Introduction
The diverse topography, geology, climate and vegetation of South Africa contribute to a rich lichen biota estimated to contain at least 2500–3000 species (Fryday Reference Fryday2015). Approximately 1750 species of lichenized and allied fungi, many of which are endemic to southern Africa, are presently recorded from the country (Crous et al. Reference Crous, Rong, Wood, Lee, Glen, Botha, Slippers, de Beer, Wingfield and Hawksworth2006; Fryday Reference Fryday2015; Ahti et al. Reference Ahti, Mayrhofer, Schultz, Tehler and Fryday2016). The majority of the at least 750–1250 undocumented or undiscovered species are expected to be crustose lichens, which have not yet received the taxonomic attention given in South Africa to macrolichen genera such as Xanthoparmelia (Fryday Reference Fryday2015).
Graphidaceae is the largest family of crustose lichens worldwide (Staiger Reference Staiger2002; Frisch Reference Frisch2006; Lücking et al. Reference Lücking, Johnston, Aptroot, Kraichak, Lendemer, Boonpragob, Cáceres, Ertz, Itati and Jia2014, Reference Lücking, Hodkinson and Levitt2017). Research on this family in South Africa has been piecemeal, beginning with the description of Thelotrema henatomma Ach. by Acharius (Reference Acharius1804). Nylander (Reference Nylander1868) published the first taxonomic paper that described new lirelliform Graphidaceae from South Africa, based on specimens collected near Pietermaritzburg by Olivia Armstrong and William Mackenzie (Medeiros Reference Medeiros2019). His article described one new species in the family and reported several records of previously described species. Additional new species were described by Müller (Reference Müller1886, Reference Müller1887, Reference Müller1895), Vainio (Reference Vainio1926) and Zahlbruckner (Reference Zahlbruckner1926, Reference Zahlbruckner1932). More recently, Egea & Torrente (Reference Egea and Torrente1996) described the new saxicolous species Gymnographopsis latispora Egea & Torrente from fresh material and Kalb et al. (Reference Kalb, Buaruang, Papong and Boonpragob2009) described the new species Acanthothecis dialeucoides Kalb & Staiger from a historical specimen. Several species described from South African type material have been revised by modern authors as part of global revisions (Frisch Reference Frisch2006; Lücking et al. Reference Lücking, Archer and Aptroot2009). The most substantial recent work on Graphidaceae in South Africa is a country-level revision of the genus Diploschistes (Guderley & Lumbsch Reference Guderley and Lumbsch1996), which has an unusual ecology within the family. Whereas most species of Graphidaceae occur on bark in shrubby or forest vegetation, Diploschistes species occur on rock or soil, often in arid or semi-arid regions (Staiger Reference Staiger2002; Frisch Reference Frisch2006; Lücking et al. Reference Lücking, Johnston, Aptroot, Kraichak, Lendemer, Boonpragob, Cáceres, Ertz, Itati and Jia2014). Moreover, the only molecular data available for South African Graphidaceae is a single nuclear large subunit ribosomal RNA (nrLSU) sequence from Diploschistes actinostoma (Ach.) Zahlbr. (Rivas Plata et al. Reference Rivas, Parnmen, Staiger, Mangold, Frisch, Weerakoon, Hernandez, Cáceres, Kalb and Sipman2013).
Throughout this literature, species descriptions and records have accumulated without any synthesis of the systematics of lirelliform Graphidaceae in southern Africa. The only attempts at summarizing these scattered data have been the lichen checklists compiled by Doidge (Reference Doidge1950) and Fryday (Reference Fryday2015), which dealt with all lichens and did not make new taxonomic conclusions. Furthermore, no recent publications have dealt with new collections of lirelliform Graphidaceae on bark, where we expect a large portion of this family's South African biodiversity. Lücking et al. (Reference Lücking, Johnston, Aptroot, Kraichak, Lendemer, Boonpragob, Cáceres, Ertz, Itati and Jia2014) estimated that the lichen biota of Namibia, Botswana and South Africa includes over 220 species of Graphidaceae, with more than 160 species either unreported or undescribed. The method Lücking et al. (Reference Lücking, Johnston, Aptroot, Kraichak, Lendemer, Boonpragob, Cáceres, Ertz, Itati and Jia2014) used to estimate Graphidaceae biodiversity excluded strictly extratropical species, but subtropical biodiversity hotspots in South Africa (Mucina & Rutherford Reference Mucina and Rutherford2006) could support substantial biodiversity in this family. The present study is an initial contribution to a modern treatment of Graphidaceae biodiversity in South Africa, focusing on the species of the tribe Graphideae that have hyaline ascospores, namely species of the lirelliform genera Allographa, Diorygma, Glyphis, Graphis, Mangoldia and Platythecium (Rivas Plata et al. Reference Rivas, Parnmen, Staiger, Mangold, Frisch, Weerakoon, Hernandez, Cáceres, Kalb and Sipman2013; Lumbsch et al. Reference Lumbsch, Kraichak, Parnmen, Rivas Plata, Aptroot, Cáceres, Ertz, Feuerstein, Mercado-Diaz and Staiger2014a). Species of Graphideae with pigmented ascospores (i.e. Phaeographis, Platygramme and related genera) will be treated in a subsequent publication.
Materials and Methods
Specimens were collected in February 2016 and May–June 2019 at forest and savannah sites in Mpumalanga, KwaZulu-Natal and Western Cape provinces (Figs 1 & 2). The forested areas were classified as Northern Mistbelt Forest, Southern Mistbelt Forest, and Southern Afrotemperate Forest (Mucina & Rutherford Reference Mucina and Rutherford2006). Additional relevant specimens were obtained from B, BOL, BM, DBN, LD, NU, PRE, SBBG, TRH, TUR, UPS, US and WIS. These included a large set of Graphidaceae from KwaZulu-Natal, collected by Ove Arbo Høeg in the 1920s, that has not previously been studied or discussed in any published work. Approximately 50 new collections and 110 herbarium specimens from South Africa, including four type specimens, were examined for this study.

Fig. 1. Map of South Africa showing collection localities for specimens in the present study. Open circles with letters A–D refer to localities depicted in Fig. 2. Filled black circles indicate other collection localities, including historical specimens. Extent of the forest biome in South Africa, Eswatini and Lesotho indicated in green (in colour) or mid grey (South African National Biodiversity Institute 2012). In colour online.

Fig. 2. Example collection sites of South African Graphidaceae. A, Northern Mistbelt Forest in a ravine at Buffelskloof Private Nature Reserve. Note planted Pinus on former natural grassland in the background. Photograph by József Geml. B, savannah in Kruger National Park. Zebras for scale. Photograph by Shuzo Oita. C, Diorygma aff. minisporum in situ on a tree trunk in Southern Mistbelt Forest in the Karkloof Nature Reserve. Photograph by Ian D. Medeiros. D, Southern Afrotemperate Forest on the coast at Nature's Valley, Garden Route National Park. Photograph by Betsy Arnold. See Fig. 1 for locations of these sites. In colour online.
Morphology and chemistry
Specimens were observed under Leica M60, Olympus SZ61, or Leica MZ6 dissecting microscopes. Photographs were taken with a Canon Rebel XSi camera attached to a Leica MZ125 dissecting microscope or with a Nikon D3200 camera with the VariMag DSLR modular imaging system attached to an Olympus SZ61 dissecting microscope. Hand-cut thin sections of apothecia were mounted in tap water for observation under either a Leica DM1000, Leica DMLB, or Olympus CH30 compound microscope. Morphological characters were documented following Lücking (Reference Lücking2009). Recently published keys (Staiger Reference Staiger2002; Lücking et al. Reference Lücking, Archer and Aptroot2009; Barcenas Peña et al. Reference Barcenas Peña, Lücking, Miranda-Gonzalez and Herrera-Campos2014; Joshi et al. Reference Joshi, Upreti, Neow and Hur2016) and species descriptions from the primary literature were used to assess whether collections represented previously described species. Genera and species have been designated as first reports for South Africa if they do not appear in the South African lichen checklist (Fryday Reference Fryday2015; Ahti et al. Reference Ahti, Mayrhofer, Schultz, Tehler and Fryday2016).
