INTRODUCTION
Whale watching has increased significantly since its inception in the late 1950s, with many countries now relying on its economic benefits. In particular, developing countries see this tourism sector as an appealing option (Hoyt, Reference Hoyt2001; Wilson & Tisdell, Reference Wilson and Tisdell2002; Cisneros-Montemayor et al., Reference Cisneros-Montemayor, Sumaila and Kaschner2010). Another benefit from this activity is its potential educational role in enhancing knowledge and conservation awareness (Jacobs & Harms, Reference Jacob and Harms2014; Johnson & McInnis, Reference Johnson, McInnis, Higham, Bejder and Williams2014). However, there are also concerns about the potential impacts of this activity on individual cetaceans and populations, especially where individuals are resident or show high site fidelity with the potential for cumulative daily disturbance by tour boats.
Various studies have been conducted to understand how tourist activities could be detrimental for cetaceans (Lusseau & Bejder, Reference Lusseau and Bejder2007; Stensland & Berggren, Reference Stensland and Berggren2007; Courbis & Timmel, Reference Courbis and Timmel2009; May-Collado et al., Reference May-Collado, Barragan-Barrera, Quinones-Lebron and Aquino-Reynoso2012). Short-term effects from boat exposure have been reported in a number of such studies. Behavioural responses include changes in dive behaviour (Ng & Leung, Reference Ng and Leung2003; Williams et al., Reference Williams, Bain, Smith and Lusseau2009; Stamation et al., Reference Stamation, Croft, Shaughnessy, Waples and Briggs2010), path direction (Timmel et al., Reference Timmel, Courbis, Sargeant-Green and Markowitz2008; Stamation et al., Reference Stamation, Croft, Shaughnessy, Waples and Briggs2010; Christiansen et al., Reference Christiansen, Rasmussen and Lusseau2011, Reference Christiansen, Rasmussen and Lusseau2013), swim speed (Williams et al., Reference Williams, Trites and Bain2002, Reference Williams, Bain, Smith and Lusseau2009; Morete et al., Reference Morete, Bisi and Rosso2008), behavioural state (Stockin et al., Reference Stockin, Lusseau, Binedell, Wiseman and Orams2008a; Arcangeli & Crosti, Reference Arcangeli and Crosti2009; Montero-Cordero & Lobo, Reference Montero-Cordero and Lobo2010; Meissner et al., Reference Meissner, Christiansen, Martinez, Pawley, Orams and Stockin2015), group cohesiveness (Bejder et al., Reference Bejder, Samuels, Whitehead and Gales2006a; Steckenreuter et al., Reference Steckenreuter, Möller and Harcourt2012), surface active behaviours (Morete et al., Reference Morete, Bisi and Rosso2008; Noren et al., Reference Noren, Johnson, Rehder and Larson2009; Cammareri & Vermeulen, Reference Cammareri and Vermeulen2010) and vocalization rate (Markowitz et al., Reference Markowitz, Richter and Gordon2011; Pirotta et al., Reference Pirotta, Milor, Quick, Moretti, Di Marzio, Tyack, Boyd and Hastie2012; Papale et al., Reference Papale, Gamba, Perez-Gil, Martin and Giacoma2015). How and whether these acute behavioural effects are associated with a long-term impact is difficult to establish although it has been suggested that they may lead to a decrease in energy uptake at the individual level. Potentially, this could lead to a decrease in fitness and ultimately changes in population levels (Williams et al., Reference Williams, Lusseau and Hammond2006; Lusseau et al., Reference Lusseau, Bain, Williams and Smith2009; Parsons, Reference Parsons2012; Christiansen et al., Reference Christiansen, Bertulli, Rasmussen and Lusseau2015).
The Azores Archipelago is established as a destination for nature tourism with whale watching being one of the primary activities (32.4%) practiced (Queiroz et al., Reference Queiroz, Guerrero and Ventura2014). Whale watching in the Azores began in the early 1990s with one operator on Pico Island and 468 tourists in 1993 (Silva, Reference Silva2015; Bentz et al., Reference Bentz, Rodrigues, Dearden, Calado and Lopes2015). In 2013, ~59,000 tourists engaged in whale watching and swim-with-dolphin tours (Bentz et al., Reference Bentz, Rodrigues, Dearden, Calado and Lopes2015). The wide variety of species that can be observed in Azorean waters has contributed to this growth. Overall, 28 species have been recorded in the region (Silva et al., Reference Silva, Prieto, Cascão, Seabra, Machete, Baumgartner and Santos2014). Some are encountered only occasionally while on migration (Visser et al., Reference Visser, Hartman, Pierce, Valavanis and Huisman2011a; Silva et al., Reference Silva, Prieto, Jonsen, Baumgartner and Santos2013, Reference Silva, Prieto, Cascão, Seabra, Machete, Baumgartner and Santos2014), while others are known to occur year-round. However, information on distribution, residency and behavioural patterns of cetaceans in the Azores remains limited (e.g. Hartman et al., Reference Hartman, Visser and Hendriks2008; Silva et al., Reference Silva, Prieto, Cascão, Seabra, Machete, Baumgartner and Santos2014; Hartman et al., Reference Hartman, Fernandez, Wittich and Azevedo2015).
Short-beaked common dolphins (Delphinus delphis, hereafter referred to as common dolphins) are the most frequently observed species in the archipelago. They occur year-round in the waters off all islands (Silva et al., Reference Silva, Prieto, Magalhães, Cabecinhas, Cruz, Gonçalves and Santos2003, Reference Silva, Prieto, Cascão, Seabra, Machete, Baumgartner and Santos2014), and are an important focus for commercial tour activities. However, although frequently encountered, baseline information including local population size, distribution and degree of site fidelity are not known. Occasionally, common dolphins are observed in mixed-species foraging associations, mostly with Atlantic spotted (Stenella frontalis) and striped dolphins (Stenella coeruleoalba) (Clua & Grosvalet, Reference Clua and Grosvalet2001; Quérouil et al., Reference Quérouil, Silva, Cascão, Magalhães, Seabra, Machete and Santos2008). Calves are observed year-round (unpublished data, MONICET database), though assumed to peak in spring-summer, as reported in other temperate common dolphin populations (Westgate & Read, Reference Westgate and Read2007; Stockin et al., Reference Stockin, Pierce, Binedell, Wiseman and Orams2008b). Similar to other small delphinids, Delphinus often form large aggregations and occasionally exhibit conspicuous behaviours above water (Ferguson et al., Reference Ferguson, Barlow, Fiedler, Reilly and Gerrodette2006), which aids their detection from land. Furthermore, common dolphins also tend to approach moving boats (Neumann & Orams, Reference Neumann and Orams2006), increasing the probability of the same groups experiencing repeated encounters, thus increasing the risk of cumulative impacts (Parsons, Reference Parsons2012; Meissner et al., Reference Meissner, Christiansen, Martinez, Pawley, Orams and Stockin2015).
