithm and the Akaike information criterion (AIC) (Lanfear et al. Reference Lanfear, Calcott, Ho and Guindon2012). Maximum likelihood (ML) analyses were carried out using a heuristic search as implemented in IQ-TREE on XSEDE and 100 bootstrap interactions on 1000 replicates to estimate branch support. Bayesian inference (BI) of the phylogenetic relationships was calculated using the Markov chain Monte Carlo (MCMC) approach as implemented in MrBayes v. 3.2.6 on XSEDE (Ronquist et al. Reference Ronquist, Teslenko, van der Mark, Ayres, Darling, Höhna, Larget, Liu, Suchard and Huelsenbeck2012), using the partitions and substitution models obtained by PartitionFinder 2. Two independent parallel runs were started, each with four incrementally heated (0.15) chains. This MCMC was allowed to run for 100 million generations, sampling every 1000th tree and discarding the first 50% of sampled trees as a burn-in factor. The analysis was stopped after 1 million generations when the standard deviation of split frequencies had dropped below 0.01. The resulting ML and BI phylogenetic trees were visualized in FigTree (http://tree.bio.ed.ac.uk/software/figtree/) and Inkscape (https://inkscape.org/). The tree was rooted using Megaspora verrucosa as the outgroup.
Thin-layer chromatography
The secondary chemistry of all samples was studied by thin-layer chromatography (TLC) following the methods of Culberson & Kristinsson (Reference Culberson and Kristinsson1970) and Orange et al. (Reference Orange, James and White2001).
Soil parameters
Soil samples were analyzed using the EDGG methodological approach (Dengler et al. Reference Dengler, Boch, Filibeck, Chiarucci, Dembicz, Guarino, Henneberg, Janišová, Marcenò and Naqinezhad2016a). A mixed soil sample of the uppermost 10 cm of the soil was taken from five random locations within the 10 m2 plot. All samples were dried at 65 °C and the following parameters were then determined in the laboratory: skeleton content (mass fraction of particles > 2 mm); the percentages of sand, clay and silt (texture class estimated with the Robinson pipette method after removing organic matter with 6% H2O2); pH (in a suspension of 10 g dry soil in 25 ml distilled water); electrical conductivity (EC) in the same pH extract (in a suspension of 10 g dry soil in 50 ml distilled water, μS cm−1); carbon total (%); CaCO3 content (%) (Schlichting et al. Reference Schlichting, Blume and Stahr1995; Wamelink et al. Reference Wamelink, van Adrichem, van Dobben, Frissel, den Held, Joosten, Malinowska, Slim and Wegman2012).
Results
The final concatenated alignment included 55 sequences of 2034 unambiguous nucleotide positions (ITS1 = 187 sites, 5.8S = 158, ITS2 = 150, nrLSU = 761, mtSSU = 778). The ML and BI analyses yielded similar topologies. Figure 1 represents the topology recovered from the BI analysis. The genus Circinaria formed a well-supported clade (PP = 1, BS = 95), sister to the clade containing Lobothallia and Aspicilia. The newly generated sequences of the Circinaria species from the sand dunes of southern Ukraine formed a highly supported separate clade with Aspicilia reptans (Looman) Wetmore (PP = 0.99, BS = 92). Morphologically and ecologically the latter species is similar to C. ucrainica, but it has thicker rhizomorphs of 200–400 μm diameter, finely developed areoles and lacks spicate prothalline tips, whereas the studied specimens grew inland on sand dunes and were characterized by narrow spicate prothalline tips and rhizomorphs. Therefore, taking into account the differences in morphological, ecological and molecular data, we describe a new Circinaria species growing on sandy soil and also propose a new combination for Aspicilia reptans.