Secondary chemistry was assessed with thin-layer chromatography (TLC) using standard methods (Culberson & Kristinsson Reference Culberson and Kristinsson1970; Orange et al. Reference Orange, James and White2010). Plates were run in solvent systems C (170 toluene: 30 glacial acetic acid) for standard analyses and G (139 toluene: 83 ethyl acetate: 8 formic acid) when necessary to separate stictic acid satellite compounds. Elix (Reference Elix2018) was consulted as an additional reference for identifying TLC spots.
Molecular phylogenetics
All new molecular data are from South African specimens except for one specimen of Platygramme pachnodes (Fée) Fée from Florida, USA (LaGreca et al. Reference LaGreca, Perlmutter, Goldman, Seavey and Seavey2021). DNA was isolated either from fresh specimens or from specimens stored at −20 °C in silica gel. Small fragments (c. 1–2 mm2) of thallus or ascomata were excised with a sterile needle. DNA extraction followed the procedure outlined in Hughes et al. (Reference Hughes, Case, Matheny, Kivlin, Petersen, Miller and Iturriaga2020) using laboratory-made extraction and dilution buffers, except that thallus fragments were frozen in the extraction buffer for 1 h at −20 °C and thawed to room temperature prior to the heating step.
The internal transcribed spacer (ITS) was amplified with primers ITS1F and LR3 (Vilgalys & Hester Reference Vilgalys and Hester1990; Gardes & Bruns Reference Gardes and Bruns1993). ITS sequences are not widely available for Graphidaceae (Cáceres et al. Reference Cáceres, Lücking, Schumm and Aptroot2020) and the few ITS sequences we obtained were not used in subsequent phylogenetic analyses. The nuclear large subunit (nrLSU) was amplified with primer pair AL2R and LR6 (Vilgalys & Hester Reference Vilgalys and Hester1990; Mangold et al. Reference Mangold, Martín, Lücking and Lumbsch2008), while the mitochondrial small subunit (mtSSU) was amplified with primer pair mrSSU1 and mrSSU3R (Zoller et al. Reference Zoller, Scheidegger and Sperisen1999). The RNA polymerase II second largest subunit (RPB2) was amplified with a nested approach, first using the fungal primers fRPB2-7cF and fRPB2-11aR (Liu et al. Reference Liu, Whelen and Hall1999) and then the internal primers GD-RPB2-7cF and GC-RPB2-11aR, which were designed specifically for Graphidaceae (Kraichak et al. Reference Kraichak, Lücking, Aptroot, Beck, Dornes, John, Lendemer, Nelsen, Neuwirth and Nutakki2015). Thermal cycler conditions followed Kraichak et al. (Reference Kraichak, Lücking, Aptroot, Beck, Dornes, John, Lendemer, Nelsen, Neuwirth and Nutakki2015). PCR products were checked on a 1% agarose gel and cleaned with exonuclease I and shrimp alkaline phosphatase (ThermoFisher Scientific, Waltham, MA, USA). Sanger sequencing was performed by Eurofins Genomics (Louisville, KY, USA) using the PCR primers. Forward and reverse reads were assembled and checked for errors in Geneious Prime v. 2022.0.1. GenBank Accession numbers for newly generated sequences are provided in Table 1 (for nrLSU, mtSSU and RPB2) or in the remarks under individual species (for ITS). We generated two new ITS sequences, 14 new nrLSU sequences, 21 new mtSSU sequences and 19 new RPB2 sequences from a total of 21 specimens.
Table 1. Voucher information and GenBank Accession numbers for new sequences of Graphidaceae generated for this study (GenBank Accession numbers in bold) and reference taxa used in the phylogenetic analysis. Dashes indicate missing data. Note that the new data include one specimen from outside South Africa (Platygramme pachnodes from Florida, USA).

The phylogenetic analyses were based on nrLSU, mtSSU and RPB2. We prepared a dataset of reference sequences (Table 1) generated in previous studies of Graphidaceae and other fungi (Kalb et al. Reference Kalb, Staiger and Elix2004; Staiger et al. Reference Staiger, Kalb and Grube2006; Mangold et al. Reference Mangold, Martín, Lücking and Lumbsch2008; Rivas Plata et al. Reference Rivas, Hernández, Lücking, Staiger, Kalb and Cáceres2011, Reference Rivas, Parnmen, Staiger, Mangold, Frisch, Weerakoon, Hernandez, Cáceres, Kalb and Sipman2013; Cáceres et al. Reference Cáceres, Rivas Plata and Lücking2012; Nelsen et al. Reference Nelsen, Lücking, Andrew, Rivas Plata, Chaves, Cáceres and Ventura2012; McDonald et al. Reference McDonald, Gaya and Lutzoni2013; Lumbsch et al. Reference Lumbsch, Parnmen, Kraichak, Papong and Lücking2014b; Fazio et al. Reference Fazio, Adler, Parnmen, Lücking and Maier2018; Vu et al. Reference Vu, Groenewald, de Vries, Gehrmann, Stielow, Eberhardt, Al-Hatmi, Groenewald, Cardinali and Houbraken2019). Species of Acanthothecis, Diploschistes and Fissurina, representing three different Graphidaceae clades outside tribe Graphideae (Lumbsch et al. Reference Lumbsch, Kraichak, Parnmen, Rivas Plata, Aptroot, Cáceres, Ertz, Feuerstein, Mercado-Diaz and Staiger2014a), were used for the outgroup. The nrLSU and mtSSU sequences were initially aligned in MAFFT with the G-INS-1 option (Katoh et al. Reference Katoh, Rozewicki and Yamada2019), while the RPB2 sequences were aligned by translated amino acids in Mesquite (Maddison & Maddison Reference Maddison and Maddison2021). Alignments were subsequently corrected and ambiguously aligned regions were delimited in Mesquite. Ambiguous regions were excluded from subsequent analyses. Complete alignments with delimited ambiguous regions are available at FigShare (DOI: 10.6084/m9.figshare.c.6007096).
Model selection and maximum likelihood tree inference were performed using IQ-TREE v. 2.1.2 (Nguyen et al. Reference Nguyen, Schmidt, Von Haeseler and Minh2015; Chernomor et al. Reference Chernomor, Von Haeseler and Minh2016) run on the CIPRES server (Miller et al. Reference Miller, Pfeiffer and Schwartz2010). We first inferred separate trees for each locus. We performed 5000 ultrafast bootstrap pseudoreplicates to calculate bipartition support (Hoang et al. Reference Hoang, Chernomor, von Haeseler, Minh and Vinh2018) and examined the trees for well-supported conflicts (i.e. ≥ 95% ultrafast bootstrap). There were no well-supported conflicts, so the nrLSU, mtSSU and RPB2 alignments were concatenated and used as input for a partitioned analysis in IQ-TREE. Our concatenated alignment included 78 specimens, including 15 South African representatives from Graphidaceae tribe Graphideae. ModelFinder (Kalyaanamoorthy et al. Reference Kalyaanamoorthy, Minh, Wong, Von and Jermiin2017) was used to optimize the partitioning scheme and determine the best substitution models (Table 2). We performed 5000 ultrafast bootstrap pseudoreplicates to calculate bipartition support for the concatenated tree topology. The alignments and three-locus phylogeny from this paper have been made available on T-BAS (Carbone et al. Reference Carbone, White, Miadlikowska, Arnold, Miller, Kauff, U'Ren, May and Lutzoni2017, Reference Carbone, White, Miadlikowska, Arnold, Miller, Magain, U'Ren and Lutzoni2019) to facilitate access to these alignments and placement of new sequence data from this clade.