Behavioural effects associated with tourism activities have been best studied in New Zealand. For example, in the Hauraki Gulf, common dolphins were shown to reduce the time spent foraging and resting (Stockin et al., Reference Stockin, Lusseau, Binedell, Wiseman and Orams2008a) when engaged with tour boats. Once disrupted, dolphins also took significantly longer to resume foraging. Similarly, in the Bay of Plenty, dolphins reduced the proportion of time spent foraging (Meissner et al., Reference Meissner, Christiansen, Martinez, Pawley, Orams and Stockin2015). A tendency to increase travelling in the presence of boats was further reported for common dolphins in Mercury Bay, although the overall activity budget was not affected. Dolphin group size also had an effect on response, with smaller groups being more likely to show boat avoidance (Neumann & Orams, Reference Neumann and Orams2006). To date in the Azores, only two studies have addressed the effects of the whale watching activities on local cetacean populations (Magalhães et al., Reference Magalhães, Prieto, Silva, Gonçalves, Afonso-Dias and Santos2002; Visser et al., Reference Visser, Hartman, Rood, Hendriks, Zult, Wolff, Huisman and Pierce2011b). Magalhães et al. (Reference Magalhães, Prieto, Silva, Gonçalves, Afonso-Dias and Santos2002) identified changes in speed and increased aerial displays in sperm whales (Physeter macrocephalus) off Pico and Faial Islands when the code of conduct was violated by tour operators. Risso's dolphins (Grampus griseus) off Pico spent less of the day resting during the season with highest traffic and rested and socialized less when more than five boats were present (Visser et al., Reference Visser, Hartman, Rood, Hendriks, Zult, Wolff, Huisman and Pierce2011b).
Knowledge of the behaviour and ecology of local populations becomes more important with increasing pressure from tourism on living resources. For this reason, the present study aims to provide first insights on the effects of whale watching on the behaviour of common dolphins off the southern coast of São Miguel, the largest and most developed island in the archipelago. Specifically, the probability that dolphins would change their current behavioural activity in the presence of tour boats was investigated using stochastic Markov chains. In addition, compliance with local whale watching regulations, specifically approach manoeuvres, speed, number of boats and encounter duration was assessed.
MATERIALS AND METHODS
Study area
The study site was located off the southern coast of São Miguel, Azores, delimited by an observation angle of 150° and a radius of ~9 km from a lookout point at Ponta Garça, at a height 93 m above sea level. This area covered ~6% of the total sea space used by the six companies operating from two harbours, Ponta Delgada and Vila Franca do Campo (Figure 1). Tour operators conduct daily whale watching and swim-with-dolphins activities throughout the year, with the main season occurring between April and October. Scuba diving and recreational private boats also use the area, but tend to stay closer to shore where common dolphins are less frequent.
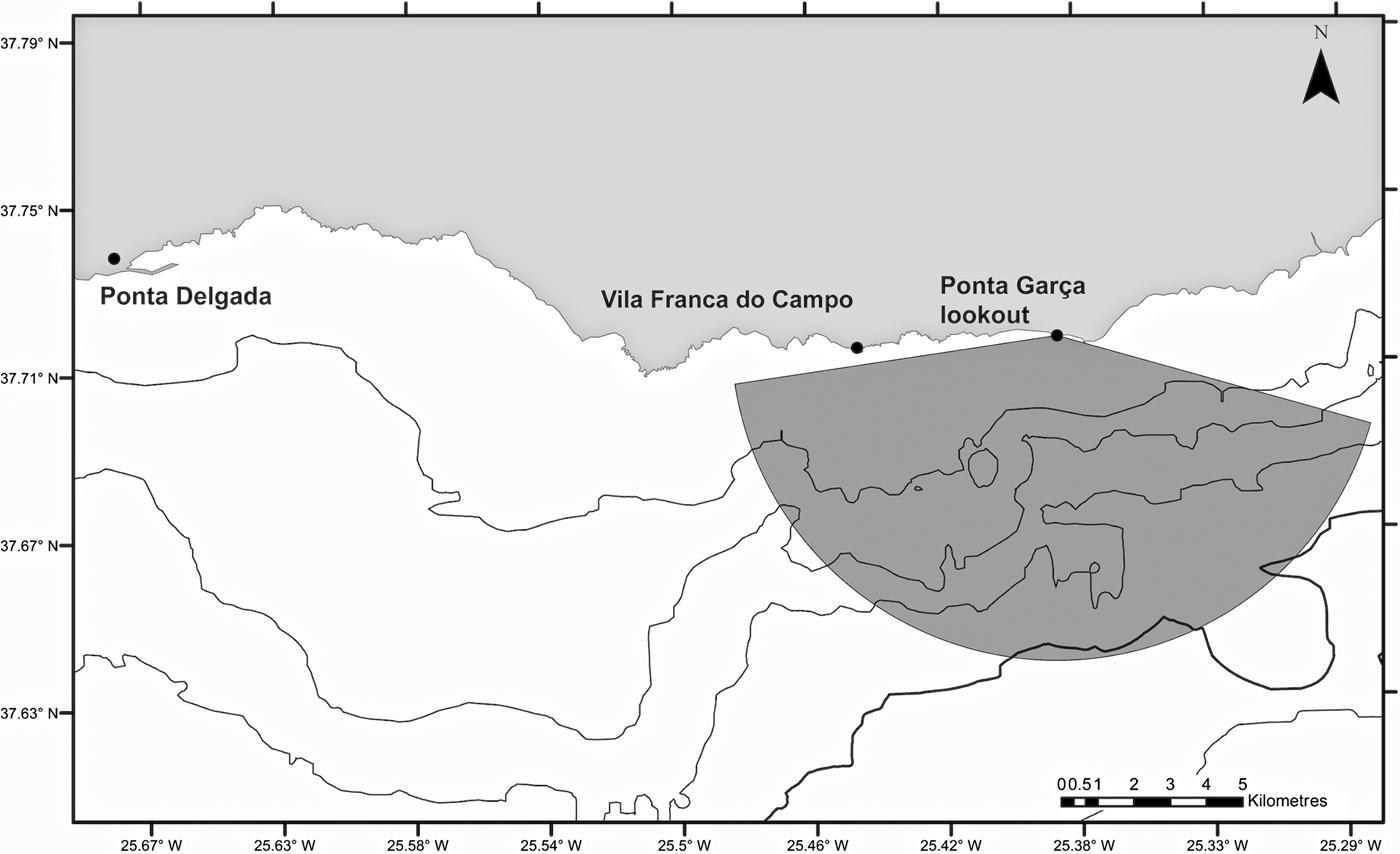
Fig. 1. Study area showing the location of the observation point in Ponta Garça (37°42′50.76″N 25°22′23.16″W) on the south coast of São Miguel Island and the observation site.
