Introduction
The ocean sunfish Mola mola (Linnaeus, Reference Linnaeus1758) is a widely distributed species, being found in waters stretching from 75°N (Norway and North America) to 55°S (South America and New Zealand; AquaMaps, 2016). While standing out from other fish due to its uncommon morphology, M. mola has been subject historically to a lack of academic interest. In fact, only in the last two decades has the scientific community truly directed its attention to this animal, in an attempt to unveil the mysteries surrounding its life (e.g. Sims & Southall, Reference Sims and Southall2002; Cartamil & Lowe, Reference Cartamil and Lowe2004; Houghton et al., Reference Houghton, Doyle, Davenport and Hays2006; Watanabe & Sato, Reference Watanabe and Sato2008; Hays et al., Reference Hays, Farquhar, Luschi, Teo and Thys2009; Syväranta et al., Reference Syväranta, Harrod, Kubicek, Cappanera and Houghton2012; Kang et al., Reference Kang, Baek, Lee and Choi2015; Nakamura et al., Reference Nakamura, Goto and Sato2015; Sousa et al., Reference Sousa, López-Castejón, Gilabert, Relvas, Couto, Queiroz, Caldas, Dias, Dias, Faria, Ferreira, Ferreira, Fortuna, Gomes, Loureiro, Martins, Madureira, Neiva, Oliveira, Pereira, Pinto, Py, Queirós, Silva, Sujit, Zolich, Johansen, De Sousa and Rajan2016a; Breen et al., Reference Breen, Cañadas, Cadhla, Mackey, Scheidat, Geelhoed, Rogan and Jessopp2017). A number of satellite tracking studies were performed on M. mola, providing solid evidence of the existence of seasonal migratory movements in distinct geographic regions (e.g. Sims et al., Reference Sims, Queiroz, Doyle, Houghton and Hays2009a; Dewar et al., Reference Dewar, Thys, Teo, Farwell, O'Sullivan, Tobayama, Soichi, Nakatsubo, Kondo, Okada, Lindsay, Hays, Walli, Weng, Streelman and Karl2010; Potter et al., Reference Potter, Galuardi and Howell2011; Thys et al., Reference Thys, Ryan, Dewar, Perle, Lyons, O'Sullivan, Farwell, Howard, Weng, Lavaniegos, Gaxiola-Castro, Miranda Bojorquez, Hazen and Bograd2015). In the northern hemisphere, northward movements are performed in late winter–spring and southward movements are performed in late summer–autumn (Sims et al., Reference Sims, Queiroz, Doyle, Houghton and Hays2009a; Dewar et al., Reference Dewar, Thys, Teo, Farwell, O'Sullivan, Tobayama, Soichi, Nakatsubo, Kondo, Okada, Lindsay, Hays, Walli, Weng, Streelman and Karl2010; Potter et al., Reference Potter, Galuardi and Howell2011; Thys et al., Reference Thys, Ryan, Dewar, Perle, Lyons, O'Sullivan, Farwell, Howard, Weng, Lavaniegos, Gaxiola-Castro, Miranda Bojorquez, Hazen and Bograd2015; Sousa et al., Reference Sousa, Queiroz, Mucientes, Humphries and Sims2016b). Though valuably contributing to the knowledge on habitat usage preferences, satellite tracking studies are inherently expensive. For this reason, analysis is generally limited to a relatively small number of specimens (i.e. 3–25 specimens; Sims et al., Reference Sims, Queiroz, Doyle, Houghton and Hays2009a; Dewar et al., Reference Dewar, Thys, Teo, Farwell, O'Sullivan, Tobayama, Soichi, Nakatsubo, Kondo, Okada, Lindsay, Hays, Walli, Weng, Streelman and Karl2010; Potter et al., Reference Potter, Galuardi and Howell2011) whose behaviour may not be representative of that of the population. As such, the use of satellite tracking data to infer population-wise temporal habitat preferences may be inappropriate. Long-term collection of abundance data in a given area, on the other hand, allows for the recognition of seasonal patterns in habitat usage at the population level (Yozzo & Smith, Reference Yozzo and Smith1995; Prista, Reference Prista2013; Licandro et al., Reference Licandro, Blackett, Fischer, Hosia, Kennedy, Kirby, Raab, Stern and Tranter2015). A few satellite tracking studies have been performed on M. mola off southern Portugal (Sims et al., Reference Sims, Queiroz, Doyle, Houghton and Hays2009a, Reference Sims, Queiroz, Humphries, Lima and Hays2009b; Sousa et al., Reference Sousa, López-Castejón, Gilabert, Relvas, Couto, Queiroz, Caldas, Dias, Dias, Faria, Ferreira, Ferreira, Fortuna, Gomes, Loureiro, Martins, Madureira, Neiva, Oliveira, Pereira, Pinto, Py, Queirós, Silva, Sujit, Zolich, Johansen, De Sousa and Rajan2016a, Reference Sousa, Queiroz, Mucientes, Humphries and Sims2016b), but information on the seasonal patterns of spatial use at the population level in this general area is lacking.
