INTRODUCTION
In species-rich tropical forests, species composition can significantly vary over a short distance between forest stands. Niche differentiation associated with habitat heterogeneity is one of the important factors accounting for such high beta diversity (Condit et al. Reference CONDIT, PITMAN, LEIGH, CHAVE, TERBORGH, FOSTER, NUÑEZ, AGUILAR, VALENCIA, VILLA, MULLER-LANDAU, LOSOS and HUBBELL2002). Habitat specialization reflecting soil properties occurs in relation to variation in topography at the local scale (Aiba et al. Reference AIBA, KITAYAMA and TAKYU2004, Harms et al. Reference HARMS, CONDIT, HUBBELL and FOSTER2001) and in geological substrates at the regional scale (Condit et al. Reference CONDIT, PITMAN, LEIGH, CHAVE, TERBORGH, FOSTER, NUÑEZ, AGUILAR, VALENCIA, VILLA, MULLER-LANDAU, LOSOS and HUBBELL2002, Pyke et al. Reference PYKE, CONDIT, AGUILAR and LAO2001). The forest structure can also vary drastically over a small distance, and this should be explained by habitat heterogeneity, including soil conditions (reflecting topographic and geological variation) and disturbance regime (often related to topography) (Ferry et al. Reference FERRY, MORNEAU, BONTEMPS, BLANC and FREYCON2010, Yamakura et al. Reference YAMAKURA, KANZAKI, ITOH, OHKUBO, OGINO, CHAI, LEE and ASHTON1996). The variation in forest structure under a similar climate is thought to reflect different forest dynamics across soil and/or topographic gradients (Ferry et al. Reference FERRY, MORNEAU, BONTEMPS, BLANC and FREYCON2010, Herwitz & Young Reference HERWITZ and YOUNG1994), which in turn may be the consequence of different species compositions under different soil conditions (Madelaine et al. Reference MADELAINE, PELISSIER, VINCENT, MOLINO, SABATIER, PREVOST and de NAMUR2007).
Many studies have shown that tropical forest communities strikingly differ between geological substrate types at similar altitudes (Aiba & Kitayama Reference AIBA and KITAYAMA1999, Proctor et al. Reference PROCTOR, ANDERSON, CHAI and VALLACK1983) and vary along the topographical gradient (Takyu et al. Reference TAKYU, AIBA and KITAYAMA2002, Tanner Reference TANNER1977). Species diversity and forest stature decrease on infertile soils found on unusual substrates and ridges. On the other hand, the relationships between forest dynamics, particularly tree mortality and recruitment, and soil variation are poorly understood. In a Bornean lowland forest, the mortality and growth rate were the lowest on the poorest soils (Russo et al. Reference RUSSO, DAVIES, KING and TAN2005). Moreover, in Amazonia, variation in tree mortality was explained by soil properties, particularly soil fertility, at the local to regional scales (Phillips et al. Reference PHILLIPS, BAKER, ARROYO, HIGUCHI, KILLEEN, LAURANCE, LEWIS, LLOYD, MALHI, MONTEAGUDO, NEILL, VARGAS, SILVA, TERBORGH, MARTÍNEZ, ALEXIADES, ALMEIDA, BROWN, CHAVE, COMISKEY, CZIMCZIK, FIORE, ERWIN, KUEBLER, LAURANCE, NASCIMENTO, OLIVIER, PALACIOS, PATIÑO, PITMAN, QUESADA, SALDIAS, LEZAMA and VINCETI2004, Toledo et al. Reference TOLEDO, MAGNUSSON, CASTILHO and NASCIMENTO2011). However, the relationships were not always determined, although tree growth usually increased with soil fertility. In central Amazonia, soil fertility was positively correlated with the mortality of smaller trees (<30 cm diameter) but not with that of larger trees, and the magnitude of the effects increased after a storm (Toledo et al. Reference TOLEDO, MAGNUSSON, CASTILHO and NASCIMENTO2011). In contrast, tree mortality did not differ systematically among topographical units in a Jamaican subtropical montane forest, although the effects of a severe hurricane confounded this finding (Bellingham & Tanner Reference BELLINGHAM and TANNER2000). Thus, the relationships between soil properties and tree mortality differ among sites and by tree size and are modulated by disturbance events. Moreover, different species composition on different soils may contribute to variations in forest dynamics. For example, a greater mortality on fertile soils may reflect a greater abundance of fast-growing, short-lived pioneer species (Ferry et al. Reference FERRY, MORNEAU, BONTEMPS, BLANC and FREYCON2010, Lawrence Reference LAWRENCE2003, Toledo et al. Reference TOLEDO, MAGNUSSON, CASTILHO and NASCIMENTO2011).