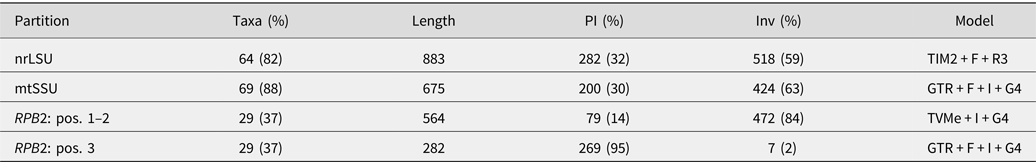
Table 2. Alignment statistics and substitution models. The full concatenated alignment included 78 specimens and 2404 sites. RPB2 was partitioned by codon position. PI = parsimony-informative sites; Inv = invariant sites.
Results
The phylogenetic analysis based on the concatenated dataset recovered hyaline-spored samples from South Africa in four highly supported clades, corresponding to the genera Allographa, Diorygma, Glyphis and Graphis s. str. (Fig. 3). We also recovered a highly supported clade containing species of the brown-spored genera Leiorreuma, Phaeographis, Pallidogramme, Platygramme and Thecaria, including three South African specimens. The monophyly of Graphideae was highly supported but relationships among genera were not, with the exception that Mangoldia was supported as sister to Allographa (Fig. 3).

Fig. 3. Maximum likelihood phylogeny of Graphidaceae tribe Graphideae based on concatenated analysis of nrLSU, mtSSU and RPB2. Specimens in bold are from South Africa. Ultrafast bootstrap (UFBoot2; Hoang et al., Reference Hoang, Chernomor, von Haeseler, Minh and Vinh2018) values ≥ 95 are indicated with thickened branches. Scale indicates substitutions per site. Loci included in the alignment are indicated for each specimen, and their GenBank Accession numbers are shown in Table 1. For information on how Graphideae is situated in a wider phylogenetic context within Graphidaceae, see Lumbsch et al. (Reference Lumbsch, Kraichak, Parnmen, Rivas Plata, Aptroot, Cáceres, Ertz, Feuerstein, Mercado-Diaz and Staiger2014a).
Morphological study of herbarium material and fresh collections yielded one species of Allographa new to science, one species based on a South African type that needed to be transferred to Mangoldia, and 12 new records for South Africa, all described below. The herbarium material also included a substantial number of Opegrapha and Enterographa specimens misidentified as Graphidaceae; these have been annotated but a complete listing of examined, non-Graphidaceae specimens is beyond the scope of this study.
Taxonomy
Note that taxonomic synonyms are provided only when they concern names described from South African material.
Allographa consanguinea (Müll. Arg.) Lücking & Kalb
Herzogia 31, 549 (2018).—Graphina consanguinea Müll. Arg., Nuov. Giorn. Bot. Ital. 21, 362 (1889).—Graphis consanguinea (Müll. Arg.) Lücking, in Lücking et al., Fieldiana, Bot. 46, 67 (2008); type: Brazil, Glaziou s. n. (G—holotype, not seen).
(Fig. 4A)

Fig. 4. Images of South African Graphidaceae. A, Allographa consanguinea (TRH L-17909). B, A. leptospora (Medeiros L449). C, A. oldayana (Medeiros L506). D, A. oldayana (Medeiros 2078—holotype). E, ascospore of A. oldayana (Medeiros L507). F, Diorygma aff. minisporum (Medeiros 2106). Scales: A–D & F = 1 mm; E = 50 μm. Images by IDM (A–E) and Thomas Barlow (F). In colour online.
Remarks
Allographa consanguinea has not previously been recorded for South Africa; it was reported from Kenya by Kirika et al. (Reference Kirika, Mugambi, Lücking and Lumbsch2012) and is otherwise known only from the Neotropics (Lücking et al. Reference Lücking, Archer and Aptroot2009). We provide the first molecular data for A. consanguinea and confirm its placement in Allographa (Table 1, Fig. 3). This corticolous species co-occurs and shares morphological similarities with Allographa oldayana, described below; see the remarks under the new species for characters that separate them. Records of Allographa acharii (Fée) Lücking & Kalb from South Africa (Nylander Reference Nylander1868; Doidge Reference Doidge1950; Almborn Reference Almborn1988) may represent A. consanguinea or A. oldayana. Allographa acharii has prominent lirellae and 2–6 large, muriform spores per ascus versus erumpent lirellae and one spore per ascus in A. consanguinea (Lücking et al. Reference Lücking, Archer and Aptroot2009).
Specimens examined
South Africa: Eastern Cape: ‘Grahamstown, natural forest just above Fern Kloof’, iii 1961, H. B. Johnston s. n. (LD 1948852). KwaZulu-Natal: ‘distr. Eshowe, on Eucalyptus along main road’, 26 viii 1919, O. A. Høeg s. n. (TRH L-17858, L-17909); ‘distr. Eshowe, S of Solheim Miss. St.’, 7 ix 1929, O. A. Høeg s. n. (TRH L-17921); uMgungundlovu District Municipality, Karkloof Nature Reserve, 29°17ʹ52ʺS, 30°13ʹ40ʺE, 2019, Medeiros 2095 (BOL). Limpopo: De Hoeck Forest, west of Tzaneen, 1953, R. Kräusel 4b [with Allographa oldayana] (B).
Allographa leptospora (Vain.) Lücking & Kalb
In Kalb et al., Phytotaxa 377, 19 (2018).—Graphis leptospora Vain., Ann. Bot. Soc. Zool.-Bot. Fenn. Vanamo 1(no. 3), 53 (1921); type: Thailand, Hosseus s. n. (TUR-V—holotype, not seen).
(Fig. 4B)
Remarks
This is the first report of this corticolous species for South Africa. It differs from the other species with a striate, completely carbonized excipulum and a complete thalline margin (A. consanguinea and A. oldayana) in having transversely septate ascospores.
Specimen examined
South Africa: Mpumalanga: Buffelskloof Private Nature Reserve, 25°15ʹ59ʺS, 30°31ʹ6ʺE, 1725 m, 2016, Medeiros L449 (BOL).
Allographa oldayana I. Medeiros sp. nov.
MycoBank No.: MB 843785
Differing from Allographa cerradensis (Marcelli et al.) Lücking & Kalb in the clear hymenium, larger ascospores, and presence of hirtifructic acid.
Type: South Africa, Mpumalanga, Buffelskloof Private Nature Preserve, 2019, Medeiros 2078 & Flakus (BOL—holotype; DUKE, PRE—isotypes).
Thallus corticolous, epiperidermal, 30–120 μm thick, continuous; surface smooth, pale green; prothallus absent. Thallus in section with prosoplectenchymatous cortex 12–30 μm thick, algal layer (Trentepohlia) 20–110 μm thick, and irregular clusters of calcium oxalate crystals.