Mola mola holds an overall low commercial value globally (Fulling et al., Reference Fulling, Fertl, Knight and Hoggard2007) and directed fisheries are largely restricted to Asian waters (Sagara & Ozawa, Reference Sagara and Ozawa2002; Liu et al., Reference Liu, Lee, Joung and Chang2009; Kang et al., Reference Kang, Baek, Lee and Choi2015). Nonetheless, high bycatch rates are reported worldwide and severe localized declines have been documented, leading the International Union for Conservation of Nature (IUCN) to recently categorize this species as ‘vulnerable’ (Liu et al., Reference Liu, Zapfe, Shao, Leis, Matsuura, Hardy, Liu, Robertson and Tyler2015). A quite recent drastic reduction in Portuguese landings of this fish from 12 metric tonnes in 1999 to about zero in 2009 (Liu et al., Reference Liu, Zapfe, Shao, Leis, Matsuura, Hardy, Liu, Robertson and Tyler2015) may indicate a severe decline in the standing stock of M. mola inhabiting Iberian Atlantic waters. As accurate knowledge on basic biological and ecological traits of any species is vital to the delineation of conservation measures and therefore its preservation, the IUCN recommends further research on the life of this fish.
In this context, the present work aimed to study, for the first time, the seasonal variation in the abundance and body size distribution of M. mola in southern waters of Portugal. Furthermore, the relationships between M. mola abundance and sea surface temperature (SST), chlorophyll a (chl a), upwelling and water transparency were also examined.
Materials and methods
Data collection
This study was carried out in southern Portugal, off Olhão, at a set-net targeting tuna (Tunipex). Mola mola abundance data (number of specimens entering the net per day as bycatch) were collected between April and November 2014. More specifically, data collection occurred throughout the months of April (5 days of sampling), May (5 days), June (18 days), July (3 days), August (10 days), September (24 days), October (12 days) and November (11 days). Additionally, whenever possible, size-related data were also obtained from the set-net daily bycatch. Due to the inherent difficulties in accurately measuring all individuals comprising the set-net daily bycatch, as noted by Nakamura (Reference Nakamura2014), crude total length (TL) measurements were mostly obtained, allowing for the assignment of specimens into 20 cm TL size classes.
Environmental data
Animal spatial usage is usually linked to the pursuit of favourable thermal and foraging conditions (Polovina et al., Reference Polovina, Howell, Kobayashi and Seki2001; Stensholt, Reference Stensholt2001; Kumari & Raman, Reference Kumari and Raman2010; Binder et al., Reference Binder, Cooke, Hinch and Farrel2011). As such, the existence of a relationship between M. mola abundance and environmental variables linked to thermal and foraging conditions was assessed.
In situ measurements of SST and water transparency (Secchi depth) were performed daily by Tunipex collaborators. Satellite-derived daily chl a data were obtained from AquaMODIS with a spatial resolution of ~4 km. A 7-day moving average was calculated and used as a proxy for primary productivity. Finally, an upwelling index (i.e. difference between coastal and offshore SST) was calculated using remotely sensed nocturnal SST (Aqua-MODIS, http://oceancolor.gsfc.nasa.gov/; Krug et al., Reference Krug, Silvano, Barbosa, Domingues, Galvão, Luis, Platt, Relvas and Sathyendranath2012; Couto et al., Reference Couto, Queiroz, Relvas, Baptista, Furtado, Castro, Nunes, Morikawa and Rosa2017).
Statistical analysis
The seasonal variation in M. mola abundance and the relationship between abundance and environmental variables were analysed using a negative binomial regression under generalized linear models (GLM) to account for overdispersion. Selection for best model was performed using Akaike Information Criterion (AIC) which balances the quality of model fitness to data and the complexity of the model (Quinn & Keough, Reference Quinn and Keough2002). Presence of outliers was assessed using Cook's distance approach (Zuur et al., Reference Zuur, Ieno and Elphick2010). No outliers were identified. The assumptions of each model were tested following Zuur et al. (Reference Zuur, Ieno and Elphick2010); independence and absence of residual patterns were verified by plotting residuals against fitted values and normality was tested with the quantile-quantile (Q-Q) plot.
To understand the relationship between M. mola abundance and environmental variables (i.e. SST, chl a (7-day moving average), upwelling index and water transparency), we applied a multistep strategy (Sikkink et al., Reference Sikkink, Zuur, Ieno and Smith2007). To correct for heterogeneity of variance in this model, data were analysed using generalized least squares (GLS) as proposed by Zuur et al. (Reference Zuur, Ieno and Elphick2010). The independent variables kept in the final model are shown in Table 1. Finally, the performance of the GLS model was evaluated by calculating the concordance index (C-index; Harrell et al., Reference Harrell, Lee, Califf, Pryor and Rosati1984) using the Hmisc package (Harrell, Reference Harrell2006) that estimates the probability of concordance between predicted and observed responses (Swets, Reference Swets1988).