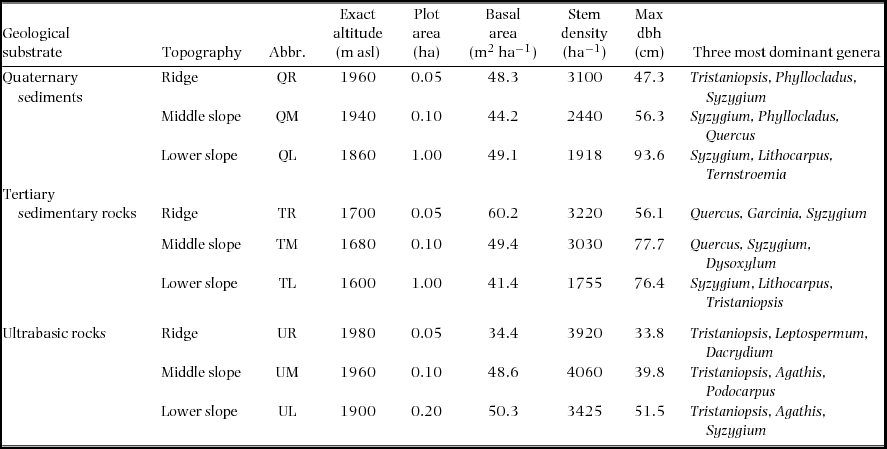
On Mount Kinabalu, Borneo, the montane forest is underlain by three types of geological substrates of different ages and/or parental rocks, which affect the availability of soil nutrients (in particular, phosphorus) derived from rock weathering. In total, nine permanent plots were established in 1997 in a matrix of three topographical units (ridge, middle and lower slopes) on each of the three geological substrates to assess the effects of substrate and topography on the forest structure and function (Takyu et al. Reference TAKYU, AIBA and KITAYAMA2002, Reference TAKYU, AIBA and KITAYAMA2003). The forest structure and function consistently changed across topography on each geological substrate, and the magnitude of the topographic changes decreased in the order of Quaternary sediments > Tertiary sedimentary rocks > ultrabasic rocks, which may be related to the different forest dynamics among geological substrates and topographies. Since then, these plots have been monitored.
In the humid tropics of South-East Asia, severe droughts associated with El Niño Southern Oscillation events increased tree mortality and suppressed growth (Leighton & Wirawan Reference LEIGHTON, WIRAWAN and Prance1986, Lingenfelder & Newbery Reference LINGENFELDER and NEWBERY2009). A severe drought occurred on Mount Kinabalu in 1997–1998 in association with an El Niño event (Aiba & Kitayama Reference AIBA and KITAYAMA2002), and the impact of the drought should be taken into account to appropriately understand forest dynamics (Phillips Reference PHILLIPS1995, Sheil Reference SHEIL1995). Moreover, sudden and often unexplained episodes of die-back of particular tree species, e.g. Metrosideros polymorpha in Hawaii (Jacobi Reference JACOBI1983, Mueller-Dombois Reference MUELLER-DOMBOIS1983), Scalesia pedunculata in Galapagos Islands (Lawesson Reference LAWESSON1988) and Terminalia quintalata in Venezuela (Dezzeo et al. Reference DEZZEO, HERNÁNDEZ and FӦLSTER1997), are known in tropical forests. Based on our long-term censuses, we can evaluate the effects of the drought and other episodic events on forest dynamics.
In the present study, we aimed to clarify the effect of soil fertility on tree community dynamics by comparing nine tropical montane forests on contrasting geological substrates and topographies. For this purpose, the mortality, recruitment and growth rates in the nine plots were calculated over 14 y (1997–2011), which included the drought period (1997–1999). In particular, we asked the following questions: (1) How do the geological substrate and topography (and soil nutrients that reflect them) affect the dynamics of a tropical montane forest? and (2) What are the impacts of an El Niño drought and other episodic events (if any)?
METHODS
Study sites
The nine permanent plots are located at 1600–1980 m asl on the south slope of Mount Kinabalu in Sabah, Malaysian Borneo (4095 m, 6°05′N, 116°33′E), which are identical to those used by Takyu et al. (Reference TAKYU, AIBA and KITAYAMA2002, Reference TAKYU, AIBA and KITAYAMA2003). The plots were established on three topographic units i.e. ridges, middle and lower slopes, on each of the three geological substrates i.e. unconsolidated Quaternary sediments, Tertiary sedimentary rocks and ultrabasic rocks of the Jurassic–Cretaceous age (Table 1). The plot area varied from 0.05 to 1.00 ha, which reflected different availability of uniform topography. The ridge plots (50 × 10 m) are on the top of small summits to sample the most stunted forest stand in the study area. The middle-slope plots (50 × 20m) are on steep upper slopes immediately (approximately 10-m elevation) below the ridge plots. These situations inevitably resulted in the small plot areas of the ridges and middle-slope plots. The size of the lower slope is 1 ha (200 × 50 m) on sedimentary rocks and Quaternary sediments and 0.2 ha (50 × 40 m) on ultrabasic rocks, where tree diversity is lower and tree density is higher. The maximum tree size (both stem diameter and height) decreased but stem density increased from the lower slope to the ridge on each substrate. When forests on different substrates were compared for each topographic unit, the maximum diameter was smaller but stem density was greater on ultrabasic rocks than on other substrates. The families Myrtaceae (particularly Syzygium and Tristaniopsis) and Fagaceae (Lithocarpus and Quercus) were dominant in all nine plots. At the three ultrabasic plots, conifers (e.g. Agathis, Dacrydium and Podocarpus) were also abundant.