Apothecia lirelliform, unbranched, erumpent to prominent, with thin to thick complete thalline margin, 1.0–7.5 mm long, 0.5–0.6 mm wide, 0.29–0.37 mm high; disc concealed; proper margin thick, labia striate, black. Excipulum striate, completely carbonized, 60–105 μm wide; hypothecium prosoplectenchymatous, 20–30 μm high, colourless to pale yellow; hymenium 100–180 μm high, colourless, clear; paraphyses unbranched, smooth; epithecium granulose, 12–25 μm high, dark olive-brown. Asci fusiform, 100–180 × 19–32 μm. Ascospores 1–4 per ascus, ellipsoid, muriform, transversely 20–30-septate, longitudinally 2–4-septate, 100–150(–175) × (16–)18–24(–27) μm, 4.5–7 times as long as wide, hyaline, I+ violet.
Conidiomata not seen.
Chemistry
Hirtifructic acid (major), cf. conhirtifructic acid (minor).
Etymology
The specific epithet honours Professor Fred C. Olday (College of the Atlantic, Bar Harbor, Maine, USA), who introduced the first author to the study of lichens.
Distribution and ecology
This species occurs in forested areas of eastern South Africa (e.g. Fig. 2A). Although fresh collections were made only in Northern and Southern Mistbelt Forest at the Buffelskloof and Karkloof reserves, respectively (Mucina & Geldenhuys Reference Mucina and Rutherford2006), one herbarium specimen from near Eshowe suggests that this species occurred in more coastal forest, at least historically (Mucina et al. Reference Mucina, Scott-Shaw, Rutherford, Camp, Matthews, Powrie, Hoare, Mucina and Rutherford2006).
Remarks
Molecular data confirm the placement of this species in Allographa (Fig. 3). Allographa elixii (A. W. Archer) Lücking & Kalb, the only other species in the genus with hirtifructic acid, differs from the new species in having an inspersed hymenium and ascospores which are only terminally muriform (Archer Reference Archer2001a). Allographa cerradensis differs in the inspersed hymenium, the presence of stictic acid instead of hirtifructic acid, and the smaller (80–100 × 15–20 μm) ascospores (Lumbsch et al. Reference Lumbsch, Ahti, Altermann, Amo de Paz, Aptroot, Arup, Bárcenas, Bawingan, Benatti and Betancourt2011). The North American Appalachian species Allographa sterlingiana (E. A. Tripp & Lendemer) Lücking & Kalb lacks lichen substances and has an inspersed hymenium (Lendemer et al. Reference Lendemer, Harris and Tripp2013).
The new species and A. consanguinea occur in the same habitats and both have a complete thalline margin, striate labia and large, muriform ascospores. They differ in secondary chemistry (no secondary metabolites in A. consanguinea), the more prominent lirellae in A. oldayana, and ascospore number (A. consanguinea has one spore per ascus). Records of Allographa acharii from South Africa (Nylander Reference Nylander1868; Steiner Reference Steiner1907; Doidge Reference Doidge1950; Almborn Reference Almborn1988) may represent A. oldayana or A. consanguinea. Allographa acharii has ascospores of similar size and number to the new species but lacks secondary compounds (Lücking et al. Reference Lücking, Archer and Aptroot2009).
Hirtifructic acid is an uncommon secondary compound in Graphidaceae, known from a handful of species in Allographa and Ocellularia (Lücking et al. Reference Lücking, Archer and Aptroot2009; Rivas Plata et al. Reference Rivas, Lücking and Lumbsch2012). This substance is rare in Lecanoromycetes more broadly and its chemical structure is unknown, although it is believed to be related to echinocarpic acid (Elix Reference Elix2018). In addition to Graphidaceae, it is also known in Parmeliaceae, where it is found in several species of Relicina and one species of Hypotrachyna (Elix Reference Elix1996; Sipman et al. Reference Sipman, Elix and Nash2009). Elix (Reference Elix1996, Reference Elix2018) does not report an R f value for this substance in solvent G; we found the R f value to be 59. Elix (Reference Elix2018) reported that there were no spot test reactions for hirtifructic acid but the new species reacts weakly K+ yellow, best observed when the secondary compounds are eluted with acetone onto filter paper. This is not diagnostic, however, as many Graphidaceae species react K+ yellow for more common substances (Lücking et al. Reference Lücking, Archer and Aptroot2009). TLC is thus required to confirm the presence of hirtifructic acid.
Additional specimens examined (paratypes)
South Africa: KwaZulu-Natal: ‘distr. Eshowe, on the trees in open forest along the small stream at Solheim’, 3 ix 1929, O. A. Høeg s. n. (TRH L-17871); uMgungundlovu District Municipality, Karkloof Nature Reserve, 29°17ʹ52ʺS, 30°13ʹ40ʺE, 2019, Medeiros 2102 (DUKE, BOL). Mpumalanga: Buffelskloof Private Nature Preserve, 25°15ʹ59ʺS, 30°31ʹ6ʺE, 1725 m, in ravine forest, on bark, 2016, Medeiros L448 (PRE); ibid., 25°17ʹ31ʺS, 30°30ʹ15ʺE, 1380 m, wet forest near bottom of ravine, 2016, Medeiros L506, L507 (PRE). Limpopo: De Hoeck Forest, west of Tzaneen, 1953, R. Kräusel 4b [with Allographa consanguinea] (B).
Allographa sp. 1
Remarks
Molecular data place the specimen cited below in Allographa without a close match to other species for which sequence data are available (Fig. 3). The specimen has a lateral thalline margin, an excipulum that is striate and apically to peripherally carbonized, and a clear hymenium. No ascospores could be found, precluding a species determination.
Specimen examined
South Africa: KwaZulu-Natal: uMgungundlovu District Municipality, Karkloof Nature Reserve, 2019, Medeiros 2099 (BOL).
Diorygma aff. minisporum Kalb, Staiger & Elix
Symb. Bot. Upsal. 34, 161 (2004); type: Guatemala, Kalb & Plöbst s. n. (WIS, hb. Kalb—holotype, not seen).
Remarks
Diorygma minisporum was described from Guatemala (Kalb et al. Reference Kalb, Staiger and Elix2004) and occurs widely in South America (Medina et al. Reference Medina, Lücking and Rojas2012; Aptroot & Cáceres Reference Aptroot and Cáceres2018; Pereira et al. Reference Pereira, Passos, Santos, Lücking and Cáceres2018). Kirika et al. (Reference Kirika, Mugambi, Lücking and Lumbsch2012) reported this corticolous species from Kenya and cited this as the first record from outside the Americas. Although the specimen cited below fits Diorygma minisporum morphologically, the molecular data suggest that our collection may not be conspecific with the neotropical material (Fig. 3).
Specimen examined
South Africa: KwaZulu-Natal: uMgungundlovu District Municipality, Karkloof Nature Reserve, 29°17ʹ52ʺS, 30°13ʹ40ʺE, on bark at base of large tree, 2019, Medeiros 2106 (BOL, DUKE).
Glyphis atrofusca (Müll. Arg.) Lücking
In Archer, Fl. Australia 57, 651 (2009).—Graphina atrofusca Müll. Arg., Flora 70, 74 (1887).—Graphis atrofusca (Müll. Arg.) Stizenb., Ber. Tätigk. St. Gallischen Naturwiss. Ges. 1889-1890, 186 (1891); type: South Africa, F. Wilms 70 (G—lectotype, not seen, designated by Lücking in A. W. Archer, Fl. Australia 57, 651 (Reference Archer and McCarthy2009)).
Graphina polycarpa Müll. Arg., Flora 70, 63 (1887); Graphis polycarpa (Müll. Arg.) Stizenb., Ber. Tät. St. Gallisch. Naturw. Gesellsch. 1889–1890, 184 (1891); type: South Africa, F. Wilms 48 (G—holotype).