Table 1. Summary of the generalized least squares (GLS) model relating the abundance of Mola mola in a tuna set-net off southern Portugal in 2014 to the independent variables kept in the final model

β, slope; SD, standard deviation; P, P-value.
Statistical analysis was performed in R (version 3.4.3; R Core Team, 2017) and data exploration and model validation used the HighstatLibV10 R library from Highland Statistics (Zuur et al., Reference Zuur, Ieno, Walker, Saveliev and Smith2009).
Results
Abundance and environmental variables
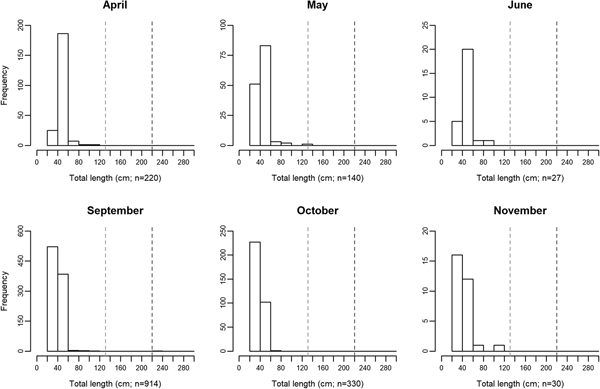
Mola mola were present in southern waters of Portugal throughout the studied period (April–November 2014). There were, however, significant seasonal differences in abundance (GLM Analysis of Deviance, df = 15, F = 9.77 P < 0.001). Two peaks were observed, one in the spring (median value of 115 specimens/day in early (days 1–15) May) and the other in the autumn (148 specimens/day in early September and 150 specimens/day in early October; Figure 1). Abundance was generally low throughout the summer, mostly below 40 specimens/day, yet, the lowest abundance values were found in early November with a median value of one specimen/day.
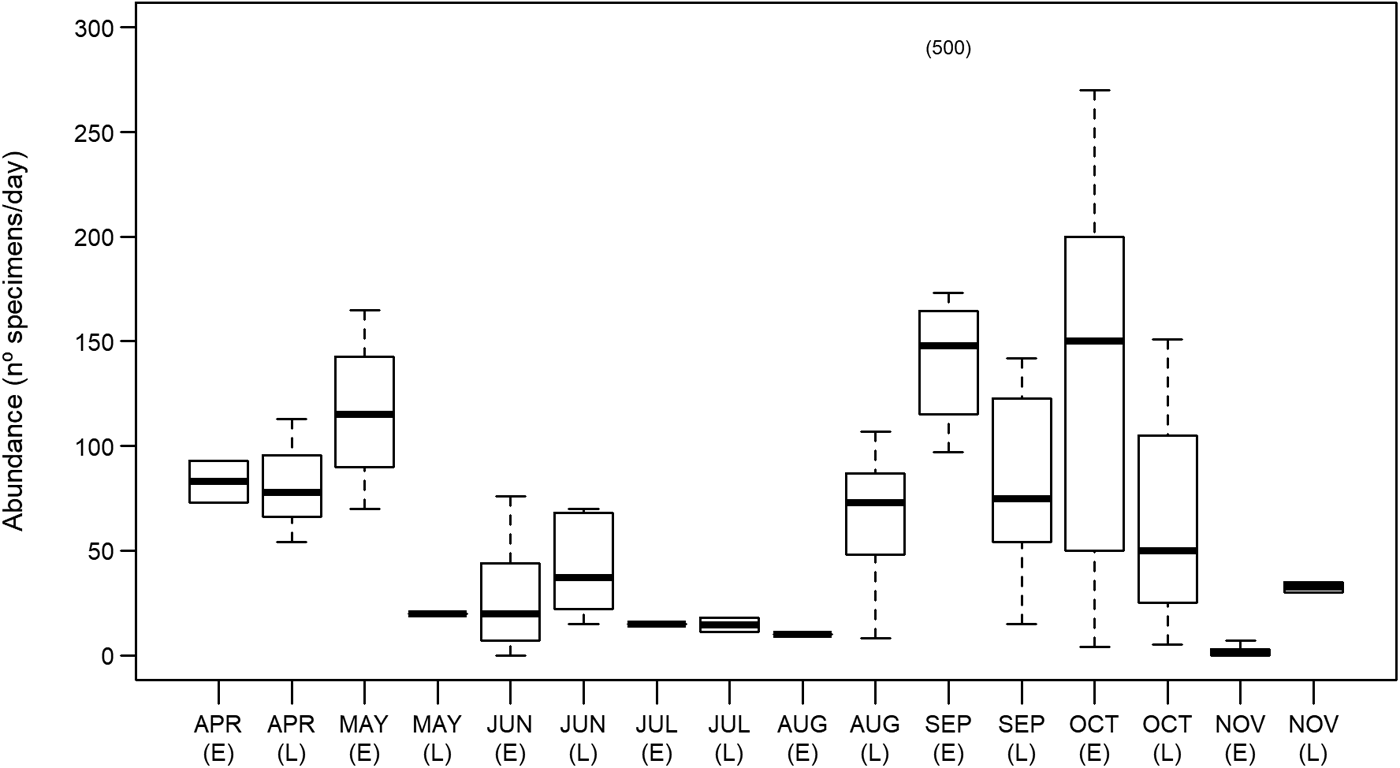
Fig. 1. Mola mola abundance between April and November 2014, in the studied location – Tunipex S.A. set-net, off Olhão, Portugal. Box-plot shows median and 25 and 75 percentiles, whiskers indicate the range. Data is pooled over fortnight periods. The number of days in which data was collected varied among fortnights: early April (2 days), late April (3 days), early May (4 days), late May (1 day), early June (13 days), late June (5 days), early July (1 day), late July (2 days), early August (1 day), late August (9 days), early September (12 days), late September (12 days), early October (5 days), late October (7 days), early November (9 days) and late November (2 days). E, early (days 1–15); L, late (days 16–30/31, depending on month). The number 500 in parentheses indicates a day (8 September 2014) when 500 specimens entered the set-net.