Table 1. Descriptions of the nine study plots, each established on the three topographies on each of three geological substrates at around 1700m asl on Mount Kinabalu.

The climate of Mount Kinabalu is humid and tropical with little yearly fluctuation. The mean annual air temperature and annual rainfall at 1560 m from June 1995 to May 1997 were 18.5°C and 2731 mm, respectively (Kitayama et al. Reference KITAYAMA, LAKIM and WAHAB1999). From the end of December 1997 to early May 1998, a severe drought occurred in association with an El Niño event, and the annual rainfall from June 1997 to May 1998 was 1036 mm (Aiba & Kitayama Reference AIBA and KITAYAMA2002). Soil nutrients and water conditions of the plots were described by Takyu et al. (Reference TAKYU, AIBA and KITAYAMA2002). Major nutrient contents (inorganic nitrogen and soluble phosphorus) decreased in the order of Quaternary sediments > Tertiary sedimentary rocks > ultrabasic rocks on each topographic unit and decreased from the lower slope to the ridge on each substrate. The soil water potential (at 15 cm depth) was measured on the ridge and lower slope of sedimentary rocks and on the ridge of ultrabasic rocks in 1997 (drought period) and 1999 (normal period). These measurements indicated that the soil water potential during the drought did not differ among topographies on the sedimentary rock, but was more negative on sedimentary rocks than on ultrabasic rocks.
Tree census
In the study plots, all trees ≥5 cm in diameter at breast height or 1.3 m above ground (dbh) were measured for trunk girth, and dbh was calculated from the girth. The measuring points without any stem irregularities were selected at around breast height (1.3 m) and marked for remeasurements. Buttressed trees were measured well above the protrusions (approximately 50 cm). Tree censuses were conducted biennially during 1997–2007 and once in 2011. Dead or missing trees and new recruits reaching ≥5 cm dbh were identified and their dbhs were recorded.
Data analysis
Mortality and recruitment rates (% y−1) were calculated for each of the six census periods (1997–1999, 1999–2001, 2001–2003, 2003–2005, 2005–2007 and 2007–2011) according to Swaine & Lieberman (Reference SWAINE and LIEBERMAN1987):
 \begin{eqnarray*}
\textrm{Mortality rate}\, (\% y^{-1}) &=& 100 \times ln \,({\it N_{b}/N_s})/{\it t}\\
\textrm{Recruitment rate}\, (\% y^{-1}) &=& 100 \times ln \,({\it N_{e}/N_s})/{\it t},
\end{eqnarray*}
\begin{eqnarray*}
\textrm{Mortality rate}\, (\% y^{-1}) &=& 100 \times ln \,({\it N_{b}/N_s})/{\it t}\\
\textrm{Recruitment rate}\, (\% y^{-1}) &=& 100 \times ln \,({\it N_{e}/N_s})/{\it t},
\end{eqnarray*}
Where Nb, Ne and Ns indicate the number of initial live individuals, final live individuals and individuals that survived to the end of each census period, respectively, and t presents the time interval (days between census midpoints divided by 365). Mortality during 2001–2005 was also calculated for ultrabasic rocks where die-back occurred. The average mortality and recruitment rates during 14 y were calculated as the average of mortality or recruitment rates of six 2- or 4-y periods. Background mortality rates were calculated as means during normal periods (excluding the 1997–1999 period for Quaternary sediments and sedimentary rocks and excluding the 1997–1999 and 2001–2005 periods for ultrabasic rocks). The growth rate (cm y−1) was calculated as the absolute difference in dbh between two censuses divided by the census interval for each census period. Stems whose measuring points were broken as well as stems that showed negative growth >5% were omitted from the calculation of the mean growth rate.
Background mortality, average recruitment and mean growth rates, which included data for several periods, were examined by one-way analysis of variance (ANOVA) with Tukey's honestly significant difference (HSD) test to calculate the statistical difference among geological substrates in each topographical unit and among topographies on each geological substrate. In addition, Pearson correlation was used to assess the relationship between soil nutrients and the average recruitment or growth rate during 14 y and that between mortality during 1997−1999 (the drought period) and the average growth rate.