(Fig. 5A)

Fig. 5. Images of South African Graphidaceae. A, Glyphis atrofusca (Medeiros 2089a). B, Gl. cicatricosa (Medeiros 2100). C, Graphis crebra (Medeiros 2088a). D, Gr. denudans (van der Bijl 126—holotype). E, Gr. dupaxana (Høeg s. n.). F, Gr. furcata (Høeg s. n.). Scales = 1 mm. Images by Willow Torrey (D) and IDM (A–C, E & F). In colour online.
Remarks
We provide molecular data (ITS, ON507259; for other loci see Table 1) for a topotype of Glyphis atrofusca, a corticolous species originally described from the general vicinity of modern-day Kruger National Park (Müller Reference Müller1887) and subsequently reported from Australia and North America (Archer Reference Archer2001b, Reference Archer and McCarthy2009; Staiger & Kalb Reference Staiger, Kalb, Nash, Ryan, Diederich, Gries and Bungartz2004; Lücking & Kalb Reference Lücking and Kalb2018; Guzmán-Guillermo et al. Reference Guzmán-Guillermo, Barrera and Llarena-Hernández2021). Lücking & Kalb (Reference Lücking and Kalb2018) noted that this species can be mistaken for a species of Graphis (its immersed, more or less elongate lirellae can suggest that genus if the presence of brown pruina is missed or indistinct) and that molecular data had not yet confirmed its placement in Glyphis. We show that Gl. atrofusca falls in Glyphis with high support (Fig. 3).
Staiger (Reference Staiger2002) established Glyphis subgenus Pallidoglyphis for two species with a non-carbonized hypothecium: Glyphis substriatula (Nyl.) Staiger and Gl. atrofusca, the latter under its synonym Gl. montoensis (A. W. Archer) Staiger. Recently, Kalb (Reference Kalb2020) described a third species from this group, Gl. frischiana Kalb. Prior to our study, only Gl. substriatula had molecular data. In our phylogenetic analysis, relationships within Glyphis were not well supported, and we can neither confirm nor refute the subgeneric classification adopted by Staiger (Reference Staiger2002).
We have only seen South African specimens of this species from savannah vegetation (Fig. 2B). It has also been found in dry, well-lit scrubland in other parts of its range (Archer Reference Archer2001b; Staiger & Kalb Reference Staiger, Kalb, Nash, Ryan, Diederich, Gries and Bungartz2004). The collection by Wilms cited below (which is from the type locality) is the basis for the record of Graphis sophistica Nyl. in Stizenberger (Reference Stizenberger1891) and therefore is also one of the records cited for Gr. platycarpa (Eschw.) Zahlbr. by Doidge (Reference Doidge1950). There are other historical records of Gr. sophistica not from the type locality of Glyphis atrofusca (Doidge Reference Doidge1950), so for the present we refrain from excluding Gr. platycarpa from the South African checklist as a misapplied name.
Specimens examined
South Africa: Mpumalanga: Kruger National Park, on bark of Spirostachys africana, 2019, Medeiros 2089a (BOL, DUKE); ‘Corticolam prope urbem Lydenburg’, s. d., F. Wilms s. n., Lichenotheca Universalis 90 (UPS L-203515).
Glyphis cicatricosa Ach.
Syn. Meth. Lich. (Lund), 107 (1814); type: Guinea, s. col. (H-ACH—holotype, not seen).
(Fig. 5B)
Remarks
This distinctive pantropical species has numerous records from KwaZulu-Natal (Nylander Reference Nylander1868; van der Byl Reference van der Byl1931; Doidge Reference Doidge1950). There is substantial morphological variation within this species (Staiger Reference Staiger2002) and previous phylogenetic analyses have failed to recover all sequenced individuals in a single, highly supported clade (Rivas Plata et al. Reference Rivas, Parnmen, Staiger, Mangold, Frisch, Weerakoon, Hernandez, Cáceres, Kalb and Sipman2013). In our phylogenetic analysis, specimens from South Africa, Kenya and El Salvador clustered together with poor support (Fig. 3). Glyphis dictyospora Staiger, described from Kenya, is externally similar to Gl. cicatricosa but has submuriform ascospores (Staiger Reference Staiger2002). It has not been found in South Africa.
Specimens examined
South Africa: Eastern Cape: ‘Alexandria Div., Alexandria State Forest’, on Pinus radiata, 60 m, G. Degelius SA-417 (UPS L-53775, with Phaeographis sp.). KwaZulu-Natal: ‘distr. Eshowe, Solheim near Eshowe’, on loquat trees, 5 ix 1929, O. A. Høeg s. n. (TRH L-17867); uMgungundlovu District Municipality, Karkloof Nature Reserve, 29°17ʹ52ʺS, 30°13ʹ40ʺE, on bark, 2019, Medeiros 2100 & Flakus (BOL); Karkloof Region, 1868, Armstrong & Mackenzie s. n. (DBN); ‘Dist. Stanger, Hill north of Umgeni River’, 1920, N. S. Pillans 10071 (BOL 216555); ‘Dist. Nongoma, Umzinene’, 18 viii 1929, O. A. Høeg s. n. (TRH L-17901).
Graphis bylii var. lividula Vain.
Ann. Univ., Fenn. Aboënsis, Ser. A 2(no. 3), 27 (1926); type: South Africa, Western Cape, Knysna, on Ficus elastica, s. d., P. A. van der Bijl 277 (TUR-V—holotype!).
Remarks
Graphis bylii var. lividula, known only from the South African type collection (Vainio Reference Vainio1926), is not closely related to Gr. bylii Vain. s. str. (see below) and is morphologically indistinguishable from the northern European species Gr. inustuloides Lücking. Both are corticolous species characterized by open, pruinose discs; an apically carbonized, entire excipulum; a clear hymenium; broadly ellipsoid, muriform spores; and the absence of secondary metabolites. The spores of the type of Gr. bylii var. lividula are 37–49 × 16–22 μm with 9–10 × 4–5 locules, while Lücking & McCune (Reference Lücking and McCune2012) give measurements of 35–50 × 15–20 μm for Gr. inustuloides.
If these are indeed conspecific, their disjunction would be comparable to that of the closely related Gr. pergracilis, which is known from South Africa, the Solomon Islands, and the north-western United States (Archer Reference Archer2007; Lücking et al. Reference Lücking, Archer and Aptroot2009; Lücking & McCune Reference Lücking, Parnmen and Lumbsch2012). Graphis bylii var. lividula, Gr. pergracilis and Gr. inustuloides, together with the Australian species Gr. coenensis A. W. Archer and the neotropical Gr. dimidiata Vain., form a species complex that requires further study (Lücking & McCune Reference Lücking, Parnmen and Lumbsch2012). We have also seen additional specimens from this group that vary slightly from the described species. For example, a South African specimen at PRE (E. Retief 551) differs from typical Gr. pergracilis in having shorter lirellae, discs that may be partially exposed, and a laterally carbonized excipulum (in some sections carbonized only in the upper two-thirds). Molecular data are not currently available for any species from this group, but such data will be essential for understanding species boundaries in what may represent a complex of cryptic species (Lücking & McCune Reference Lücking, Parnmen and Lumbsch2012; van der Pluijm Reference van der Pluijm2014). A precedent for such a pattern can be found in the Graphis scripta complex (Neuwirth & Aptroot Reference Neuwirth and Aptroot2011; Kraichak et al. Reference Kraichak, Lücking, Aptroot, Beck, Dornes, John, Lendemer, Nelsen, Neuwirth and Nutakki2015).
Graphis crebra Vain.
Hedwigia 38, 256 (1899); type: Guadeloupe, Duss 541 (TUR-V 27617, not seen).