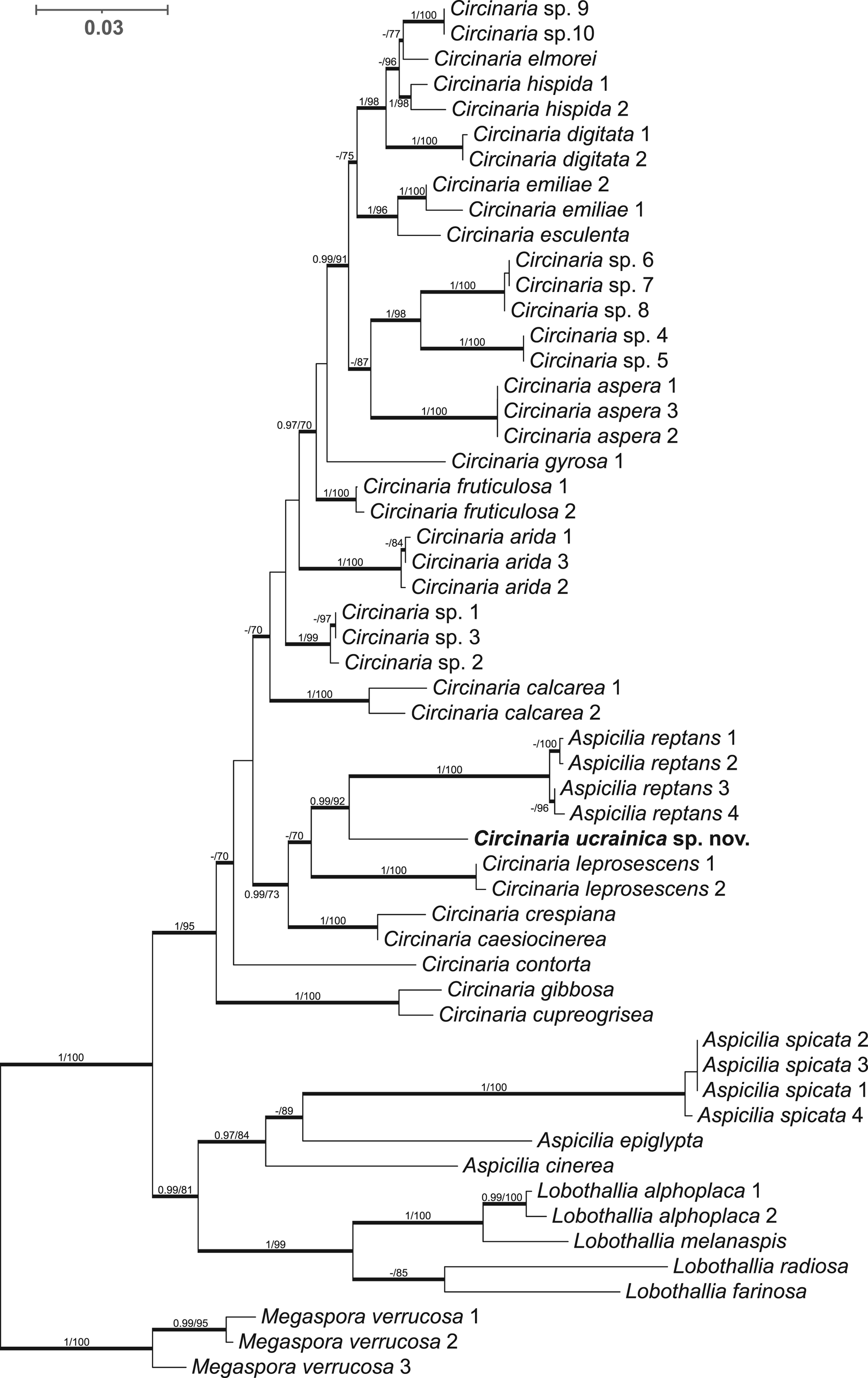
Figure 1. Phylogenetic placement of Circinaria ucrainica inferred from Bayesian inference (BI) analyses of the combined nrITS, nrLSU and mtSSU data set. Megaspora verrucosa was used as the outgroup. Bold branches represent either maximum likelihood (ML) bootstrap values ≥ 70 and/or Bayesian posterior probabilities ≥ 0.97. BI/ML values are indicated on branches. The new species is shown in bold.