For mortality during 1997–1999 (drought period) and 2001–2005 (die-back period), logistic regression analysis (generalized linear model with a logit link) with a binomial error distribution was performed for continuous variables and the chi-square test was performed for categorical variables to assess the effects of the geological substrate, topography and tree size (dbh in the initial census). In logistic regression, tree mortality represented a binary categorical response variable. Trees alive at the beginning of the period were coded as one if they were dead at the end of the period and as zero if they survived during the period. The significance of explanatory variables was tested using the Wald test, which uses Z-statistics, squared values of which follow a chi-square distribution with one degree of freedom. In addition, the influence of soil nutrient concentrations (inorganic nitrogen and soluble phosphorus; Takyu et al. Reference TAKYU, AIBA and KITAYAMA2002) on mortality during 1997–1999 (drought period) was examined using logistic regression analysis. In these analyses, univariate models were constructed because the explanatory variables are often correlated with one another.
Among mortality and growth rates calculated for each of the nine plots, small ridge plots occasionally showed extreme values. Portions of the larger plot may also show similarly extreme values; however, these will be masked by the average values at the plot level. Thus, we also showed mortality and average growth rates for 0.05-ha subplots (50 × 10 m, longer side parallel to contour lines) for 1- to 0.1-ha plots. In this study, three extreme values were found for ridge plots: mortality rates during 1997–1999 and 2001–2005 were strikingly high in the ridge plots on Quaternary sediments and ultrabasic rocks, respectively, and the growth rate during 1997–1999 was very low in the Quaternary ridge plot. To evaluate whether these values are indeed extreme even when comparing the 0.05-ha subplots, one 0.05-ha subplot (full plot for three ridge plots) was selected from each of the nine plots in all possible combinations. This yielded 12800 combinations of subplots [(1 × 2 × 20) × (1 × 2 × 20) × (1 × 2 × 4)]. For each combination of subplots, we checked whether the above-mentioned ridge plots showed the highest (mortality) or lowest (growth rate) values of the nine subplots and whether the difference among subplots was statistically significant. For statistical analysis, the chi-square test was used for mortality rates and Kruskal–Wallis test was used for the growth rates. All statistical analyses were performed using R software version 2.14.1 (R Development Core Team).
RESULTS
Change over time in basal area and stem density
Patterns of change over time in the basal area and stem density differed among geological substrates but were more or less similar among topographical units of each substrate (Figure 1). The basal area and stem density decreased synchronously during the 1997–1999 period, including the drought, and the 2001–2005 period. From 1997 to 1999, the basal area and stem density decreased in almost all the plots, particularly in the Quaternary ridge plot. The basal area decreased from 2001 to 2005 in all the three plots on ultrabasic rocks, and both the basal area and stem density dramatically decreased in the ridge plot.

Figure 1. Changes over 14 y (1997–2011) in basal area (m2 ha−1) and stem density (ha−1), in nine study plots of tropical montane forests on Mount Kinabalu, Borneo, placed on three topographies (ridge, middle and lower slopes) of three geological substrates: Quaternary sediments, (a) and (d); Tertiary sedimentary rocks, (b) and (e); ultrabasic rocks, (c) and (f). Red, green and blue symbols indicate the values on the ridge, middle-slope and lower-slope plots, respectively.
Mortality and recruitment
Synchronous changes across nine plots were found for the mortality rate but not for the recruitment rate (Figure 2). Plot-level mortality rates during 1997–1999 were relatively high in all the plots, excluding the ridge and middle-slope plots on ultrabasic rocks, and those during 2001–2005 were strikingly and moderately high in the same ultrabasic plots, respectively. Background mortality rates did not differ significantly among geological substrates of each topographic unit as well as among topographies on each geological substrate (Table 2, P > 0.05), ranging from 0.89% y−1 to 1.39% y−1.
Table 2. Background mortality rates (% y−1) as means of mortality rates during normal periods (excluding the 1997–1999 period for all plots and the 2001–2005 period for ultrabasic plots) in the nine study plots. Differences in background mortality rates were tested by one-way analysis of variance (ANOVA); ns, not significant (P > 0.05).
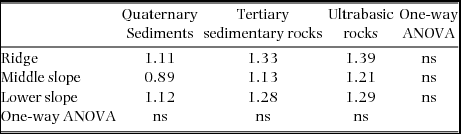

Figure 2. Changes over 14 y in mortality rates (% y−1) and recruitment rates (% y−1) in the nine study plots on three topographies (ridge, middle and lower slopes) of three geological substrates: Quaternary sediments, (a) and (d); Tertiary sedimentary rocks, (b) and (e); ultrabasic rocks, (c) and (f). Red, green and blue symbols indicate the values on the ridge, middle-slope and lower-slope plots, respectively. Dark- and light-coloured symbols indicate the values for undivided plots and 0.05-ha subplots, respectively.