Field data collection
Data were collected from a land-based station using Steiner 15 × 80 binoculars mounted on a rotating platform. A team of two trained observers undertook dedicated watches between 0800 and 1700 h from July to September 2013 and April to October 2014. Surveys were conducted in ≤3 Beaufort, in good visibility (>10 km) and in the absence of precipitation or fog using focal group follows to sample the predominant group activity (Altmann, Reference Altmann1974; Mann Reference Mann1999). Common dolphins, like many other small delphinids, tend to form large fluid groups making it impossible to track individuals (Neumann Reference Neumann2001; Stockin et al., Reference Stockin, Binedell, Wiseman, Brunton and Orams2009). However, the substantial elevation did provide a good vantage point from which to observe the extent of even large focal groups (Martinez, Reference Martinez2010). The subject for the first focal follow of each day was the dolphin group first observed during scan sampling. This a priori rule limited bias due to group size or behaviour. Subsequent encounters were selected based on direction and distance from the original group, to minimize the probability of pseudo-replication.
Based on preliminary behavioural observations, four behavioural categories were identified: foraging, travelling, high surface activity and low surface activity. Behavioural definitions applied were a simplified version of the states described by Neumann (Reference Neumann2001) and Stockin et al. (Reference Stockin, Binedell, Wiseman, Brunton and Orams2009) (Table 1). Low surface activity included both resting and milling behaviours previously described by Neumann (Reference Neumann2001) and Stockin et al. (Reference Stockin, Binedell, Wiseman, Brunton and Orams2009), while high surface activity included individuals engaged in behavioural events such as breaching, porpoising and head slapping, which are previously described indicators of social behaviour. These pooled categories were used because it was difficult to reliably identify and discriminate between the previously described states at a distance. All behavioural observations of dolphins were performed by the same observer (AC) to avoid inter-individual variability.
Table 1. Definition of behavioural categories considered in the present study adapted from Neumann (Reference Neumann2001) and Stockin et al. (Reference Stockin, Binedell, Wiseman, Brunton and Orams2009).
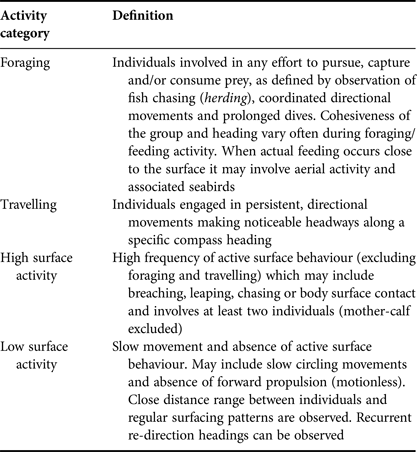
The behaviour of a group being followed was allocated to one of the four categories every 2 min. Behaviour was based upon the activity in which >50% of the group was engaged (Stockin et al., Reference Stockin, Binedell, Wiseman, Brunton and Orams2009). Data were recorded until the focal group was lost from sight or the reliability of data was compromised due to distance or ambiguity of view. Any sampling unit in which either the behaviour or proportion of group engaged was considered ambiguous was omitted from analysis.
Boat manoeuvres during encounters were recorded to allow compliance with the requirements of local whale watching guidelines (Decreto Legislativo Regional 9/99/A, 10/2003/A) to be assessed. For each encounter, the angle of boat approach was classified into three categories (parallel and behind, parallel and ahead and transversal), and the speed of the boat was noted as idle, equal to that of the dolphins or exceeding that of the dolphins.
Data were recorded using the Epicollect+ (version 1.5, http://www.epicollect.net/) Android App. When not recording data, the second observer assisted with searching for dolphin groups in order to reduce observer fatigue. Additional search support was given by the whale watch lookout operating from the same station. This further reduced the probability of resampling the same group.
The duration of the encounter was based on the number of 2 min intervals the boats were present and interacting with the dolphins group. An interaction was defined as boats slowing down and stopping within the vicinity of, or moving amongst dolphins.
Guidelines for swim-with-dolphin activities do not include type approach and maximum encounter duration. Whale watching companies were aware at the time of observation that a study to assess effects of dolphin tourism was in progress.
Data analysis
Control and interaction intervals were identified in each focal group follow based on the presence or absence of tour boats interacting with the group of dolphins (either observing or placing swimmers in the water). Boats other than tour vessels were occasionally present in the area but did not specifically target or interact with dolphins, and thus are not considered here.
A Markov chain analysis, which allows for the dependence of an event with preceding ones to be assessed, was used (Guttorp, Reference Guttorp1995). A first-order Markov chain analysis, where a category is only dependent on the immediately preceding one, was applied to derive transition probabilities between mutually exclusive behavioural categories and used to develop two-way contingency tables as described in Lusseau (Reference Lusseau2003). Hence, within each focal follow if no tour boat interaction occurred between two samples, the transition between the two categories was tallied in the control table. If an interaction occurred, it was tallied in the interaction table. In instances where it was difficult to decide whether a transition was to be considered within a control or interaction sequence, e.g. arrival and departure of a boat (Lusseau, Reference Lusseau2003), a conservative approach was followed (Meissner et al., Reference Meissner, Christiansen, Martinez, Pawley, Orams and Stockin2015). Hence, to avoid ambiguity, transitions occurring between a sample after an interaction and the following sample were removed (Lusseau, Reference Lusseau2003; Stockin et al., Reference Stockin, Lusseau, Binedell, Wiseman and Orams2008a; Meissner et al., Reference Meissner, Christiansen, Martinez, Pawley, Orams and Stockin2015).
Transition probabilities for control and interaction chains were calculated by:
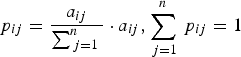 $$p_{ij} = \displaystyle{{a_{ij}} \over {\sum\nolimits_{\,j = 1}^n {}}} \cdot a_{ij}, \sum\limits_{\,j = 1}^n {\,p_{ij}} = 1$$
$$p_{ij} = \displaystyle{{a_{ij}} \over {\sum\nolimits_{\,j = 1}^n {}}} \cdot a_{ij}, \sum\limits_{\,j = 1}^n {\,p_{ij}} = 1$$where i is the preceding behavioural category and j is the succeeding behavioural category (ranging from 1 to 4, total number of categories observed and included in the analysis), a ij is the number of transitions observed from category i to category j and p ij is the transition probability from i to j in the Markov chain. A two-tailed Z-test for proportions was then used to compare each transition between control and interaction (Fleiss et al., Reference Fleiss, Levin and Paik2003).