The GLS model revealed statistically significant relationships between M. mola abundance and two environmental variables. Positive relationships were found with SST (which ranged between 14 and 22°C; β = 8.93, SD = 3.93, P = 0.026) and chl a (7-day moving average; 0.2–2.4 mg m−3; β = 40.78, SD = 19.24, P = 0.038; Table 1). No significant relationship was found between M. mola abundance and water transparency (which ranged between 4 and 17 m; Table 1).
Body size
Measured ocean sunfish specimens ranged between 31.8 and 230 cm TL, with the second largest animal registering 120.3 cm TL. The use of crude TL measurements for the assignment of fish into different size classes is a relevant limitation of the present study and may not allow for the accurate description of biologically relevant features. Nonetheless, some patterns became available in the present study. Overall, 98% of all sampled specimens measured between 31.8 and 59.9 cm TL. Specimens larger than 80 cm were extremely rare, accounting for less than 0.7% of sampled M. mola. There was an apparent seasonal shift in size distribution around the 40 cm TL mark. Size class ‘40–59.9 cm’ was more abundant between April and June (75% of total specimens), while class ‘20–39.9 cm’ attained slightly greater numbers between September and November (60% of total specimens; Figure 2).
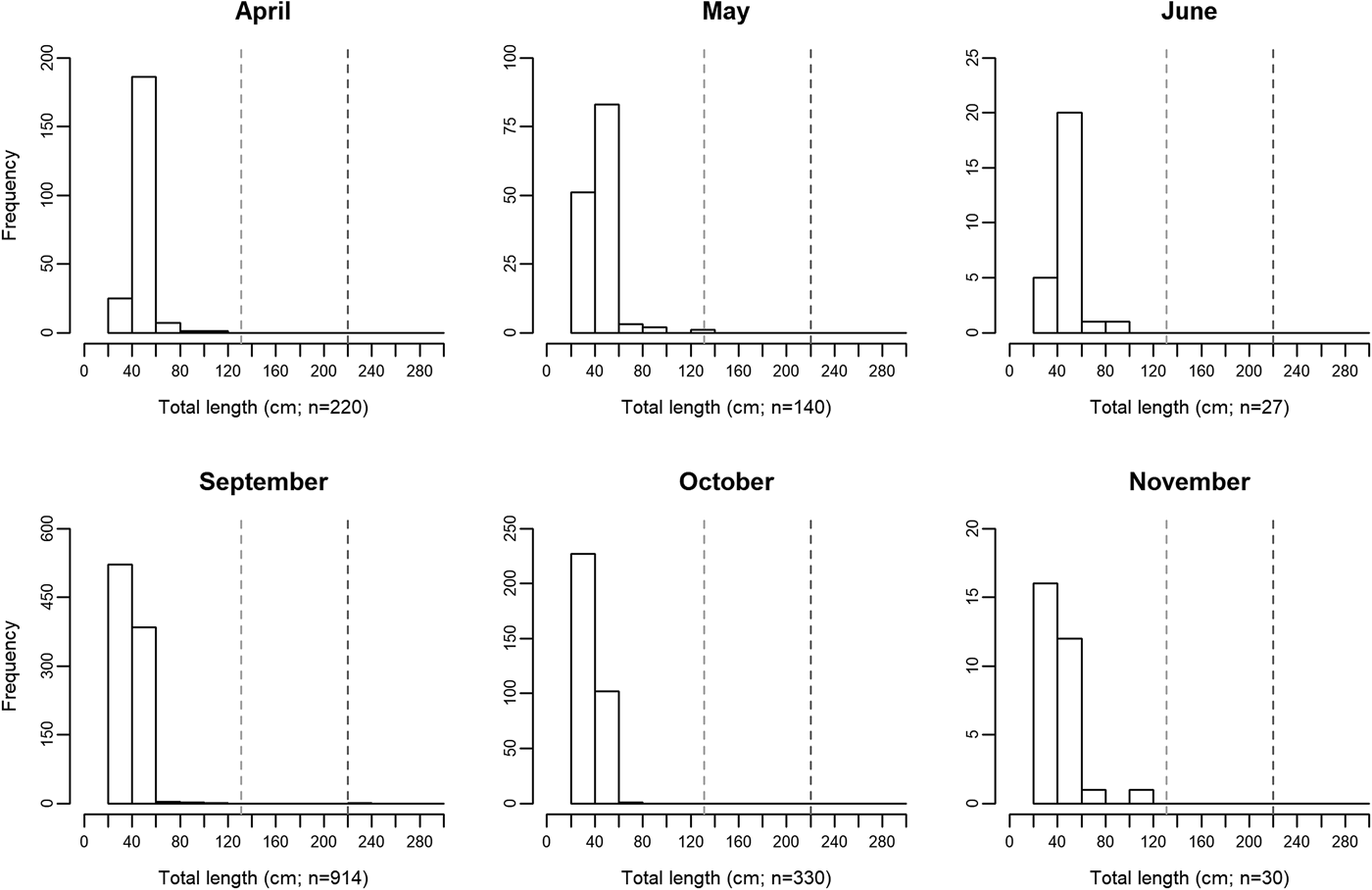
Fig. 2. Monthly changes in Mola mola body size distribution between April and November, 2014, in the studied location – Tunipex S.A. set-net, off Olhão, Portugal. The vertical dashed lines indicate size at maturity for males (131 cm; light grey) and females (220 cm; dark grey) obtained from Kang et al. (Reference Kang, Baek, Lee and Choi2015).
Discussion
Seasonal changes in abundance and body size distribution
Information on seasonal variations in habitat use provides critical data needed when planning for species conservation, stock management and establishment of marine protected areas (West et al., Reference West, Dytham, Righton and Pitchford2009; Chapman et al., Reference Chapman, Hulthén, Brodersen, Nilsson, Skov, Hansson and Brönmark2012). The present study showed that the presence of Mola mola in southern waters of Portugal is recurrent between April and November (study period). Moreover, ocean sunfish probably occur in the area throughout the year since SST in this area generally varies between 13 and 23°C year-round (Tunipex, 2017), a range of temperatures in which ocean sunfish are found worldwide (Sims et al., Reference Sims, Queiroz, Doyle, Houghton and Hays2009a; Dewar et al., Reference Dewar, Thys, Teo, Farwell, O'Sullivan, Tobayama, Soichi, Nakatsubo, Kondo, Okada, Lindsay, Hays, Walli, Weng, Streelman and