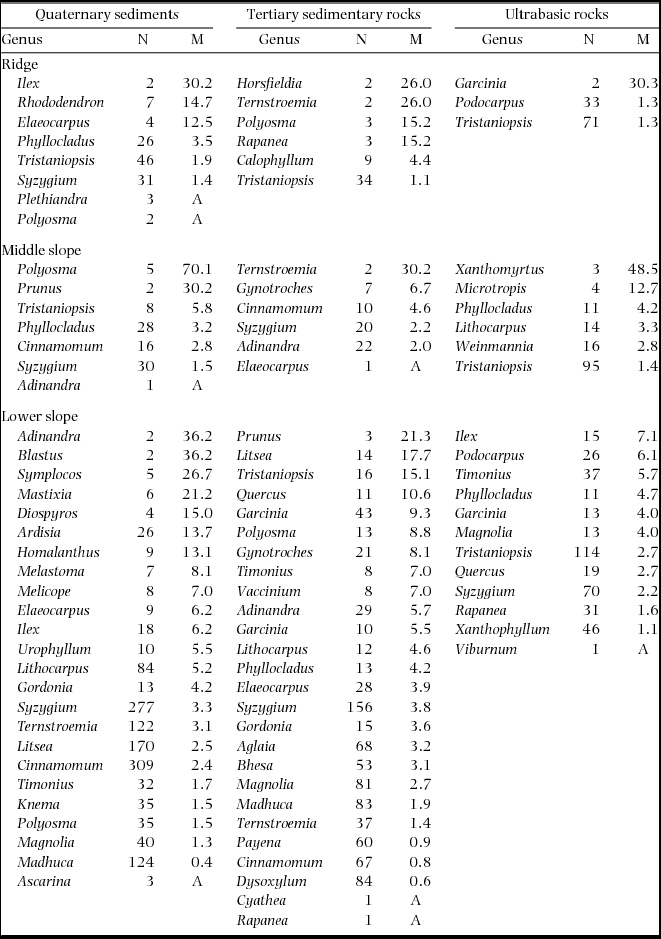
Plot-level mortality rates during 1997–1999 increased in the order of ultrabasic rocks < sedimentary rocks < Quaternary sediments on each topographic unit (Figure 2). When compared among topographical units on each geological substrate, plot-level mortality rates tended to increase in the order of ridge < middle slope < lower slope on sedimentary and ultrabasic rocks but increased in the order of middle slope < lower slope < ridge on Quaternary sediments, although the Quaternary lower-slope plots showed large variation among 0.05-ha subplots. The plot-level mortality of the Quaternary ridge plot appeared to be particularly high; however, this was not observed when compared with the 0.05-ha subplots of other plots. The Quaternary ridge plot showed significantly high mortality in only 77 (6%) of 12800 combinations of 0.05-ha subplots. In logistic regression analysis, the geological substrate showed a significant effect on tree mortality during 1997–1999 (Table 3). Furthermore, tree mortality decreased with the tree size (dbh in 1997) and increased with the soil phosphorus concentration. Stems belonging to diverse genera died during 1997–1999, and no genera consistently showed high mortality in multiple plots (Appendix 1).
Table 3. The effect of each variable on tree death during 1997–1999 in univariate logistic regression analysis. The χ2 value for continuous values (diameter at breast height (dbh) in 1997, Inorganic N and soluble P) showed the square of Z-statistics in logistic regression analysis. Cat, categorical variable.

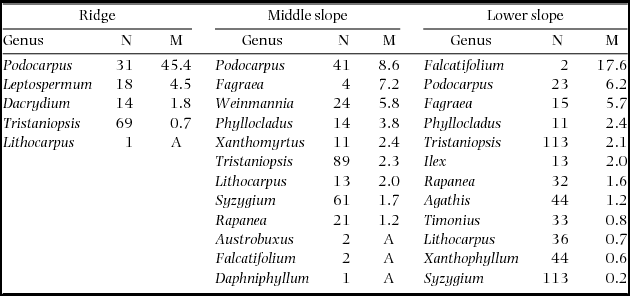
During 2001–2005, the plot-level mortality rate decreased in the order of ridge > middle slope > lower slope on ultrabasic rocks (Figure 2), and this was observed even when the lower-slope plots were divided in 0.05-ha subplots. The plot-level mortality rate of the ultrabasic ridge plot was strikingly high among the nine plots, and this was observed even when compared with 0.05-ha subplots of other plots. The Quaternary ridge plot showed significantly high mortality in all the 12800 combinations of subplots. Logistic regression also revealed a significant effect of topography on mortality on ultrabasic rocks during 2001–2005 (χ2 = 38.0, df = 2, P < 0.001); however, tree size did not affect mortality (χ2 = 0.33, df = 1, P = 0.564). Podocarpus (represented by a single species P. gibbsiae) showed high mortality rates on all topographies of ultrabasic rocks during the 2001–2005 period (Appendix 2), and mortality rates for Podocarpus during this period were higher than those during other periods (data not shown). Recruitment rates for six 2- or 4-y periods did not show a consistent pattern across substrates or topographies (Figure 2), and the average recruitment rates over 1997–2011 did not show any correlation with mortality rates during 1997–1999 or with background mortality or mortality over 1997–2011 (R = 0.37–0.43, all P > 0.1).