Following Lusseau (Reference Lusseau2003), the activity budget in control and interaction scenarios was derived from the left eigenvector of the dominant eigenvalue of the transition matrices using the Excel add-in PopTools (version 3.0.3, CSIRO, www.cse.csiro.au/poptools). A two-tailed Z-test for proportion was used to compare control and interaction activity budgets and 95% CI were calculated. The time to return to a preceding activity after a change occurred was calculated for both control and interaction scenarios and for each activity category (Stockin et al., Reference Stockin, Lusseau, Binedell, Wiseman and Orams2008a; Meissner et al., Reference Meissner, Christiansen, Martinez, Pawley, Orams and Stockin2015):
where E(T j) is the number of transitions, which when multiplied by the time unit (2 min interval in the present study) gives the time taken by the dolphins to return to the initial activity j), and πj is the steady-state probability of each activity in the chain. The average bout length for each category in control and interaction scenarios was approximated, following Lusseau (Reference Lusseau2003), from the mean of the geometric distribution of p ii (Guttorp, Reference Guttorp1995):
where p ii is the probability to stay within the same behavioural category i. A Mann–Whitney test was subsequently applied to compare average bout lengths for both scenarios. The cumulative activity budget, i.e. the time dolphins could potentially be exposed within the season was derived following Lusseau (Reference Lusseau2003); Christiansen et al. (Reference Christiansen, Lusseau, Stensland and Berggren2010) and Meissner et al. (Reference Meissner, Christiansen, Martinez, Pawley, Orams and Stockin2015) using the following formula:
where I is the proportion of time common dolphins spend with interacting tour boats and C is the proportion of time dolphins spend without interacting tour boats (hence C = 1–I). I equals 0 when there is no interaction with tour boats, and the cumulative budget corresponds to the control budget. If interaction with tour boats was continuous, I equals 1 and the cumulative budget corresponds to the interaction budget.
A two-tailed Z-test for proportion was used to compare cumulative and control budget for each behavioural category. The difference between the Z-test P-values of control and cumulative budgets were used to explore the effects of cumulative budget based on the intensity of tour boat traffic.
RESULTS
Field effort
Data were collected during 83 days, corresponding to a total effort of 599 h, of which 157 were spent engaged in group focal follows. A total of 3357 control and 419 interaction transitions were recorded during 154 and 25 follow sequences, respectively. Control sequences lasted on average 46 min (median = 36 min, SD = 17.12, range = 14–215 min), while interaction sequences averaged 34 min (median = 32 min, SD = 33.9, range = 14–78 min). Of the 25 interaction sequences, 11 involved whale watching only, seven only swimming with dolphin activities and a further seven sequences included both activities. The maximum number of tour boats observed during interaction sequences was three (4% of transitions, N = 419), while most of the interaction sequences involved only one boat (79% of transitions, N = 419).
Effects of boat interactions
Transition probabilities were highest between the same behavioural category for both control and interaction scenarios (Figure 2). The category transitions which were significantly affected by the presence of tour boats (Figure 3) were: foraging to high surface activity (Z = 5.15, P < 0.001) and travelling to high surface activity (Z = 2.14, P = 0.032), which increased from 0.3 to 4.8% and from 7 to 12%, respectively, while travelling to foraging decreased from 4.9 to 0.7% (Z = 2.26, P = 0.023) when boats were interacting with the dolphins.
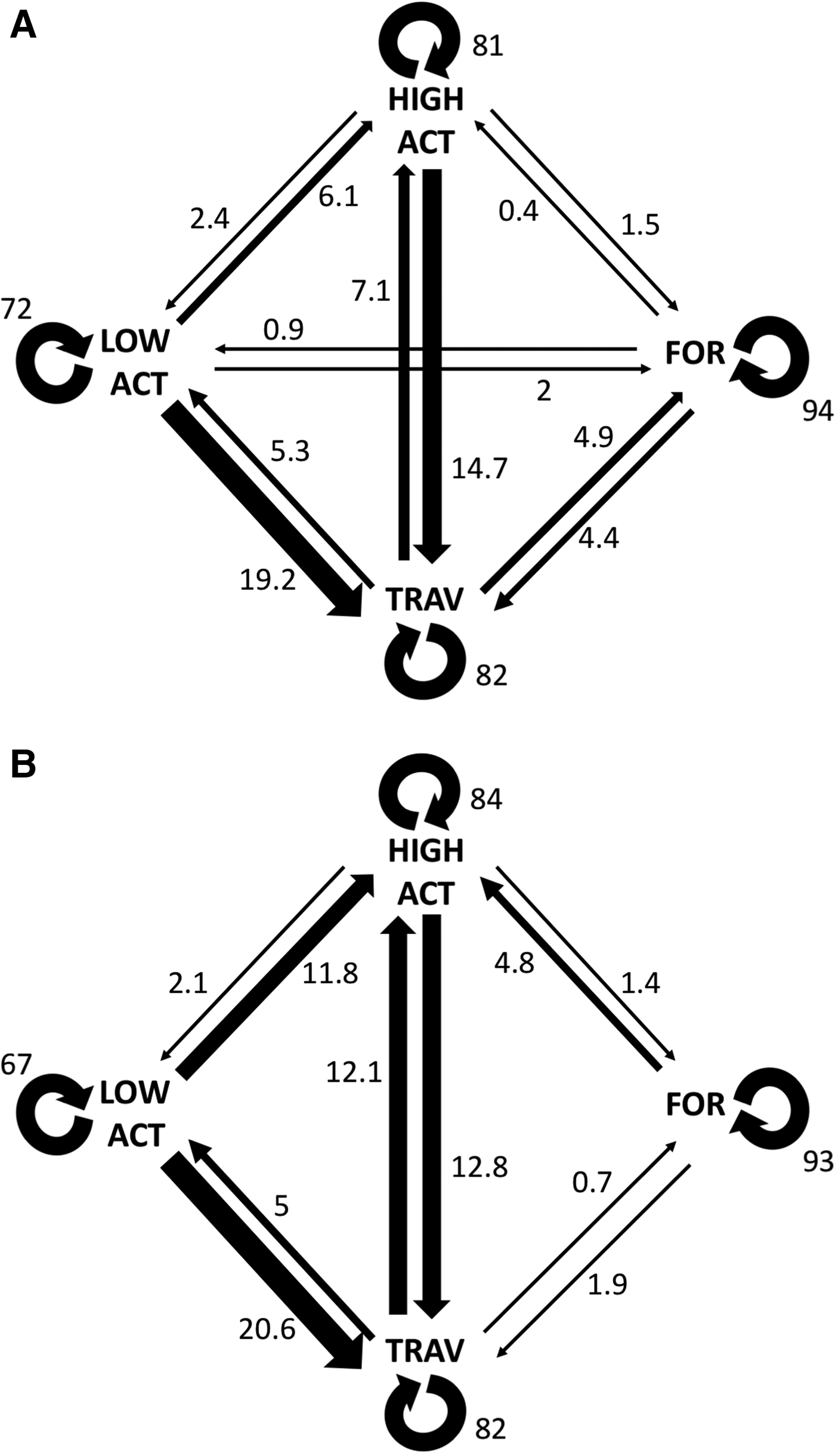
Fig. 2. Transition probabilities calculated for both control (a) and interaction (b) scenarios. Thicker arrows indicate transitions. Values shown are percentages.