Karl2010; Potter et al., Reference Potter, Galuardi and Howell2011; Thys et al., Reference Thys, Ryan, Dewar, Perle, Lyons, O'Sullivan, Farwell, Howard, Weng, Lavaniegos, Gaxiola-Castro, Miranda Bojorquez, Hazen and Bograd2015; Sousa et al., Reference Sousa, Queiroz, Mucientes, Humphries and Sims2016b). Interestingly and contrarily to other large migratory fish such as bluefin tuna (Thunnus thynnus), meagre (Argyrosomus regius) and basking shark (Cetorhinus maximus) that show only one peak of abundance in this area (mid-summer for tuna, late-summer for meagre and mid-spring for basking shark; Prista, Reference Prista2013; Santos et al., Reference Santos, Rosa, Coelho and Lino2016; Couto et al., Reference Couto, Queiroz, Relvas, Baptista, Furtado, Castro, Nunes, Morikawa and Rosa2017), M. mola revealed two peaks – spring and autumn (greater values being registered in early May and both early September and early October). More so, the existence of spring and autumn abundance peaks is recurrent in this area (Poço, personal communication, 24 August 2017), and agrees with the seasonal latitudinal movements reported for ocean sunfish in the region (Sims et al., Reference Sims, Queiroz, Doyle, Houghton and Hays2009a; Sousa et al., Reference Sousa, Queiroz, Mucientes, Humphries and Sims2016b). This finding is indicative of the relevance of southern waters of Portugal in the migratory ecology of M. mola in the North-east Atlantic. A comprehensive number of studies performed on the horizontal movements of mostly ≤150 cm TL M. mola (e.g. Hays et al., Reference Hays, Farquhar, Luschi, Teo and Thys2009; Potter et al., Reference Potter, Galuardi and Howell2011; Thys et al., Reference Thys, Ryan, Dewar, Perle, Lyons, O'Sullivan, Farwell, Howard, Weng, Lavaniegos, Gaxiola-Castro, Miranda Bojorquez, Hazen and Bograd2015; Sousa et al., Reference Sousa, Queiroz, Mucientes, Humphries and Sims2016b) has allowed for the identification of migration patterns that appear confined to coastal areas (Thys et al., Reference Thys, Ryan, Dewar, Perle, Lyons, O'Sullivan, Farwell, Howard, Weng, Lavaniegos, Gaxiola-Castro, Miranda Bojorquez, Hazen and Bograd2015). Indeed, movement ranges generally do not exceed 500 km away from shoreline and fish largely remain within 300 km of the shore. Migratory movements of smaller ocean sunfish (≤150 cm TL) seem therefore dependent on coastal proximity and while the use of alternative more oceanic migration routes is not viable, the presently studied area should be pivotal in the northward-southward migration pattern described for the species in the North-east Atlantic (Sims et al., Reference Sims, Queiroz, Doyle, Houghton and Hays2009a, Reference Sims, Queiroz, Humphries, Lima