Taxonomy
Circinaria ucrainica Khodos. & Darmostuk sp. nov.
MycoBank No.: MB 853496
Differing from Aspicilia spicata by the presence of finely developed dark grey to brown spicate prothalline tips, 50–150 μm diam., (0.5–)1–2.5(–3) mm long, poorly developed grey areoles, a thinner epicortex and the presence of aspicilin.
Type: Ukraine, Kherson Region, Kherson District, near Oleshky, Landscape Reserve ‘Sagy’, 46°36ʹ40.7ʺN, 32°51ʹ28.8ʺE, 12 m, 11 June 2021, A. Khodosovtsev (KHER 15091—holotype; KHER 15092, 15093, KRAM L- 74394—isotypes). GenBank Accession nos: PP515449 (nrITS), PP515450 (nrLSU), PP515451 (mtSSU).
(Fig. 2)
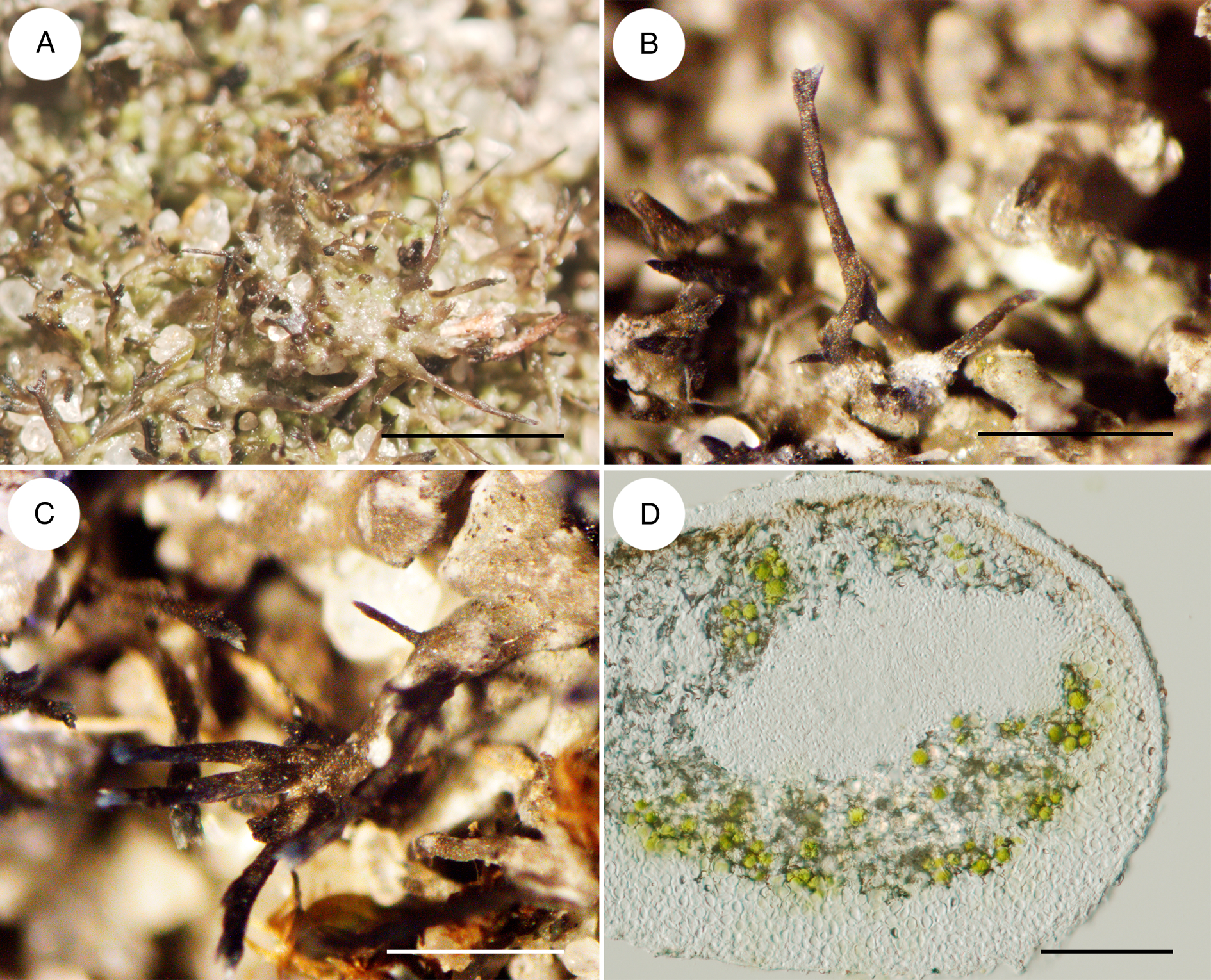
Figure 2. Circinaria ucrainica. A, general habitat of wet lichen thalli. B, spicate prothalline tips. C, elongated areoles with spicate prothalline tips. D, cross-section through almost cylindrical areoles. Scales: A = 2 mm; B & C = 1 mm; D = 100 μm. In colour online.
Thalli crustose, cushion-like, relatively large, 2–10 cm diam. and 0.2–0.5 cm high, consisting of dispersed or rarely overlapping areoles, interconnected by abundant mycobiont rhizomorphs. Areoles rarely finely developed and overlapping, (120–)150–200(–220) μm thick, more or less isodiametric, 0.2–1.0 mm wide or elongated, 0.1–0.2 × 0.5–1.0 mm, flat, slightly convex or almost cylindrical, grey or greyish green, sometimes with a brown tinge above and whitish below. Spicate prothalline tips