(Fig. 5C)
Remarks
Graphis crebra has a pantropical distribution (Lücking et al. Reference Lücking, Archer and Aptroot2009). This is the first report of this corticolous species for South Africa, and we provide the first molecular data for the species (Table 1, Fig. 3). Graphis crebra and Gr. handelii (newly reported from South Africa below) both have open discs, an inspersed hymenium, hyaline, transversely septate ascospores c. 25 μm in length, and contain norstictic acid. The hyaline ascospores separate these two taxa from species of Phaeographis or Platygramme with open discs, whereas these two Graphis species can be distinguished from one another by the discs that are white pruinose in Gr. crebra and epruinose in Gr. handelii. Glyphis atrofusca was found at the same savannah site as Gr. crebra (Fig. 1B) and also has open discs, but can be readily distinguished by the brown pruina on the discs, clear hymenium, and muriform ascospores. Graphis pyrrhocheiloides Zahlbr., which was reported from South Africa by Lücking et al. (Reference Lücking, Archer and Aptroot2009) as an accessory species on the type of Gr. pergracilis, is similar to Gr. crebra in having open, pruinose discs, a laterally carbonized excipulum, transversely septate ascospores and norstictic acid, but differs in the clear hymenium.
Specimen examined
South Africa: Mpumalanga: Kruger National Park, on bark of Euclea divinorum, 2019, Medeiros 2088a (BOL, DUKE).
Graphis denudans Vain.
Ann. Univ., Fenn. Aboënsis, Ser. A 2(no. 3), 27 (1926); type: South Africa, KwaZulu-Natal, near Durban, s. d., P. A. van der Bijl 126 (TUR-V—holotype!).
(Fig. 5D)
Remarks
This corticolous species is characterized by very short (< 1 mm), unbranched to sparsely branched, erumpent lirellae with lateral thalline margin and entire (or weakly striate?) labia, discs that are often partially exposed, a completely carbonized excipulum (sometimes weakly at base) and a clear hymenium; ascospores 8 per ascus, hyaline, transversely 5–6-septate, 17–22(–25) × 7–8 μm; and the presence of norstictic acid. Graphis denudans is known only from the holotype and has not been reported since its original description by Vainio (Reference Vainio1926). It may be related to G. schiffneri Zahlbr., an eastern palaeotropical species that differs in having longer lirellae, obscurely striate labia and slightly longer ascospores (Lücking et al. Reference Lücking, Archer and Aptroot2009); additional material might show the two to be conspecific.
Graphis dupaxana Vain.
Ann. Acad. Sci. Fenn., Ser. A 15(no. 6), 241 (1921); type: Philippines, McGregor 14313 (TUR-V—lectotype, not seen).
(Fig. 5E)
Remarks
Graphis dupaxana has a pantropical distribution (Lücking et al. Reference Lücking, Archer and Aptroot2009). This is the first report of this corticolous species for South Africa. It differs from other species of Graphis in South Africa by having a completely carbonized, striate excipulum, a combination of characters more associated with Allographa (Lücking & Kalb Reference Lücking and Kalb2018). It differs from South African species of Allographa in lacking a thalline margin and having a distinctly white (not greenish) thallus.
Specimen examined
South Africa: KwaZulu-Natal: ‘distr. Eshowe, in a small krantz at Inyezane R., between the Mtunzeni Road and S. Siding’, very dense vegetation of small trees, 31 viii 1929, O. A. Høeg s. n. (TRH L-17906).
Graphis furcata Fée
Essai Crypt. Exot. (Paris), 40 (1825); type: South America, s. col. (G—holotype, not seen).
(Fig. 5F)
Remarks
Graphis furcata has a pantropical distribution (Lücking et al. Reference Lücking, Archer and Aptroot2009). This is the first report of this corticolous species for South Africa. See remarks under Gr. pinicola for how to differentiate these two species. Graphis furcata is known only from a single historical collection and its current status in South Africa is uncertain.
Specimen examined
South Africa: KwaZulu-Natal: ‘distr. Durban, Salisbury Island’, small trees in rather dense vegetation outside the mangrove, 20 ix 1929, O. A. Høeg s. n. (TRH L-17915).
Graphis handelii Zahlbr.
Symb. Sinic. 3, 44 (1930); type: China, Handel-Mazzetti 12788 (W—lectotype, not seen).
(Fig. 6A)

Fig. 6. Images of South African Graphidaceae. A, Graphis handelii (Killick 535). B, Gr. librata (Garside s. n.). C, Gr. longula (Medeiros L497). D, Gr. pinicola (Sipman 20.181). E, Gr. proserpens (Medeiros L444). F, Gr. subhiascens (Medeiros 2088b). Scales = 1 mm. All Images by IDM. In colour online.
Remarks
Graphis handelii has a pantropical distribution (Lücking et al. Reference Lücking, Archer and Aptroot2009). This is the first report of this corticolous species for South Africa. It is very close to Gr. crebra and in the field could also be mistaken for a species of Phaeographis; characters to separate these taxa are provided above in the remarks for Gr. crebra. We have seen Graphis handelii only in historical specimens from KwaZulu-Natal, where it was found in both coastal and inland forest vegetation. We lack recent collections from the Indian Ocean Coastal Belt vegetation of this province, a threatened vegetation type (Jewitt Reference Jewitt2018), so the current status of this species in South Africa is uncertain.
Specimens examined
South Africa: KwaZulu-Natal: ‘distr. Durban, summit of the Bluff, on trees in dense vegetation’, 22 ix 1929, O. A. Høeg s. n. (TRH L-17922); ‘distr. Umgeni, at Petermaritzburg, near Town Bush Road. On small tree (Solanum) along small stream’, 29 ix 1929, O. A. Høeg s. n. (TRH L-17908); ‘distr. Eshowe, Solheim near Eshowe’, on branches of loquat trees, 5 ix 1929, O. A. Høeg s. n. (TRH L-17899, mixed collection with other Graphis spp.); ‘Pietermaritzburg Division, Table Mountain, epiphloeodal on Croton sylvaticus growing along forest margin, 2000 ft’, 1948, D. J. B. Killick 535 (BOL 207564).
Graphis librata C. Knight
Trans. Proc. New Zeal. Inst. 16, 404 (1884); type: New Zealand, Knight 67:23 (WELT—lectotype, not seen, designated by Hayward, New Zealand J. Bot. 15, 571 (Reference Hayward1977)).
Graphis diaphoroides Müll. Arg., Flora (Regensburg) 69(20), 316 (1886); type: South Africa, Mpumalanga, near Lydenburg, 1884, Wilms 11 [Lojka Lichenothecum Universalis 91] (G—holotype, not seen; BM, M, MICH, NY—isotypes, not seen; US—isotype!).
(Fig. 6B)
Remarks
Lücking et al. (Reference Lücking, Archer and Aptroot2009) synonymized Graphis diaphoroides, a corticolous species described from South African material (Müller Reference Müller1886), with G. librata. As currently circumscribed, this species is pantropical; it is one of the most common and widely distributed species of Graphis in South Africa. We obtained molecular data from one specimen of G. librata. In our phylogenetic analysis, this specimen was recovered distant to G. librata from El Salvador but without strong support (Fig. 3). Molecular data for Graphis librata from other locations, especially the type locality in New Zealand, will be necessary to understand the delimitation of this species.
Specimens examined
South Africa: KwaZulu-Natal: Karkloof, on bark, 2019, Medeiros 2112 (BOL). Western Cape: Kirstenbosch, on Brabejum, ii 1946, S. Garside s. n. (BOL 207569); Kirstenbosch, on bark, 2019, Medeiros 2145 (BOL); Table Mountain National Park, Silver Mine pond, on bark of tree at base of dam, 2019, Medeiros 2143 (BOL); ‘S. Cape, Zitzikamma forest near Coldstream, on twigs of fruit trees’, vii 1944, J. Pont s. n. (PRE 766676); Nature's Valley, on vining Euphorbiaceae, 2019, Medeiros 2134 (BOL).