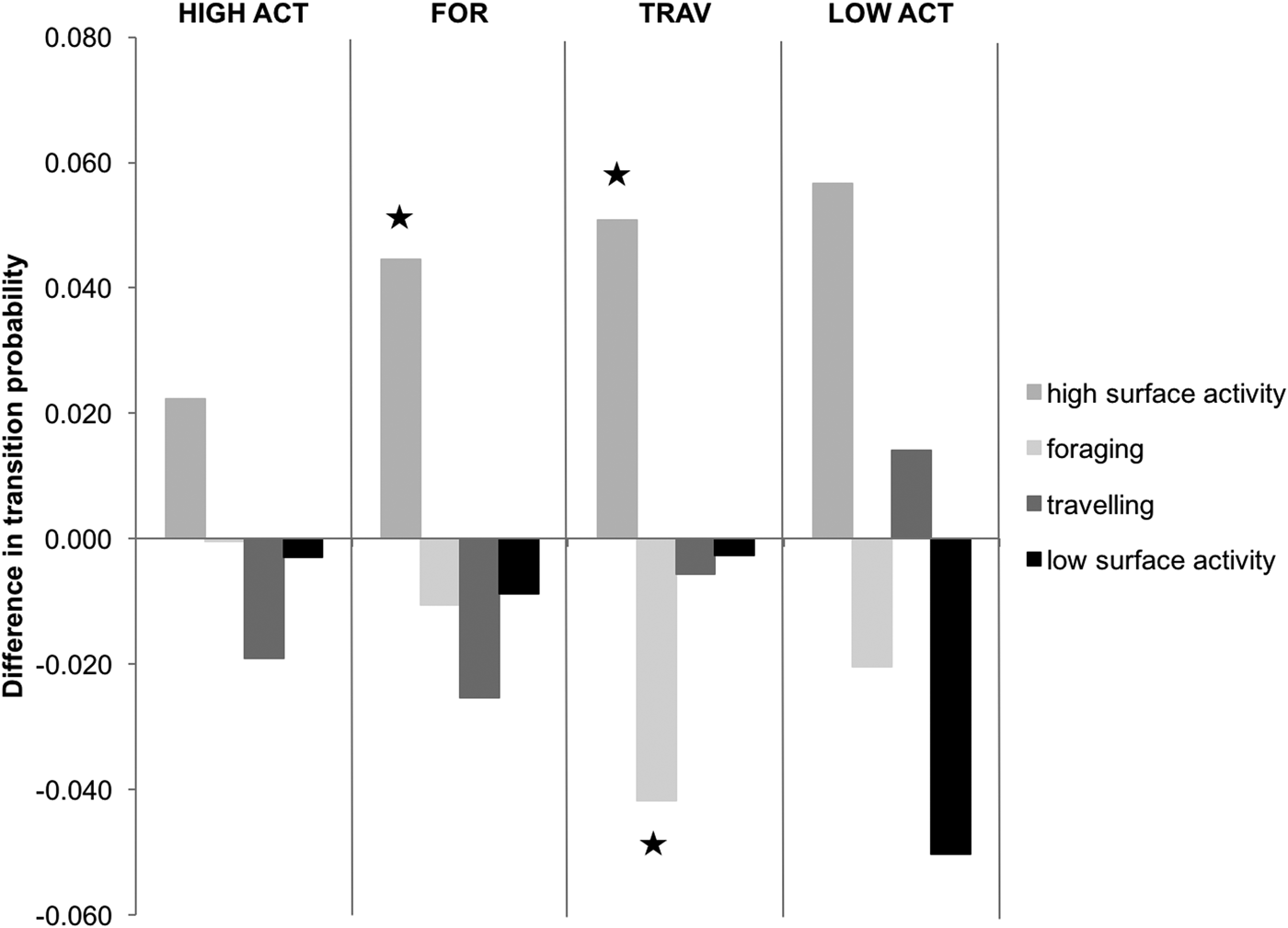
Fig. 3. Effect of boat interactions on activity transitions, based on differences in transition probabilities. The graph is divided into four parts delimited by vertical lines each representing a different preceding behavioural activity. Bars indicate succeeding behavioural activities. Those marked by ★ are significantly different. Negative values indicate that the transition probability of the control chain is higher to that of the interaction chain.
The proportion of time spent in high surface activity and foraging differed significantly between control and interaction sequences (Figure 4), with dolphins engaging more in high surface activity (39% vs 17%, Z = 6.822, P < 0.001) and less in foraging (12.4% vs 38%, Z = 3.78, P < 0.001) when boats were present. In the presence of tour boats, low surface activity and travelling decreased (9.6% to 8.6%) and increased (35% to 39%), respectively.