formed on the edges of areoles, (2–)3–5(–7) per areole, dark grey to brown, 50–150 μm diam., horizontally or vertically oriented, (0.5–)1–2.5(–3) mm long, single or dichotomously branched, fan-shaped, extended and bluish at the tips. Rhizomorphs hyaline, 100–150 μm diam., up to 10 mm long, branched and forming a loose network. Epicortex hyaline, consisting of dead cells, without crystals, (5–)7–9(–12) μm deep (n = 10). Upper cortex hyaline or light brown in external parts, (20–)30–50(–65) μm (n = 20) thick, paraplectenchymatous, consisting of rounded cells with lumina (4–)5.0–8.0(–9.0) μm (n = 30) diam., completely covered by cylindrical areoles (Fig. 2D). Algal plectenchyma c. 30–50 μm thick, more or less continuous or with clusters deep in medulla; algae Trebouxia-type, (8–)9.5–13.5(–20) μm diam. Medulla white, loose, prosoplectenchymatous, c. 80–120 μm thick. Lower cortex not developed. Rhizomorphs and spicate prothalline tips covering hyaline layers c. 10–15 μm thick, without algae, prosoplectenchymatous, with lumina (1.5–)2.0–4.0(–4.5) μm (n = 15) wide.
Apothecia, pycnidia, pseudocyphellae and vegetative diaspores absent.
Chemistry
TLC: aspicilin. Spot tests negative.
Etymology
The species is named after the Ukraine, where the Eurasian Dry Grassland Group (https://edgg.org) organized a research expedition to the steppe and coastal habitats in May–June 2021, during which the species was discovered.