Graphis longula Kremp.
Flora 59, 414 (1876); type: Brazil, Glaziou 5497 (M—lectotype, not seen).
(Fig. 6C)
Remarks
Graphis longula has a pantropical distribution (Lücking et al. Reference Lücking, Archer and Aptroot2009). This is the first report of this corticolous species from South Africa.
Specimen examined
South Africa: Mpumalanga: Buffelskloof Private Nature Preserve, 25°17ʹ31ʺS, 30°30ʹ15ʺE, 1380 m, wet forest near bottom of ravine, 2016, Medeiros L497 (BOL).
Graphis pinicola Zahlbr.
In Handel-Mazzetti, Symb. Sinic. 3, 43 (1930); type: China, Handel-Mazzetti 2829 (W—holotype, not seen; US—isotype, not seen).
(Fig. 6D)
Remarks
Graphis pinicola has a pantropical distribution (Lücking et al. Reference Lücking, Archer and Aptroot2009). This is the first report of this corticolous species from South Africa, although it is a widespread species with numerous historical and modern collections. Graphis pinicola is similar to Gr. furcata; however, the former has a corticate thallus and well-defined thalline margin, while the latter is ecorticate and the thalline margin slopes gradually into the thallus. Graphis librata differs from Gr. pinicola in producing norstictic acid.
Specimens examined
South Africa: KwaZulu-Natal: ‘distr. Eshowe, S. of Solheim’, on trees, 7 ix 1929, O. A. Høeg s. n. (TRH L-17911); ‘Solheim near Eshowe’, on branches of loquat trees, 5 ix 1929, O. A. Høeg s. n. (TRH L-17899, mixed collection with other Graphis spp.); Karkloof region, Armstrong & Mackenzie s. n., 1866 (DBN). Western Cape: Cape Town, east slope of Table Mtn at Kirstenbosch Botanical Garden, 400 m, 1986, H. Sipman 20.181 (B); Garden Route National Park, Nature's Valley, 2019, Medeiros 2135a (with Phaeographis sp.) (BOL).
Graphis proserpens Vain.
Botanisk Tidsskrift 29, 132 (1909).—Graphis disserpens Vain., Acta Soc. Fauna Flora Fenn. 7(2), 123 (1890), nom. illeg. (non Graphis disserpens Nyl.); type: Brazil, Vainio s. n. (TUR-V—holotype, not seen).
(Fig. 6E)
Remarks
Graphis proserpens has not previously been reported from South Africa, although this corticolous species is probably pantropical (Lücking et al. Reference Lücking, Archer and Aptroot2009). Historical references to Gr. striatula Ach. (Nylander Reference Nylander1868) probably represent this species (see discussion of misapplied names, below). Diederich et al. (Reference Diederich, Lücking, Aptroot, Sipman, Braun, Ahti and Ertz2017) recently reported this species as new to the Seychelles. In our phylogenetic analysis, Gr. proserpens from South Africa was recovered as sister to Gr. proserpens from the Philippines (Fig. 3). The GenBank Accession number for the ITS sequence of Medeiros 2105 is ON507258.
Specimens examined
South Africa: KwaZulu-Natal: Karkloof, on bark, 2019, Medeiros 2105 (BOL, DUKE). Mpumalanga: Buffelskloof Private Nature Preserve, 25°15ʹ59ʺS, 30°31ʹ6ʺE, 1725 m, 2016, Medeiros L444 (PRE), L447 (BOL); 25°17ʹ31ʺS, 30°30ʹ15ʺE, 1380 m, wet forest near bottom of ravine, 2016, Medeiros L498 (PRE).
Graphis subhiascens (Müll. Arg.) Lücking
In Lücking et al., Fieldiana, Bot. 46, 111 (2008).—Graphina subhiascens Müll. Arg., Bot. Jahrb. 20, 2811 (1894); type: Tanzania, Holst 696 (G—holotype, not seen).
(Fig. 6F)
Remarks
Graphis subhiascens has not previously been reported from South Africa, although this corticolous species is pantropical (Lücking et al. Reference Lücking, Archer and Aptroot2009). We provide the first molecular data for this species (Table 1, Fig. 3). It has medium-sized, muriform ascospores and a complete thalline margin, and can be distinguished from Allographa consanguinea and A. oldayana on the basis of the larger ascospores and striate, completely carbonized excipulum in those species. Other South African species with an entire, laterally carbonized excipulum (Gr. crebra, Gr. furcata, Gr. handelii and Gr. pinicola) have transversely septate ascospores.
Specimen examined
South Africa: Mpumalanga: Kruger National Park, on bark of Euclea divinorum, 2019, Medeiros 2088b (BOL).
Mangoldia bylii (Vain.) I. Medeiros comb. nov.
MycoBank No.: MB 843786
Graphis bylii Vain., Ann. Univ., Fenn. Aboënsis, Ser. A 2(no. 3), 27 (1926).—Graphina bylii (Vain.) Zahlbr., Cat. Lich. Univers. 8, 604 (1932); type: South Africa, Western Cape, Knysna, on bark of Ocotea bullata, 1922, P. A. van der Byl 265 (TUR-V—holotype!).
Syn. nov.: Graphina atronitens A. W. Archer, Mycotaxon 77, 162 (2001).—Thalloloma atronitens (A. W. Archer) A. W. Archer, Telopea 11, 77 (2005).—Mangoldia atronitens (A. W. Archer) Lücking et al., Phytotaxa 69, 4 (2012); type: Australia, New South Wales, Black Rock, c. 7 km S of Brunswick Heads, 5 November 1998, A. W. Archer G292 (NSW—holotype, not seen).
(Fig. 7A)

Fig. 7. Images of South African Graphidaceae. A, Mangoldia bylii (van der Bijl 265—holotype). B, Platythecium sp. (Høeg s. n.). Scales = 1 mm. Images by Willow Torrey (A) and IDM (B). In colour online.
Remarks
Lücking et al. (Reference Lücking, Parnmen and Lumbsch2012) established the genus Mangoldia for two Australian species with Phaeographis-like ascomata but Graphis-like ascospores. Mangoldia is closely related to Allographa (Fig. 3). The holotype of Graphis bylii closely matches the description of Graphina atronitens from Archer (Reference Archer2001b); the ascospores of G. bylii are slightly larger (41–51 × 12–18 μm versus 30–45 × 10–15 μm) but the difference is not sufficient to treat them as separate species. The previously unrecognized presence of this genus in South Africa highlights biogeographical connections between southern Africa and Australasia (Almborn Reference Almborn1988; Galley & Linder Reference Galley and Linder2006; McCarthy Reference McCarthy2006). We have not seen any specimens other than the holotype, and the present status of this species in South Africa is therefore unknown.
Platythecium sp.
(Fig. 7B)
Remarks
The only specimen of Platythecium we have seen from South Africa could not be determined to species because mature spores were not seen and none of the TLC spots could be positively identified; secondary chemistry is important for species determination in this genus (Neuwirth et al. Reference Neuwirth, Aptroot and Stocker-Wörgötter2017). We include it here because this genus has not otherwise been reported from South Africa. In South Africa, Platythecium is probably restricted to the vegetation of the Indian Ocean Coastal Belt, which is a threatened vegetation type (Jewitt Reference Jewitt2018) that lacks recent lichen sampling.
Specimen examined
South Africa: KwaZulu-Natal: ‘distr. Eshowe, rather dense and dark situation in the indigenous forest’, 26 viii 1929, O. A. Høeg s. n. (TRH L-17910).