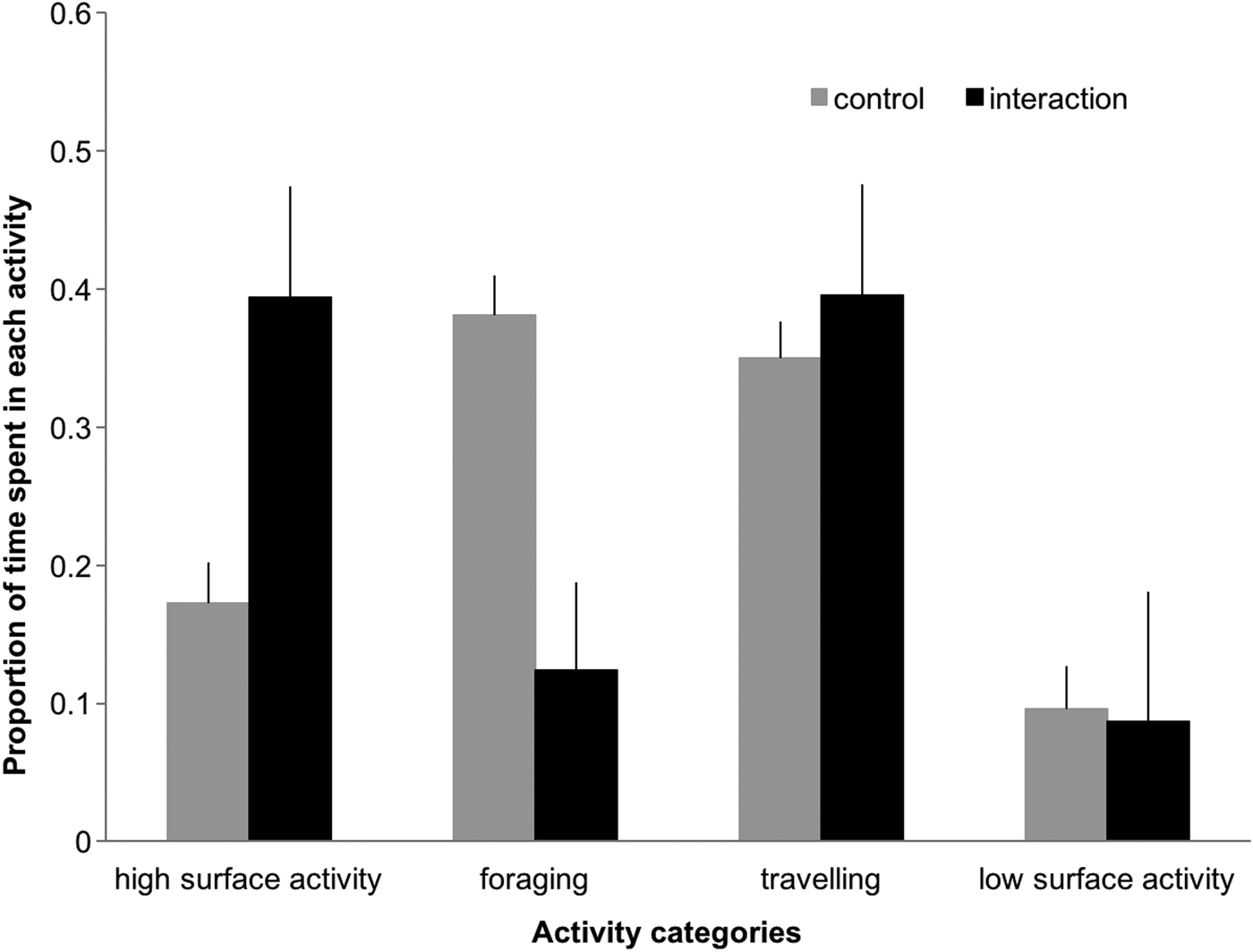
Fig. 4. Proportion of time (min) dolphins spent in each activity during control and interaction scenarios. Error bars represent 95% confidence intervals.
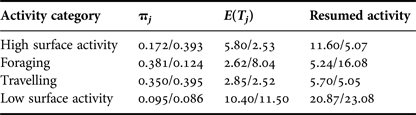
Time taken to return to the preceding activity differed during interaction with tour boats. Dolphins engaged in foraging and low surface activity before an interaction, took longer to return to their initial activity, 10.8 and 2.2 min more, respectively. While engaged in high surface activity, dolphins took less time (6.5 min difference) to return to their initial activity after interacting with tour boats (Table 2).
Table 2. Probabilities of staying in a particular behavioural category (πj), relative average number of time units E(T)j taken to return to an activity after boat approached and time needed to return to that activity. Control/interaction values are reported.
The average length of bouts of consistent behaviour varied significantly between control and interaction sequences (Table 3). Bout length increased 13% for groups in high surface activity (V = 1658.5, P < 0.0001), while it decreased 15% for foraging (V = 2994.5, P < 0.0001) and low surface activity groups (V = 1420, P < 0.0001), and by 3.1% for travelling dolphins (V = 4945.5, P < 0.0001).
Table 3. Average bout length, t ii (min) for each behavioural category in both control and interaction scenarios.

The cumulative interaction time analysis (Figure 5) shows that high surface activity and foraging by common dolphins could be affected when 44% and 48% of the time, respectively, is spent interacting with tour boats. In the present study, dolphin groups were observed spending only 10.5% of their time interacting with tour boats.