Ecology
The species grows in the sand dunes of the Lower Dnipro valley, Ukraine, where it can cover a significant area (up to 20%). It is part of the biological crust dominated by the moss Syntrichia ruraliformis (up to 40%), along with other lichens such as Cladonia fimbriata (L.) Fr., C. foliacea (Huds.) Willd., Placynthiella uliginosa (Schrad.) Coppins & P. James s. lat., Xanthoparmelia camtschadalis (Ach.) Hale and the mosses Bryum caespiticium Hedw. and Ceratodon purpureus (Hedw.) Brid. Vascular plants in the biodiversity plot were represented by 32 species with the highest cover by Poa bulbosa L. (15%), Helichrysum arenarium (L.) Moench (5%) and Stipa borysthenica Klokov ex Prokudin (5%) (Table 2).
Table 2. Diversity and cover of vascular plants, mosses and lichens adjacent to the EDGG biodiversity plot UAS046 with Circinaria ucrainica (L = lichen, M = mosses). The table is ordered according to Dengler et al. (Reference Dengler, Biurrun, Apostolova, Baumann, Becker, Berastegi, Boch, Dembicz, Dolnik and Ermakov2016b). Organisms are arranged in the order in which they are identified in the corner of the site.
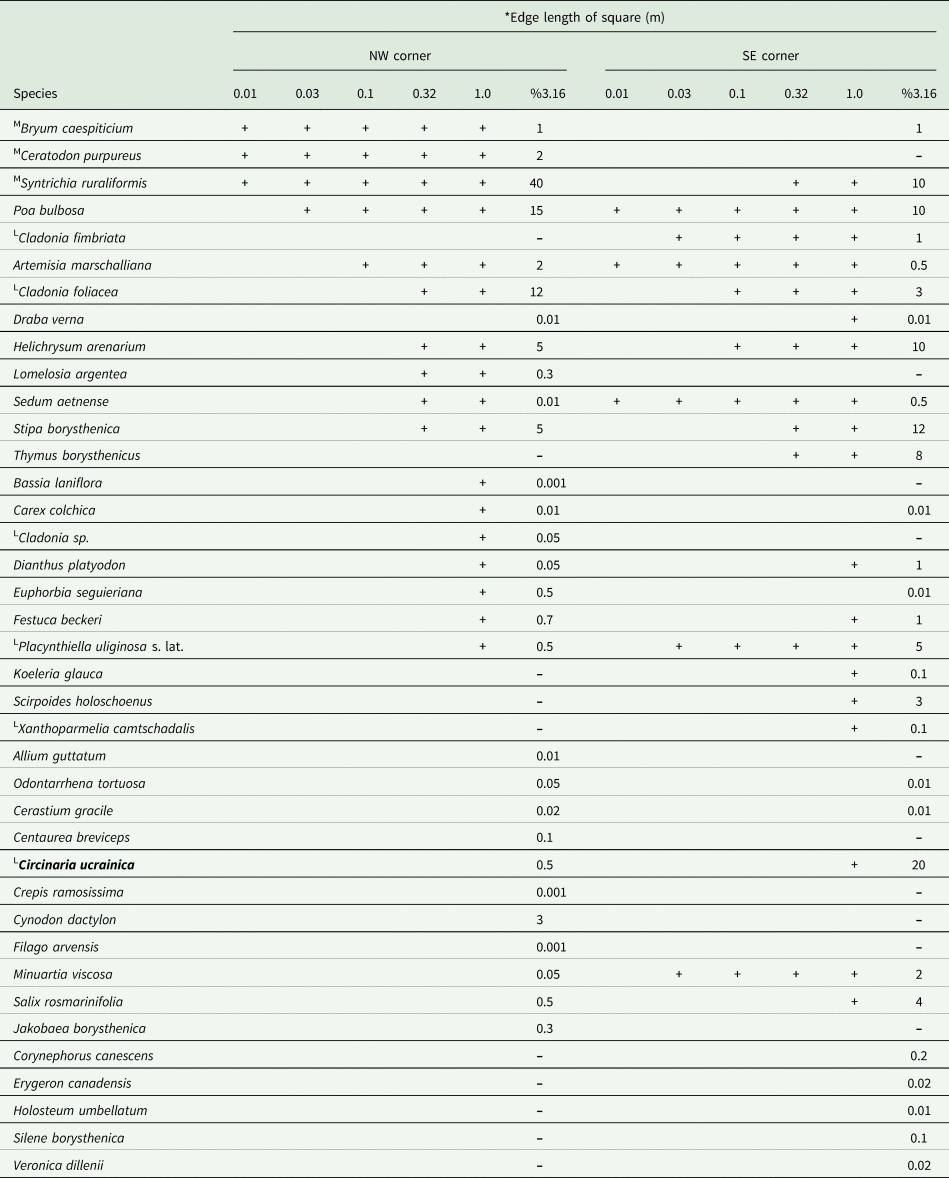
* Astragalus varius was found only in 100 m2
The rhizomorphs of Circinaria ucrainica grew closely attached to sand grains of stable small dunes. The main soil parameters in the habitat are: proportion of skeleton – 0.6%; soil texture class – sand; proportion sand – 99.0%; proportion silt – 1.0%; proportion of clay – 0.02%; pH (H2O) – 6.97; electrical conductivity – 64.4 μS cm−1; carbon total – 0.33%; CaCO3 content – 12.5%.
Specimen examined
Ukraine: Kherson region, Kherson district, near Oleshky, Landscape Reserve ‘Sagy’, 46°36ʹ40.6ʺN, 32°51ʹ28.3ʺE, 12 m, biodiversity plot UAS046, 2 vi 2021, I. Dembicz, J. Dengler & I. Moysiyenko (KHER 15094—paratype).
New combination
Circinaria reptans (Looman) Khodos. & Darmostuk comb. nov.
MycoBank No.: MB 853497
Basionym: Lecanora reptans Looman, Bryologist 65, 301 (1963).
Discussion