Misapplied names
Allographa striatula (Ach.) Lücking & Kalb
In Kalb et al., Phytotaxa 377, 26 (2018).—Opegrapha striatula Ach., Syn. Meth. Lich. (Lund), 74 (1814).—Graphis striatula (Ach.) Spreng., Syst. Veg., Edn 16 4, 250 (1827); type: Guinea, s. col. (H-ACH 629—holotype, not seen).
Remarks
This species is known for South Africa only from Nylander (Reference Nylander1868). Several specimens examined by Nylander are in the Armstrong collection at DBN. A small number lack spores and are undeterminable, while several others represent Graphis proserpens.
Graphis analoga Nyl.
Annls Sci. Nat., Bot., Sér. 4 11, 244 (1859); type: Tahiti, Viellard & Planchet G13:8 (H-NYL 7432—holotype, not seen).
Remarks
This species is known for South Africa only from Nylander (Reference Nylander1868). The single specimen in the Armstrong collection at DBN is in very poor condition, but the excipulum is striate and completely carbonized. We could not find ascospores, although Nylander's notes on the specimen give spore dimensions of 46–80 μm × 18–30 μm. The excipulum characters and ascospore size contradict the description of this species provided by Lücking et al. (Reference Lücking, Archer and Aptroot2009).
Graphis scripta (L.) Ach.
K. Vetensk-Acad. Nya Handl. 30, 145 (1809).—Lichen scriptus L., Spec. Plant. 2, 1140 (1753); type: Sweden, Malme s. n. [Lich. Suec. Exs. 47] (UPS—epitype, not seen).
Remarks
This is primarily a temperate, Northern Hemisphere species (Kraichak et al. Reference Kraichak, Lücking, Aptroot, Beck, Dornes, John, Lendemer, Nelsen, Neuwirth and Nutakki2015), although there are a small number of confirmed records from the Southern Hemisphere (Neuwirth & Aptroot Reference Neuwirth and Aptroot2011). Frequent records of this species in older literature (see Doidge (Reference Doidge1950) and citations therein) date to an era when Graphidaceae biodiversity was poorly known. All South African specimens we have seen identified as Graphis scripta are misidentifications of other taxa.
Discussion
The species outlined above consist mostly of pantropical taxa, especially in Glyphis and Graphis s. str. Conversely, Allographa oldayana is a candidate southern African endemic, and Mangoldia bylii represents a biogeographical connection between South Africa and Australia. Several species known only from their type specimens (e.g. Graphis bylii var. lividula and Gr. denudans) will require additional study to resolve their taxonomy and biogeography.
The forest habitats preferred by many Graphidaceae species are a small proportion of the total land cover in South Africa (Fig. 1; Mucina & Geldenhuys Reference Mucina, Geldenhuys, Mucina and Rutherford2006), and their natural patchiness might contribute to both the evolution of endemic species and the susceptibility to disturbance. More than one third of the species we treat in this paper are known from South Africa only from pre-1950s collections. Their current status in the country is uncertain given high levels of forest habitat loss, especially in the Indian Ocean Coastal Belt (Mucina & Geldenhuys Reference Mucina, Geldenhuys, Mucina and Rutherford2006; Jewitt Reference Jewitt2018).
In addition to the new species and records listed in this paper, we have seen many additional specimens that cannot clearly be assigned to a known species and several putative new species known only from a single specimen. For the time being, we refrain from describing these new species pending the availability of additional specimens and molecular data. The present paper is only a first step towards a modern understanding of lirelliform Graphidaceae in South Africa, and we hope it will be a starting point for more researchers to investigate taxonomic problems such as the Graphis pergracilis complex and the description of additional new species.
The separation of Allographa and Graphis s. str. (Lücking & Kalb Reference Lücking and Kalb2018) resolved one of the broader systematic issues in Graphideae: the polyphyly of Graphis s. lat. (Berger et al. Reference Berger, Stamatakis and Lücking2011; Rivas Plata et al. Reference Rivas, Hernández, Lücking, Staiger, Kalb and Cáceres2011). Two major problems remain. First, Thalloloma is a junior synonym of Diorygma, but the two genera have not yet been formally merged. When these genera were resurrected in the modern understanding of Graphidaceae (Staiger Reference Staiger2002; Kalb et al. Reference Kalb, Staiger and Elix2004), they were recognized as close relatives. Molecular data now strongly support a single genus for this group (Fig. 3) but, to avoid excessive name changes, more molecular data from atypical species of Thalloloma are necessary before the genera are formally synonymized. Second, and a greater problem, phylogenetic relationships and generic boundaries among the genera with pigmented ascospores (Leiorreuma, Pallidogramme, Phaeographis, Platygramme, Sarcographa and Thecaria) are unresolved or not well supported (Fig. 3). This group has a complicated nomenclatural and taxonomic history (Staiger Reference Staiger2002; Lücking et al. Reference Lücking, Kalb, Staiger and McNeill2007). Phaeographis has long been known to be polyphyletic (Rivas Plata et al. Reference Rivas, Parnmen, Staiger, Mangold, Frisch, Weerakoon, Hernandez, Cáceres, Kalb and Sipman2013), and our new data on Platygramme pachnodes suggests that Platygramme is also polyphyletic (Fig. 3). Genera in this clade have probably been oversplit; for example, there is much more sequence variation within Graphis or Allographa than there is among Leiorreuma, Pallidogramme and Thecaria (Fig. 3). We will address some of these taxonomic and nomenclatural issues in a subsequent paper on the South African species of Graphidaceae tribe Graphideae with pigmented ascospores, which will also include a complete key to Graphideae in South Africa.
Acknowledgements
Specimens were collected under permits from South African National Parks (CRC/2019-2020/020–2018/V1), Ezemvelo KZN Wildlife (OP 1404/2019), Mpumalanga Parks and Tourism Agency, and CapeNature (CN35-31-9213). We thank Alicia Ibáñez for managing the research permits. The curators of B, BM, BOL, DBN, LD, NU, PRE, SBBG, TRH, TUR, UPS, US and WIS are gratefully acknowledged for the loan of specimens and for facilitating in-person visits to collections. Scott LaGreca and Geneva Langley provided invaluable assistance with loans and collections management. Terry Hedderson, Stefan Sibert, Nishanta Rajakaruna, Alan Fryday, Nate Pope, Arnold Frisby, Adam Flakus, Reinaldo Vargas Castillo, Betsy Arnold, Louise Lewis, Eric Hom, Maya Kaup, Nicolas Magain, Elizaveta Terlova, József Geml and Jolanta Miadlikowska participated in fieldwork during which collections for this project were made. John Burrows permitted collecting at the Buffelskloof Nature Reserve and shared his extensive knowledge of the vegetation of South Africa. We thank Robert Lücking for many helpful discussions on the taxonomy of Graphidaceae and John Elix for providing a control sample of hirtifructic acid. Thomas Barlow, Willow Torrey, Shuzo Oita, Betsy Arnold and József Geml are thanked for providing photographs. Several specimens cited in this study were collected during fieldwork supported by the National Geographic Society grant #9774-15, awarded to Nishanta Rajakaruna. Funding for this research was provided by the Department of Biology at Duke University and the United States National Science Foundation through grant number DEB 1541548 to FL. This material is based upon work supported by the National Science Foundation Graduate Research Fellowship Program under grant number DGE 1644868.
Author ORCIDs
Ian D. Medeiros, 0000-0003-2179-0745; François Lutzoni, 0000-0003-4849-7143.
Competing Interests
The authors declare none.