Growth rates
Patterns of change over time in the growth rate were basically similar across all the plots, particularly among the plots on the same geological substrate (Figure 3). The results of multiple comparisons showed a significant difference in the average growth rate during 1997–2011 among geological substrates for middle and lower slopes but not for ridges (Table 4). For middle and lower slopes, the average was significantly higher on Quaternary sediments than on ultrabasic rocks (P < 0.05), and the average on sedimentary rocks showed intermediate values. For sedimentary and ultrabasic rocks, the average growth rate was higher on the lower slope than on the ridge or middle slope (P < 0.05), and the values of the latter two topographic units did not differ (P > 0.05). For Quaternary sediments, the average growth rates increased from the ridge to lower slope; however, the differences were not significant (P > 0.05). The 0.05-ha subplots in larger plots generally showed the same pattern. These trends indicated that the average growth rates tended to increase along the gradient of soil nutrient concentrations, although there was no significant correlation with soil nutrients (R = 0.33, P = 0.39 for nitrogen; R = 0.61, P = 0.08 for phosphorus). Average growth rates over 1997–2011 showed a positive correlation with mortality during 1997–1999 among the nine plots (Figure 4, R = 0.71, P = 0.03), which indicates that forests with more fertile soils where trees grew faster were more severely impacted by the drought. A similar relationship was found when 0.05-ha plots were used for correlation analysis (R = 0.33, P = 0.02).
Table 4. Mean growth rates (cm y−1) during 1997–2011 in the nine study plots. SD, standard deviation. Differences in mean growth rates were tested by one-way analysis of variance (ANOVA) with Tukey's honestly significant difference (HSD) test. The different capital and lower case letters show significant difference among geological substrates of each topography and among topographies on each geological substrate, respectively (P < 0.05).


Figure 3. Changes over 14 y in mean growth rates of diameter at breast height (cm y−1) in the nine study plots on three topographies (ridge, middle and lower slopes) of three geological substrates: Quaternary sediments (a), Tertiary sedimentary rocks (b) and ultrabasic rocks (c). Red, green and blue symbols indicate the values on the ridge, middle-slope and lower-slope plots, respectively. Dark- and light-coloured symbols indicate the values for undivided plots and 0.05-ha subplots, respectively.

Figure 4. Relationship between average growth rate (cm y−1) during 1997–2011 and mortality (% y−1) during 1997–1999 in the nine study plots. See Table 1 for plot abbreviations. Black and grey symbols indicate the values calculated for undivided plots and 0.05-ha subplots, respectively. R and P statistics show the results of correlation analysis for undivided plots (N = 9).
During the 1997–1999 period, including the drought, growth rates of all the plots were low (Figure 3). In particular, the average growth rate in the Quaternary ridge plot was negative, and this was because 10 and 74 of 140 stems (excluding those with negative growth >5% and/or with broken stems) showed zero and negative growth, respectively. Compared with 0.05-ha subplots of other plots, the growth rate in the Quaternary ridge plot was significantly lower in all the 12800 combinations. The growth rates during 2005–2007 synchronously decreased at all topographic positions on ultrabasic rocks and were higher on the lower slope than on the ridge or middle slope. However, the decreases during 2005–2007 were observed not only on ultrabasic rocks but also on the other substrates (Figure 3).
DISCUSSION
The present results demonstrated that the tree community dynamics of tropical montane forests on Mount Kinabalu varied by topography and geological substrates and that the effects of geological substrates were generally stronger than those of topography. This may reflect that geological substrates affect soil property more strongly than topography. Parent rocks affect the availability of soil nutrients because soil nutrients are mainly derived from weathering of parent materials, except for nitrogen that is scarce in rocks. In our study plots, three plots on ultrabasic rocks showed consistently low concentration of soluble phosphorus (Takyu et al. Reference TAKYU, AIBA and KITAYAMA2002), and soils in lower slope plots had a greater amount of labile phosphorus on Quaternary sediments than on sedimentary rocks (Kitayama et al. Reference KITAYAMA, AIBA, TAKYU, MAJALAP and WAGAI2004). Therefore, soil phosphorus derived from parent rocks appears to be the strongest determinant of soil fertility in our plots, thereby affecting forest dynamics. Similarly, Mage & Porder (Reference MAGE and PORDER2013) showed that parent material largely determined the variation in the soil phosphorus concentration and topography explained the additional variance in subtropical forests of Puerto Rico.