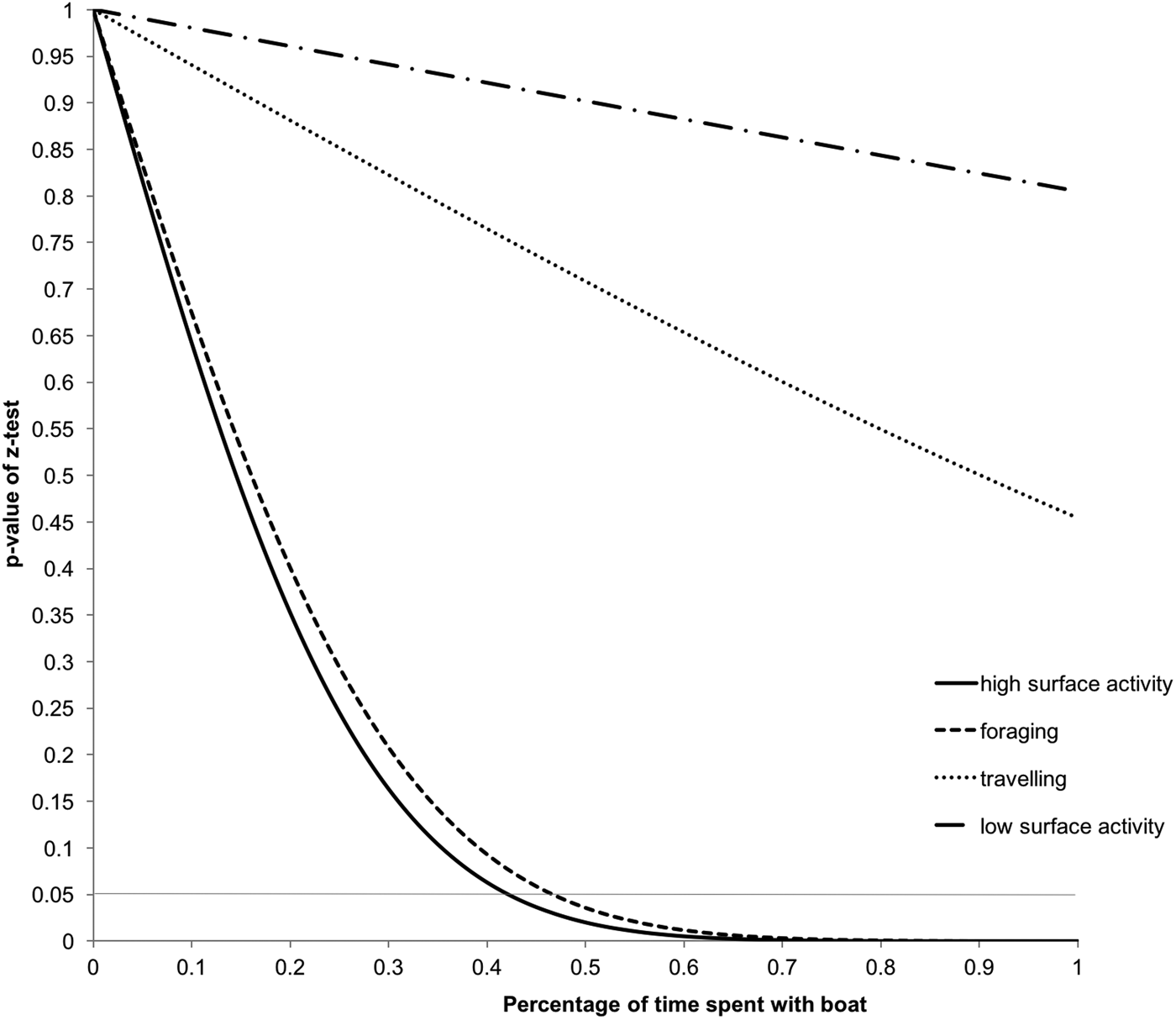
Fig. 5. Effect of tour boat traffic intensity on common dolphin activity budget. Z-test P-values of the difference between cumulative and control budgets. The dotted horizontal line indicates the level of significance set at P < 0.05.
Compliance with whale watching guidelines
Whale watching tour boats demonstrated compliance by approaching and manoeuvring around dolphins on 90.4% (N = 197) of encounters. However, during 9.6% (N = 197) of cases, boats approached the group either transversally, parallel and ahead or moved into the middle of the pod, or moved too quickly nearby the dolphins. Duration of encounters ranged between 2 and 53 min (mean = 22.67; SD = 14.97; median = 19), exceeding the 30 min permitted time limit in 27% (N = 18) of cases.
DISCUSSION
Transition probability analysis revealed that interactions with tour boats affected the behaviour of common dolphins off São Miguel, Azores. In the presence of tour boats, common dolphins spent less time foraging and more time in high surface activity. Furthermore, the time required to resume foraging after a tour boat interaction was higher.
Feeding is a biologically significant activity so any disruption may reduce energy intake and pose risk to the wellbeing and potential survival of individuals which could in turn lead to consequences at population level (Christiansen et al., Reference Christiansen, Rasmussen and Lusseau2011, Reference Christiansen, Rasmussen and Lusseau2013). The reduction of time allocated to foraging observed in this study could result in a reduction in feeding rate. Oceanic delphinids are reported to feed mostly at dusk and during the early hours of the morning (Neumann, Reference Neumann2001; Ringelstein et al., Reference Ringelstein, Pusineri, Hassani, Meynier, Nicolas and Ridoux2006; Pusineri et al., Reference Pusineri, Magnin, Meynier, Spitz, Hassani and Ridoux2007, Reference Pusineri, Chancollon, Ringelstein and Ridoux2008), i.e. when whale watching activities do not occur. If the same diurnal pattern of foraging occurred in the Azores then it might be argued that disruption of foraging during daylight hours might have a minor consequence on the total daily food input. However, within this study, foraging frequently occurred associated with diving Cory's shearwaters (Calonectris diomedea borealis) indicating that actual feeding during daylight hours did indeed occur. Moreover, whale watch operators anecdotally report the observation of bait balls during such encounters with foraging dolphins. Therefore, although the extent to which common dolphins off São Miguel feed at night remains unknown, foraging bouts during daylight hours do appear profitable. Significant amounts of daytime foraging has certainly been reported in Delphinus observed within the Hauraki Gulf, New Zealand (Stockin et al., Reference Stockin, Binedell, Wiseman, Brunton and Orams2009). A decrease in feeding activity is a particular concern for common dolphins given their need to cover high metabolic costs resulting from their small size and active swimming habits (Spitz et al., Reference Spitz, Mourocq, Leauté, Quéro and Ridoux2010). Further, food availability in oceanic habitats is typically patchily distributed, creating a need for considerable movements in order to find resources (Benoit-Bird & Au, Reference Benoit-Bird and Au2003). Common dolphins, like other small delphinids, are reported to use cooperative foraging techniques, for example to gather bait-balls (Gallo Reynoso, Reference Gallo Reynoso1991; Neumann & Orams, Reference Neumann and Orams2003; Benoit-Bird & Au, Reference Benoit-Bird and Au2009; Vaughn et al., Reference Vaughn, Würsig and Packard2010). Such complex and cooperative behaviour is likely to be particularly vulnerable to disruption.