Rhizomorphs and spicate prothalline tips are rare and specific structures in Aspicilia s. lat. Rhizomorphs are linear mycelial aggregates produced from the lower cortex of crustose lichens (Sanders & Rico Reference Sanders and Rico1992; Sanders & Ascaso Reference Sanders and Ascaso1997; Sanders Reference Sanders1999; McCune & Di Meglio Reference McCune and Di2021). They are mainly characteristic of species that overgrow loose substrata, such as mosses or soil. In Megasporaceae, these structures are rare and found only in a small number of species of Aspicilia s. lat. (incl. Circinaria) (McCune & Di Meglio Reference McCune and Di2021). In Europe, Circinaria crespiana (V.J. Rico) Sohrabi & V.J. Rico was described on mosses from the Mediterranean (Rico Reference Rico1999). It has thick underground rhizomorphs, up to 1.5 mm wide (in Circinaria ucrainica up to 0.15 mm), lacks spicate prothalline tips and has better developed squamulose areoles, up to 5 mm in width (in C. ucrainica up to 1 mm). Another species with rhizomorphs is Circinaria reptans, reported from calcareous soil and plant detritus in North America (Canada, USA) and Asia (Iran) (Lumbsch et al. Reference Lumbsch, Ahti, Altermann, Amo de Paz, Aptroot, Arup, Pena, Bawingan, Benatti and Betancourt2011; McCune & Di Meglio Reference McCune and Di2021). This species is similar to C. ucrainica but has thicker rhizomorphs of 200–400 μm diameter, finely developed areoles and lacks spicate prothalline tips. Recently, rhizomorphs were found in Aspicilia diploschistiformis McCune & J. Di Meglio, A. papilliformis McCune & J. Di Meglio, A. subcontinua McCune & J. Di Meglio and A. wyomingensis McCune & J. Di Meglio (McCune & Di Meglio Reference McCune and Di2021), but these species lack spicate prothalline tips like those found in Circinaria ucrainica. These species resolved in different clades in the phylogenetic analysis provided by McCune & Di Meglio (Reference McCune and Di2021), phylogenetically different from the Circinaria reptans clade.