and Hays2009b; Sousa et al., Reference Sousa, Queiroz, Mucientes, Humphries and Sims2016b). Seasonal changes in abundance are sometimes related to reproductive processes and, in the presently studied general area, episodes of greater bluefin tuna abundance occur as a result of migration into the Mediterranean Sea for spawning (Santos et al., Reference Santos, Rosa, Coelho and Lino2016). Regarding M. mola, however, as the overwhelming majority (~99.9%) of specimens inhabiting the southern waters of Portugal were immature (see Figure 2; Nakatsubo et al., Reference Nakatsubo, Kawachi, Mano and Hirose2007; Kang et al., Reference Kang, Baek, Lee and Choi2015), the presently revealed seasonal differences in abundance should not be related to spawning (Dewar et al., Reference Dewar, Thys, Teo, Farwell, O'Sullivan, Tobayama, Soichi, Nakatsubo, Kondo, Okada, Lindsay, Hays, Walli, Weng, Streelman and Karl2010). Conversely, the presence of smaller M. mola in the studied area has been attributed to its value as a developmental habitat for this species (Sousa et al., Reference Sousa, Queiroz, Mucientes, Humphries and Sims2016b). A seasonal shift in size distribution around the 40 cm TL mark was apparent in the present study, with slightly greater numbers of smaller specimens later in the year. While sampling limitations (i.e. usage of crude TL measurements for size class assignment) do not allow solid conclusions to be drawn from this pattern, it resembles that observed by Sawai et al. (Reference Sawai, Yamanoue, Yoshita, Sakai and Hashimoto2011) in north-eastern Japanese waters. Future work should attempt to assess the actual existence of a seasonal size shift as increases in the relative abundance of smaller sized fish are most likely indicative of recruitment success and a shift in habitat use. In loggerhead turtles (resembling ocean sunfish in regard to seasonality and habitat use; Kenney, Reference Kenney, Keinath, Barnard, Musick and Bell1996), smaller juveniles were found to occupy oceanic habitats whereas larger ones occupy coastal habitats (Bowen et al., Reference Bowen, Bass, Chow, Bostrom, Bjorndal, Bolten, Okuyama, Bolker, Epperly, Lacasella, Shaver, Dodd, Hopkins-Murphy, Musick, Swingle, Rankin-Baransky, Teas, Witzell and Dutton2004). It is possible that young ocean sunfish adopt a similar strategy in spatial use, initially inhabiting oceanic waters and switching to a more coastal habitat upon reaching 20 cm TL, size of the smallest specimens observed in coastal waters (Silvani et al., Reference Silvani, Gazo and Aguilar1999; Thys et al., Reference Thys, Ryan, Dewar, Perle, Lyons, O'Sullivan, Farwell, Howard, Weng, Lavaniegos, Gaxiola-Castro, Miranda Bojorquez, Hazen and Bograd2015).