Other studies have indicated that tour boats may affect foraging in a number of cetacean species (Williams et al., Reference Williams, Lusseau and Hammond2006; Dans et al., Reference Dans, Degrati, Pedraza and Crespo2012; Steckenreuter et al., Reference Steckenreuter, Möller and Harcourt2012), a finding reflected in Senigaglia et al. (Reference Senigaglia, Christiansen, Bejder, Gendron, Lundquist, Noren, Schaffar, Smith, Williams, Martinez, Stockin and Lusseau2016), which revealed decreased foraging as one of the most consistent responses to whale watching vessels. For instance, foraging common dolphins in New Zealand responded to commercial tourism boats by reducing time spent in this activity and by delaying their feeding bouts (Stockin et al., Reference Stockin, Lusseau, Binedell, Wiseman and Orams2008a; Meissner et al., Reference Meissner, Christiansen, Martinez, Pawley, Orams and Stockin2015). The main concern for the population in the Hauraki Gulf was linked to its elevated site fidelity, potentially leading to higher long-term effects (Stockin et al., Reference Stockin, Lusseau, Binedell, Wiseman and Orams2008a; Hupman, Reference Hupman2016) compared with a neighbouring site in the Bay of Plenty (Meissner et al., Reference Meissner, Christiansen, Martinez, Pawley, Orams and Stockin2015). In the Azores, common dolphins are observed year-round, although no information is available about their site fidelity or breeding cycles. Concern, in this case, would be associated with the frequent presence of calves during summer months, when the peak of the tourism activities occurs. Common dolphins were also observed increasing high surface activity in the presence of the tour boats, in particular increasing the transitions from foraging to high surface activity and from travelling to high surface activity. The high surface activity category probably included socializing, a state which is biologically important in ensuring better cooperation during group foraging, as a means of defence strategy and for enhancing reproductive success (Silk, Reference Silk2007; Schülke et al., Reference Schülke, Bhagavatula, Vigilant and Ostner2010). The limitation in being able to distinguish among such functions makes the results problematic to interpret, especially given the likelihood that high surface activity in the presence of boats vs control scenarios could have been different. Dolphins engaged in high surface activity are often performing aerial behaviours, possibly functioning as a means of communication. During interaction with the tour boats, dolphins could have increased their surface activity to improve communication as a response to disorientation and noise perturbation (Lusseau, Reference Lusseau2006; Noren et al., Reference Noren, Johnson, Rehder and Larson2009). From a metabolic perspective, two possible inferences could be derived from these findings. Either tourism activities are not affecting energetics and thus dolphins can still engage in costly behaviours or alternatively, dolphins not only reduce energy intake as a consequence of foraging disruption, but also increase their energy costs by engaging more in high surface activity behaviours.
With respect to the former point, it is notable that short-term responses to disturbance do not necessarily match stress levels as these may be delayed in their expression (Holmes et al., Reference Holmes, Giese and Krikwoken2005). This would challenge the interpretation of immediate reactions deemed either as ‘negative’ or ‘positive’. For instance, the approach behaviour of dolphins towards oncoming vessels gives an impression of lack of disturbance and is usually understood as a positive response. However, dolphins engaged in a particular activity may stop in order to bow-ride, reducing the time used for the initial activity. Similarly, an evidently negative reaction could lack biological significance in the long term (Blumstein & Fernández-Juricic, Reference Blumstein and Fernández-Juricic2010). Although categories of activity states used in the present study were pooled to minimize bias, results presented here still require cautious interpretation.
Effects of cetacean tourism are likely to be cumulative rather than acute (Bejder et al., Reference Bejder, Dawson and Harraway1999). In the present study, the cumulative effects on dolphins engaged in foraging and high surface activity were predicted to occur above 48% and 44% of tour boat exposure, respectively. Common dolphins within the study area were observed spending only ~10% of their daytime interacting with tour boats. This is lower than other exposed populations (e.g. 28.9% in Hauraki Gulf, New Zealand, Stockin et al., Reference Stockin, Lusseau, Binedell, Wiseman and Orams2008a; 21% Bay of Plenty, New Zealand, Meissner et al., Reference Meissner, Christiansen, Martinez, Pawley, Orams and Stockin2015), which could potentially allow for recovery between interactions (Christiansen & Lusseau, Reference Christiansen, Lusseau, Higham, Bejder and Williams2014). However, the area surveyed is only a small portion of the coast of São Miguel used by tour boats, suggesting dolphin exposure reported here is underestimated, at least during April–October when operations are mostly prolific. Unfortunately, the lack of data assessing population size and site fidelity of common dolphins in the Azores prevents clarity on the potential for cumulative effects.
Tour operators rarely violated the approach guidelines or the observation time limits. Typically, only one boat at a time approached dolphins, although on occasion as many as three vessels were recorded in proximity of a dolphin group, three being the maximum limit set by the guidelines. When a breach of the guidelines did occur, no change in dolphin behavioural category was recorded. The small sample size relative to interaction sequences of the present study emphasizes the need for further monitoring, covering a larger area. Bentz et al. (Reference Bentz, Rodrigues, Dearden, Calado and Lopes2015), for instance, working from a much larger dataset, reported that in over 20% of cetacean encounters, the limit of three boats was exceeded. Future studies could also specifically address other proxies such as direction patterns and breathing rates (Hastie et al., Reference Hastie, Wilson, Tufft and Thompson2003; Lusseau, Reference Lusseau2006), allowing a more comprehensive insight to the full effects of dolphin tourism in this region.
We cannot know whether the behavioural changes revealed by this study have any biological significance, especially given (i) the relatively small sample size of interaction data and (ii) the small proportion of time dolphins were exposed to tourism in this study. Indeed, establishing that whale watching has biologically significant impacts has rarely been achieved, and only in very well studied locally resident populations (e.g. Bejder et al., Reference Bejder, Samuels, Whitehead, Gales, Mann, Connor, Heithaus, Watson-Capps, Flaherty and Krützen2006b; Tyne et al., Reference Tyne, Johnston, Christiansen and Bejder2017). Given this, it is appropriate to apply caution in the interpretation of findings presented here, especially since the overall effect on the population remains unknown, and may not as has been observed in other species, be necessarily an indicator that current levels of tourism are not sustainable (Filby et al., Reference Filby, Christiansen, Stockin and Scarpaciin press). Nonetheless, the development of effective guidelines should be considered crucial. Future research should focus on the development of best practice boat approaches to reduce disturbance reported in this study. Until the effects reported here are proven as having no demonstrable biological impact on the broader population, we recommend the precautionary principle, and no further licencing for dolphin tourism be issued in this region.
ACKNOWLEDGEMENTS
We would like to thank all the volunteers involved in data collection as well as Terra Azul whale watching company for extra support during data collection. The authors also acknowledge two anonymous reviewers whose comments improved earlier versions of this manuscript. The map of the study area was produced by Marc Fernandez.
FINANCIAL SUPPORT
This research was partially supported by the European Regional Development Fund (ERDF) through the COMPETE – Operational Competitiveness Programme and national funds through FCT – Foundation for Science and Technology, under the project PEst-C/MAR/LA0015/2013, by the Strategic Funding UID/Multi/04423/2013 through national funds provided by FCT – Foundation for Science and Technology and European Regional Development Fund (ERDF), in the framework of the programme PT2020 and by cE3c funding (Ref:UID/BIA/003329/2013). It was also partly supported by CIRN (Centro de Investigação de Recursos Naturais, University of the Azores), and CIIMAR (Interdisciplinary Centre of Marine and Environmental Research, Porto, Portugal). A. Cecchetti was supported by the Regional Fund for Science scholarship M.3.1.2/F/036/2011.