The term ‘spicate prothalline tips’ was introduced by McCune & Di Meglio (Reference McCune and Di2021) to describe fungal structures formed by the prothallus. These structures were found in Aspicilia californica Rosentr. and A. filiformis Rosentr. from arid habitats in the United States (Sanders Reference Sanders1999). However, these species differ from C. ucrainica by their fruticose growth. The recently described crustose Aspicilia spicata McCune & J. Di Meglio with rhizomorphs (McCune & Di Meglio Reference McCune and Di2021) has finely developed spicate prothalline tips and is morphologically very close to C. ucrainica. However, A. spicata has finely developed, beaded-lobate or stringy to reticulate-lobate or warty-areolate brown areoles with a thick epicortex (in contrast to C. ucrainica, with poorly developed greyish areoles and a thin epicortex). Aspicilia californica, A. filiformis and A. spicata belong to the Aspicilia filiformis clade (McCune & Di Meglio Reference McCune and Di2021), which is phylogenetically distant from the Circinaria reptans clade. Circinaria aspera (Mereschk.) Sohrabi & Şenkard. rarely has rhizomorphs and spicate prothalline tips (McCune & Di Meglio Reference McCune and Di2021) but differs by a dimorphic thallus with crustose and fruticose parts (in contrast to the poorly developed thallus of C. ucrainica), rounded or isidioid prothalline tips (vs spicate tips in C. ucrainica), as well as a thicker epicortex, 12–50 μm (vs (5–)7–9(–12) μm in C. ucrainica). Aspicilia albonota McCune & J. Di Meglio has very small spicate prothalline tips, but this species has no rhizomorphs and forms pseudocyphellae on the areoles (McCune & Di Meglio Reference McCune and Di2021). The poorly studied Aspicilia terrestris Tomin (Tomin Reference Tomin1956), from arid salt habitats of Kazakhstan (Lake Inder) and Russia (Lake Baskunchak), lacks spicate prothalline tips and is morphologically similar to Circinaria reptans.
Based on current knowledge, Circinaria ucrainica has a very narrow distribution range but is quite abundant locally. It has been found only on a small number of dunes, even though 140 relevés of terricolous bryophyte and lichen communities had previously been conducted in the area without encountering the species (Khodosovtsev et al. Reference Khodosovtsev, Boiko, Nadyeina and Khodosovtseva2011). Phytosociologically, the plots with Circinaria ucrainica can, in accordance with the ‘EuroVegChecklist’ (Mucina et al. Reference Mucina, Bültmann, Dierßen, Theurilla, Raus, Čarni, Šumberová, Willner, Dengler and Gavilán García2016), be assigned to the class Koelerio-Corynephoretea canescentis, the order Festucetalia vaginatae, and the alliance Festucion beckeri. According to the ‘Prodrome of the Vegetation of Ukraine’ (Dubyna et al. Reference Dubyna, Dzuba, Іemelianova, Bagrycova, Borysova, Borsukevych, Vynokurov, Gapon, Gapon and Davydov2019), the assignment is somewhat different due to a different system of higher units of psammophyte vegetation. There it belongs to the class Festucetea vaginatae but the same order and alliance as in the EuroVegChecklist. In terms of floristic composition, the stand is closest to the association Centaureo brevicipiti-Festucetum beckeri. If we consider the mosaic of cryptogam communities within the ‘biological crust’, then the crust can be attributed to the class Ceratodonto purpurei-Polytrichetea piliferi, the alliance Cladonion rei and close to the association Syntrichietum ruraliformis (Khodosovtsev et al. Reference Khodosovtsev, Boiko, Nadyeina and Khodosovtseva2011). This is especially evident at small scales within the biodiversity plot (Table 2). Following the EUNIS habitat classification (Schaminée et al. Reference Schaminée, Chytrý, Hennekens, Janssen, Knollová, Rodwell and Tichý2018; Chytrý et al. Reference Chytrý, Tichý, Hennekens, Knollová, Janssen, Rodwell, Peterka, Marcenò, Landucci and Danihelka2020), the site belongs to the habitat type ‘R11-Pannonian and Pontic sandy steppe’, while in the national habitat classification of Ukraine (Kuzemko et al. Reference Kuzemko, Didukh, Onyshchenko and Sheffer2018) it corresponds to ‘Т1.1.2-Sandy grasslands on neutral substrata’.