Relationships between abundance and environmental variables
Typically, migration patterns follow seasonal changes in temperature and productivity as animals seek favourable thermal and foraging conditions (Polovina et al., Reference Polovina, Howell, Kobayashi and Seki2001; Stensholt, Reference Stensholt2001; Kumari & Raman, Reference Kumari and Raman2010; Binder et al., Reference Binder, Cooke, Hinch and Farrel2011). As fluctuations in the abundance of an organism at a given location are in all likelihood related to seasonal migratory movements, a relationship between abundance and those environmental factors should also be verified. Accordingly, the present investigation found SST and chl a (a widely recognized indicator of productivity; e.g. Breen et al., Reference Breen, Cañadas, Cadhla, Mackey, Scheidat, Geelhoed, Rogan and Jessopp2017; Couto et al., Reference Couto, Queiroz, Relvas, Baptista, Furtado, Castro, Nunes, Morikawa and Rosa2017) as determinants of M. mola abundance in southern waters of Portugal (Table 1). Mola mola abundance was positively correlated with both environmental variables. In the studied area, greater abundance was found upon higher SSTs (between 18.0 and 21.5°C). While in agreement with the work of Fulling et al. (Reference Fulling, Fertl, Knight and Hoggard2007) where greater ocean sunfish presence was verified at SSTs around 20.5°C, the presently obtained results differ from those of Hahlbeck et al. (Reference Hahlbeck, Scales, Dewar, Maxwell, Bograd and Hazen2017) and Nakamura & Sato (Reference Nakamura and Sato2014) who observed higher M. mola abundance associated with SSTs below 17–18°C. The discrepancy in these observations provides an indication that the relationship between spatial use and temperature is not straightforward. Indeed, even though abundance was shown to increase with temperature (Table 1), abundance peaks took place under differing SST scenarios: spring (April–May; 16.46 ± 1.54°C) and autumn (September–October; 20.63 ± 1.39°C; Figure 3). Conversely, chl a was also found as a determinant of M. mola abundance in the southern waters of Portugal (Table 1). Chlorophyll a is widely recognized as an indicator of productivity (Sims et al., Reference Sims, Southall, Richardson, Reid and Metcalfe2003; Breen et al., Reference Breen, Cañadas, Cadhla, Mackey, Scheidat, Geelhoed, Rogan and Jessopp2017; Couto et al., Reference Couto, Queiroz, Relvas, Baptista, Furtado, Castro, Nunes, Morikawa and Rosa2017) and an association between predators and productive areas is frequently observed (Polovina et al., Reference Polovina, Howell, Kobayashi and Seki2001; Kumari & Raman, Reference Kumari and Raman2010) as these areas should provide enhanced foraging opportunities. Concomitantly, M. mola abundance peaks coincided with the seasons exhibiting regular occurrence of phytoplankton blooms in the North Atlantic – spring and autumn (Lalli & Parsons, Reference Lalli, Parsons, Lalli and Parsons2006; Longhurst, Reference Longhurst2007). While M. mola has not been shown to seek highly productive areas, it appears to avoid oligotrophic areas (Sousa et al., Reference Sousa, Queiroz, Mucientes, Humphries and Sims2016b), being mostly associated with chl a concentrations in the range 0.5–2.5 mg m−3 (Phillips et al., Reference Phillips, Reid, Thys, Harrod, Payne, Morgan, White, Siobhán and Houghton2017). Accordingly, in the present work, greater M. mola abundance was observed above 0.6 mg m−3. Curiously, no effect of chl a was found on M. mola abundance in western USA coastal waters (Hahlbeck et al., Reference Hahlbeck, Scales, Dewar, Maxwell, Bograd and Hazen2017). It is possible that the implementation of different methodologies and/or consideration of diverse spatial and temporal scales may explain the contrasting results. The presently found relationship between abundance and productivity in southern waters of Portugal, is indicative of the relevance of this general area (i.e. Gulf of Cadiz), as a feeding ground for ocean sunfish during their seasonal migratory movements in the North-east Atlantic. Additionally, the observed association of M. mola abundance with both temperature and productivity provides an indication that a combination of these environmental factors dictates spatial use, in agreement with the hypothesis put forth by Thys et al. (Reference Thys, Ryan, Dewar, Perle, Lyons, O'Sullivan, Farwell, Howard, Weng, Lavaniegos, Gaxiola-Castro, Miranda Bojorquez, Hazen and Bograd2015) regarding seasonal migrations. The visual acuity of juvenile M. mola (42–46 cm TL) was found to be similar to that of adult cetaceans (Kino et al., Reference Kino, Miayzaki, Iwami and Kohbara2009). Additionally, 95% of feeding events by ocean sunfish take place during the day (Nakamura et al., Reference Nakamura, Goto and Sato2015). Such observations point to vision as playing an important role in the foraging ecology of M. mola and as such, it would make sense that this animal attempted to maximize prey capture chances by actively seeking high visibility waters. Surprisingly, however, water transparency was found to have no effect on M. mola abundance and therefore spatial use. As both productivity and water transparency provide information on potential foraging success, the apparent disregard for the latter may indicate a strategy in M. mola spatial use favouring a medium-term steady food supply over short-term improved feeding opportunities.