In the present study, mortality rates synchronously increased in all the plots during 1997–1999 (the drought period) and only in the three plots on ultrabasic rocks during 2001–2005 when the die-back of P. gibbsiae occurred. Mortality rates during the drought and die-back periods were greatly affected by the geological substrate and topography, although background mortality during the normal periods was not affected. In contrast, growth rates varied by the geological substrate and topography throughout the study period. The implication of these findings are discussed below.
Firstly, precaution is needed to interpret recruitment and mortality rates that greatly fluctuate in a stochastic manner when calculated for small sample sizes during short time intervals (Aiba et al. Reference AIBA, TAKYU and KITAYAMA2005). Numbers of trees are relatively few in our plots on ridges and middle slopes (N = 142–406), and this may be the reason why background mortality and recruitment rates did not show a clear pattern across soil gradients. Recruitment and mortality rates should be balanced in climax forests at the steady state; however, this was not found over 1997–2011 in our results. It appears that the die-back of P. gibbsiae had not been compensated by sufficient recruitment on ultrabasic rocks and that some forests were undergoing self-thinning as stem density continued to decrease over 14 y (Figure 1).
Mortality rates during 1997–1999 increased in the order of ultrabasic rocks < sedimentary rocks < Quaternary sediments on the same topographic unit and in the order of ridge < middle slope < lower slope on each geological substrate, except on Quaternary sediments. Logistic regression analysis showed a positive effect of soil nutrient (soluble phosphorus) on tree mortality (Table 3), implying that trees on more fertile soils are less drought-tolerant. In general, faster-growing species are dominant on fertile soils. Such species often have wider vessels or tracheids, which have advantages in water transportation and photosynthesis in tropical and temperate rain forests (Brodribb & Feild Reference BRODRIBB and FEILD2000) but increase the risk of embolism and cavitation (Davis et al. Reference DAVIS, SPERRY and HACKE1999, Sperry et al. Reference SPERRY, NICHOLS, SULIVAN and EASTLACK1994). Indeed, a positive correlation was found between the mortality during 1997–1999 and average growth during 1997–2011 in our study (Figure 4). Moreover, smaller stems showed a higher probability of death during the 1997–1999 period (Table 3), which may indicate that smaller stems are more vulnerable to drought damage due to shallower root systems (Becker et al. Reference BECKER, SHARBINI and YAHYA1999). This also suggests that the above-mentioned correlation between drought mortality and growth rates is not the result of enhanced tree growth under improved light conditions because the death of small trees would not greatly change the understorey light condition. In contrast, during 1997–1999, mortality rates were lower on ultrabasic rocks than on other substrates and on ridges than on slopes of sedimentary rocks and ultrabasic rocks. Dominant trees on these sites had smaller and thicker leaves (Takyu et al. Reference TAKYU, AIBA and KITAYAMA2002, Reference TAKYU, AIBA and KITAYAMA2003), which are considered as an adaptation to water stress (Jones Reference JONES1992) and nutrient shortage (Buckley et al. Reference BUCKLEY, CORLETT and GRUBB1980). Therefore, trees on infertile soils may have been pre-adapted to drought stress (Aiba & Kitayama Reference AIBA and KITAYAMA2002).
Unexpectedly, mortality rates during 1997–1999 tended to be low on ultrabasic rocks and on ridges of each substrate (except Quaternary sediments) despite the fact that soils on ultrabasic rocks are generally permeable to water (Proctor & Woodell Reference PROCTOR and WOODELL1975) and soils are drier on ridges than on slopes (Yanagisawa & Fujita Reference YANAGISAWA and FUJITA1999). However, the measurements of soil conditions in our study plots showed that water potentials did not differ among topographical units even during the drought and were less negative on ultrabasic rocks than on sedimentary rocks (Takyu et al. Reference TAKYU, AIBA and KITAYAMA2002). This is in line with the tendency of mortality rates not to increase on ridges and/or on ultrabasic rocks in the present study. Moreover, as stated above, the mortality rate in the Quaternary ridge plot during the 1997–1999 period was particularly high (although not significantly higher than that in 0.05-ha subplots of other plots) during the 1997–1999 and the growth rate was extremely low (Figures 2, 3). These may reflect that the intensity of the drought may have been more severe in the Quaternary ridge plot than in the other plots. The Quaternary ridge plot may have been more exposed to dry air during the drought because it was located on a more isolated peak than the other ridge plots. In addition, the porous structure of the young sediments may have accentuated the drought.
During 2001–2005, the die-back of P. gibbsiae accounted for increased mortality in the ridge and middle-slope plots on ultrabasic rocks. In particular, the relative basal area of P. gibbsiae strikingly decreased from 9.6% in 1997 to 1.8% in 2011 in the ultrabasic ridge plot. Thus, this episodic event drastically changed the structure and composition of the tree community. The growth rate decreased during 2005–2007 after the die-back of P. gibbsiae. However, this occurred in almost all the plots, irrespective of substrates, and it is therefore unlikely to be related to the die-back. Our climatic measurements at 1560 m asl during 1997–2011 showed that the mean photosynthetically active radiation calculated at annual intervals was the lowest in the first half of the 2005–2007 period (195 μmol m−2 d−1 from August 2005 to July 2006), which is substantially lower than the average over the 14 y (258 μmol m−2 d−1) (K. Kitayama, unpubl. data; the data during 2001–2004 and 2008 were lost). This may explain the decreased growth rate during 2005–2007.