While most lichen species have large distribution ranges, some vagrant and terricolous Circinaria species from arid habitats are local endemics. For example, Circinaria tominii (Oxner) Sohrabi was collected only from two nearby localities in the Czuensi Desert (Altai, Russia) (Sohrabi et al. Reference Sohrabi, Stenroos, Myllys, Søchting, Ahti and Hyvönen2013) and C. aschabadensis (J. Steiner) Sohrabi is known from a single locality in the Kopet-Dagh Desert (Turkmenistan). Circinaria ucrainica is probably a local endemic of the dunes of the Lower Dnipro and needs to be protected. The population grows in the Landscape Reserve ‘Sagy’, but it is situated only 150–200 m from the main roads to Oleshky, Kakhovka and Crimea, where the Russian invasion of Ukraine took place in 2022. The single known population of the species is under threat of complete destruction and possible extinction.
The international research expeditions of the Eurasian Dry Grassland Group, conducted since 2009 in grassland and other non-forest ecosystems throughout the Palearctic, do not only yield standardized high-quality biodiversity data of vascular plants, but also of terricolous bryophytes and lichens, and sometimes animal taxa, together with soil and other environmental variables (Dengler et al. Reference Dengler, Boch, Filibeck, Chiarucci, Dembicz, Guarino, Henneberg, Janišová, Marcenò and Naqinezhad2016a, Reference Dengler, Biurrun, Apostolova, Baumann, Becker, Berastegi, Boch, Dembicz, Dolnik and Ermakovb). Careful sampling of survey plots of defined areas by international experts, particularly for understudied taxa such as lichens, provides not only valuable references for species diversity in different grassland types and regions (Dengler et al. Reference Dengler, Biurrun, Apostolova, Baumann, Becker, Berastegi, Boch, Dembicz, Dolnik and Ermakov2016b; Biurrun et al. Reference Biurrun, Pielech, Dembicz, Gillet, Kozub, Marcenò, Reitalu, Van Meerbeek, Guarino and Chytrý2021), but has repeatedly led to first records of lichen species for countries or other larger geographical areas. Circinaria ucrainica is already the second species new to science reported on an EDGG expedition, after the spider Pulchellodromus navarrus found on the EDGG expedition in Navarre, Spain (Kastrygina et al. Reference Kastrygina, Kovblyuk and Polchaninova2016).
Acknowledgements
We thank the Eurasian Dry Grassland Group for financial support to the Ukrainian participants of the expedition, and also Marina Zakharova, Olena Schepeleva and Anna Tavrovetska for help during the field excursion. Alexander Khodosovtsev is grateful to researchers from the Center for Molecular Medicine (UMC Utrecht, The Netherlands), especially Prof. Saskia van Mil and Dr Anna Mukha for the donation of a ‘Zeiss Axioscope’ microscope to Kherson State University (Ukraine) which made it possible to continue working in Kyiv during the temporary occupation of Kherson by Russian troops.
Author ORCIDs
Alexander Khodosovtsev, 0000-0002-5906-9876; Valerii Darmostuk, 0000-0003-1430-1755; Iwona Dembicz, 0000-0002-6162-1519; Jürgen Dengler, 0000-0003-3221-660X; Ivan Moysiyenko, 0000-0002-0689-6392; Anna Kuzemko, 0000-0002-9425-2756.