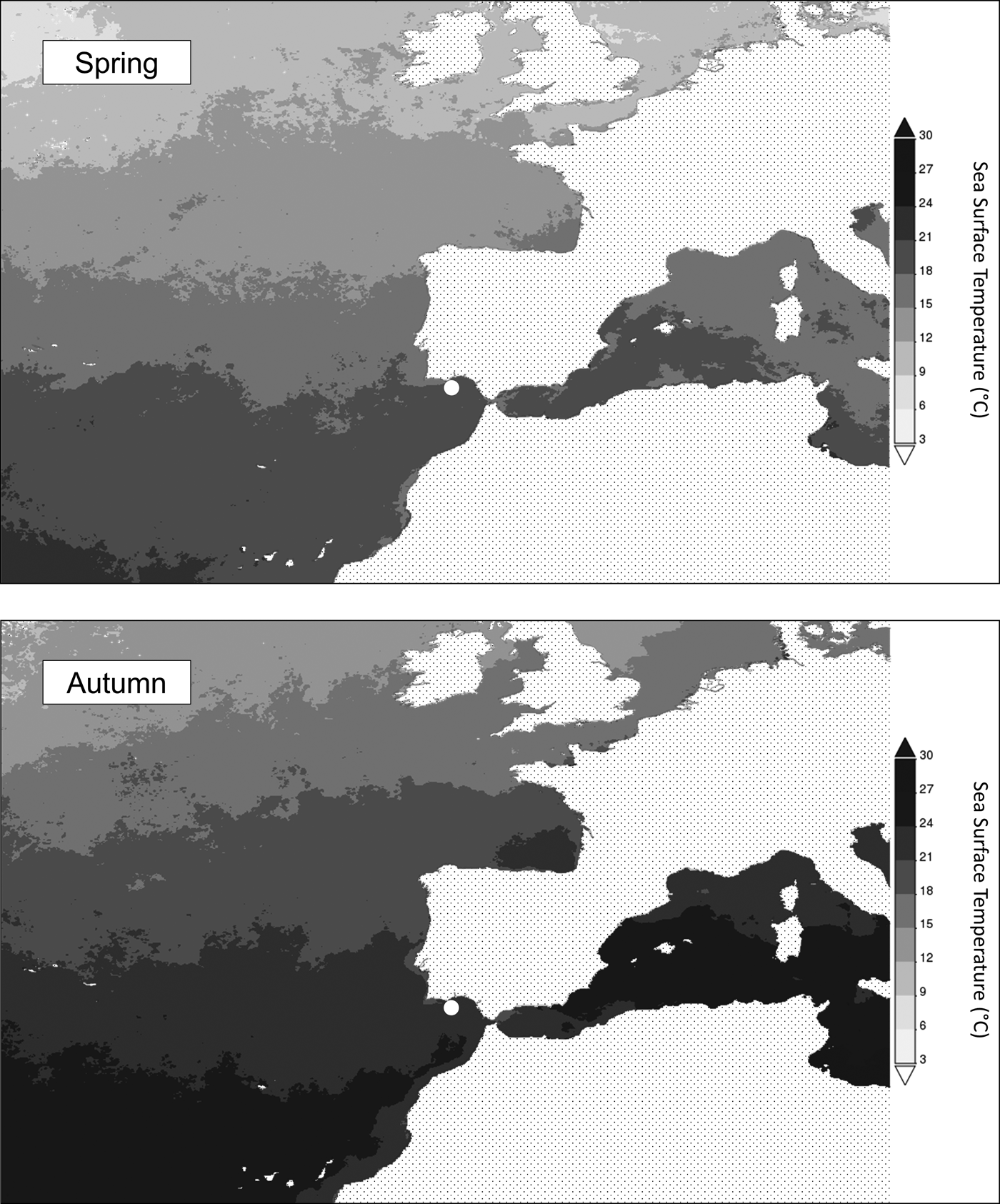
Fig. 3. MODIS Aqua average sea surface temperature in the studied location and surrounding areas in the spring (April–May) and autumn (September–October) of 2014. Data obtained from Giovanni (Acker & Leptoukh, Reference Acker and Leptoukh2007). The white filled circle indicates the studied location.
Concluding remarks
The present study supports the notion of seasonal latitudinal movements in the North-eastern Atlantic and highlights the importance of southern waters of Portugal (and potentially Gulf of Cadiz) in the migratory ecology of M. mola. As the vast majority of specimens inhabiting the studied area were immature, such horizontal movements should not be related to spawning. Instead, the observed relationship between M. mola abundance and both temperature and productivity provides indication that a combination of these environmental factors dictates spatial use. Complementarily, the absence of a relationship between abundance and water transparency may reveal a strategy in spatial use favouring a medium-term steady food supply over short-term improved feeding opportunities.
Author ORCIDs
Miguel Baptista, 0000-0001-8833-4766.
Acknowledgements
We would like to thank the staff of Tunipex for their invaluable help throughout this study, especially Dr Hirofumi Morikawa and Captain Alfredo Poço for allowing access to the set-net and providing logistical support. Figure 3 was produced with the Giovanni online data system, developed and maintained by the NASA GES DISC. We also acknowledge the MODIS mission scientists and associated NASA personnel for the production of the data used.
Financial support
The Portuguese Foundation for Science and Technology (FCT) supported this study through the PhD scholarship awarded to M. Baptista (SFRH/BD/88175/2012), the Postdoctoral Grant awarded to J. Raimundo (SFRH/BPD/91498/2012), the FCT Investigator Fellowships awarded to R. Rosa (IF/01373/2013) and N. Queiroz (IF/01611/2013), and the strategic project UID/MAR/04292/2013 granted to MARE. This study was also supported by Programa Operacional Pesca 2007-2013 – Promar through the project MOLA (31-03-05-FEP-0037) awarded to R. Rosa.