Episodic and extensive mortality of particular tree species is explained by endogenous factors such as synchronous cohort senescence (Mueller-Dombois Reference MUELLER-DOMBOIS1983, Ward Reference WARD1982) or exogenous factors such as pathogens and herbivores (Castello et al. Reference CASTELLO, LEOPOLD and SMALLIDGE1995). In particular, cohort senescence has been proposed to be the cause of die-back in Pacific forests in Hawaii and Galapagos Islands. For example, the die-back of Metrosideros polymorpha in Hawai in the 1960s and 1970s (Jacobi Reference JACOBI1983, Mueller-Dombois Reference MUELLER-DOMBOIS1983) is well known. Metrosideros polymorpha seedlings and saplings were abundant in most die-back stands and mortality was restricted to canopy trees, from which die-back was explained by cohort senescence and considered as a form of succession (Mueller-Dombois Reference MUELLER-DOMBOIS1983). In our study, mortality rates of P. gibbsiae did not differ among size classes. Thus, die-back is not likely to be related to senescence in our case. Growth rates of P. gibbsiae were lower on the ridge (data not shown), implying that the growth condition for P. gibbsiae was poorer on the ridge. Thus, defoliation by pests or diseases may have interacted with site conditions and led to higher mortality rates on the ridge. Conspecific density may also explain the topographic variation in die-back because the mortality of P. gibbsiae was greater where the population density was higher (Appendix 2). However, what caused the die-back of P. gibbsiae on Mount Kinabalu remains debatable.
How are the above-mentioned findings of tree community dynamics related to the variation in the forest structure and diversity across soil gradients? In our study sites, the forest stature and maximum tree size increased along the gradients of increasing soil nutrients (Takyu et al. Reference TAKYU, AIBA and KITAYAMA2002), which was parallel to the variation in the growth rate found in the present study. The growth variation across soil gradients should reflect that tree species occurring on fertile soils intrinsically grow faster and that individuals of widespread species also grow faster on more fertile soils. Thus, we suggest that a decreased growth rate (across and within species) accounts for the stunted forest structure on poorer soils. In addition, it is known that species richness is positively correlated with forest turnover (mean mortality and recruitment rates) across tropical forests worldwide (Phillips et al. Reference PHILLIPS, HALL, GENTRY, SAWYER and VÁSQUEZ1994). A higher turnover is associated with a more frequent disturbance, and fast-growing species are dominant in such forests. Species richness in our study sites was higher on more fertile soils (Takyu et al. Reference TAKYU, AIBA and KITAYAMA2002), where mortality rates during the drought period were high. This indicates that the disturbance by occasional droughts may play a role in the maintenance of high species diversity on fertile soils. Increased mortality on poorer soils was suggested to be a cause of stunted structure elsewhere in tropics (Herwitz & Young Reference HERWITZ and YOUNG1994); however, this was not the case in our forests. On the other hand, environmental filtering may be responsible for the lowered species diversity on poorer soils.
This long-term study enabled us to evaluate the effects of topography and geological substrate and the impact of drought and other episodic events on community dynamics in tropical montane forests on Mount Kinabalu. The drought increased mortality, and the die-back of a particular tree species drastically changed the composition of the tree community. In particular, the drought was an important factor that emphasizes the effects of the geological substrate and topography on forest dynamics. Differences in forest dynamics should be related to the variation in the forest structure and diversity across the soil gradient, although disentangling the causal relationship is difficult. A severe drought was recorded only once during the 14 y of our study, and die-back was observed for only one particular species restricted to an ultrabasic substrate. The generality of our findings should be tested by continuous monitoring of our study plots, which may encounter another drought or die-back in the future. In addition, we need to study the physiological responses of species to environmental factors to understand the mechanism of tree mortality.
ACKNOWLEDGEMENTS
We thank staff members of the Sabah Parks for their continuous support during the fieldwork throughout the study. For the English language review, we would like to thank Enago (www.enago.jp). This study was supported by Japan Society for the Promotion of Science KAKENHI grant numbers 18255003, 22255002 and 23255003.
Appendix 1. Number of stems in 1997 (N) and mortality rates (M, % y−1) during 1997–1999 (drought period) by genera in nine plots. Genera are arranged by descending order of mortality. A: all stems died.

Appendix 2. Number of stems in 2001 (N) and mortality rates (M, % y−1) during 2001–2005 by genera on three topographical units of the ultrabasic rock. Genera are arranged by descending order of mortality. A: all stems died.