INTRODUCTION
Octopus populations show large reproductive plasticity and are known to have variable sizes-at-maturity between and within genders, high individual growth plasticity, short life, and the majority of their biological processes strongly influenced by temperature and diet (Leporati et al., Reference Leporati, Pecl and Semmens2008). Substantial changes in cephalopod biological traits have been reported at different time scales in response to environmental conditions (Quetglas et al., Reference Quetglas, Rueda, Alvarez-Berastegui, Guijarro and Massutí2016), which provides challenges for the management of their fisheries (Rodhouse et al., Reference Rodhouse, Pierce, Nichols, Sauer, Arkhipkin, Laptikhovsky, Lipinski, Ramos, Gras, Kidokoro, Sadayasu, Pereira, Lefkaditou, Pita, Gasalla, Haimovici, Sakai and Downey2014). Despite a large body of recently published literature on octopus reproductive biology, interannual or spatial differences in parameters such as size at maturity have rarely been assessed (Carvalho & Sousa Reis, Reference Carvalho and Sousa Reis2003; Storero et al., Reference Storero, Ocampo-Reinaldo, González and Narvarte2010, Reference Storero, Narvarte and González2012; Lourenço et al., Reference Lourenço, Moreno, Narciso, González and Pereira2012).
The Mayan octopus (Octopus maya Voss & Solís, 1966) is a holobenthic octopus, endemic to shallow waters of the Campeche Bank, in the south-eastern Gulf of Mexico (Solís-Ramírez, Reference Solís-Ramírez, Lang and Hochberg1997; Rosas et al., Reference Rosas, Gallardo, Mascaró, Caamal-Monsreal, Pascual, Iglesias, Fuentes and Villanueva2014). A small-scale fishery targets it from Sabancuy, Campeche, to Holbox, northern Quintana Roo, including all the western and northern shore of the Yucatan peninsula (Figure 1). It is the main fishery resource along its distribution range and the third marine fishery resource by value and the seventh by volume in Mexico (CONAPESCA, 2014). It is the largest octopus fishery in the Americas and one of the largest worldwide (FAO, 2011–2016). Fishing is manually performed from small vessels using lines baited with dead fresh crabs that drift over the soft bottom. Octopus take and hold the crabs while they are dragged on board. This gear based on feeding behaviour precludes breeding females from being caught (Solís-Ramírez, Reference Solís-Ramírez1962, Reference Solís-Ramírez, Lang and Hochberg1997, Reference Solís-Ramírez1998).

Fig. 1. Campeche State shoreline showing Octopus maya landing localities. Depth in meters. Inset shows the states on the Yucatan peninsula, Mexico: CAM, Campeche; YUC, Yucatan and QR, Quintana Roo. Arrows show west (Sabancuy) and east (Holbox) limits of localities with commercial landings.
Studies based on 20,000 octopus, taken from 1974–1983, established the current regulation in 1984 (Solís-Ramírez, Reference Solís-Ramírez1988, Reference Solís-Ramírez, Lang and Hochberg1997): a fishing closure from 15 December to 31 July, minimum legal size of 110 mm mantle length (ML) and the prohibition of using hooks or harpoons (DOF, 2014). However, no detailed information has been published on these studies, such as the criteria to define the minimum legal size. Otherwise few works describe size structure of O. maya (Arreguín-Sánchez, Reference Arreguín-Sánchez1992; Arreguín-Sánchez et al., Reference Arreguín-Sánchez, Solís, Sánchez, Valero and González1996, Reference Arreguín-Sánchez, Solís-Ramírez and González de la Rosa2000; González-de la Rosa et al., Reference González-de la Rosa, Santos-Valencia and Solís-Ramírez1998; Hernández-Sánchez & De Jesús-Navarrete, Reference Hernández-Sánchez and De Jesús-Navarrete2010; Cabrera et al., Reference Cabrera, Ramos-Miranda, Salas, Flores-Hernández and Sosa-López2012), none of them linked to sex or maturity.
As holobenthic octopus are more prone to localized impacts than merobenthic species (Narvarte et al., Reference Narvarte, Gonzalez and Fernandes2006; Leporati et al., Reference Leporati, Pecl and Semmens2008), detailed description of their reproductive characteristics and their variations is essential for a successful management (Storero et al., Reference Storero, Narvarte and González2012). This paper deals with the population dynamics of O. maya from six localities of the state of Campeche during five fishing seasons (2005–2009). The aim is to provide basic information on its reproductive traits such as population structure, sex ratios, maturation and size at maturity and evaluate changes with locality and season.
MATERIALS AND METHODS
Octopus maya landing places in Campeche state were visited: Isla Arena, Campeche (including neighbouring Lerma), Seybaplaya, Villamadero (which reports catches at Seybaplaya), Champoton and Sabancuy (Figure 1). The Campeche Bank is a shallow and wide shelf of calcareous sand and lime-mud deposition. The shoreline is marked by unpopulated mangrove swamps between Campeche and Isla Arena, while south of Campeche rocky headlands alternate with sandy beaches (Vokes & Vokes, Reference Vokes and Vokes1983). The soft-bottom subtidal is prominent in extensive Thalassia testudinum seagrass beds, although local particularities regarding environmental properties are unknown.
Octopus landings (>80%) are concentrated in central localities (Campeche to Seybaplaya, Figures 1 & 2). There, illegal fishing methods such as diving with hooks and rudimentary pot lines which may target breeding females have been widespread in recent years. In peripheral localities (Isla Arena and Sabancuy) such illegal practices are virtually unknown.
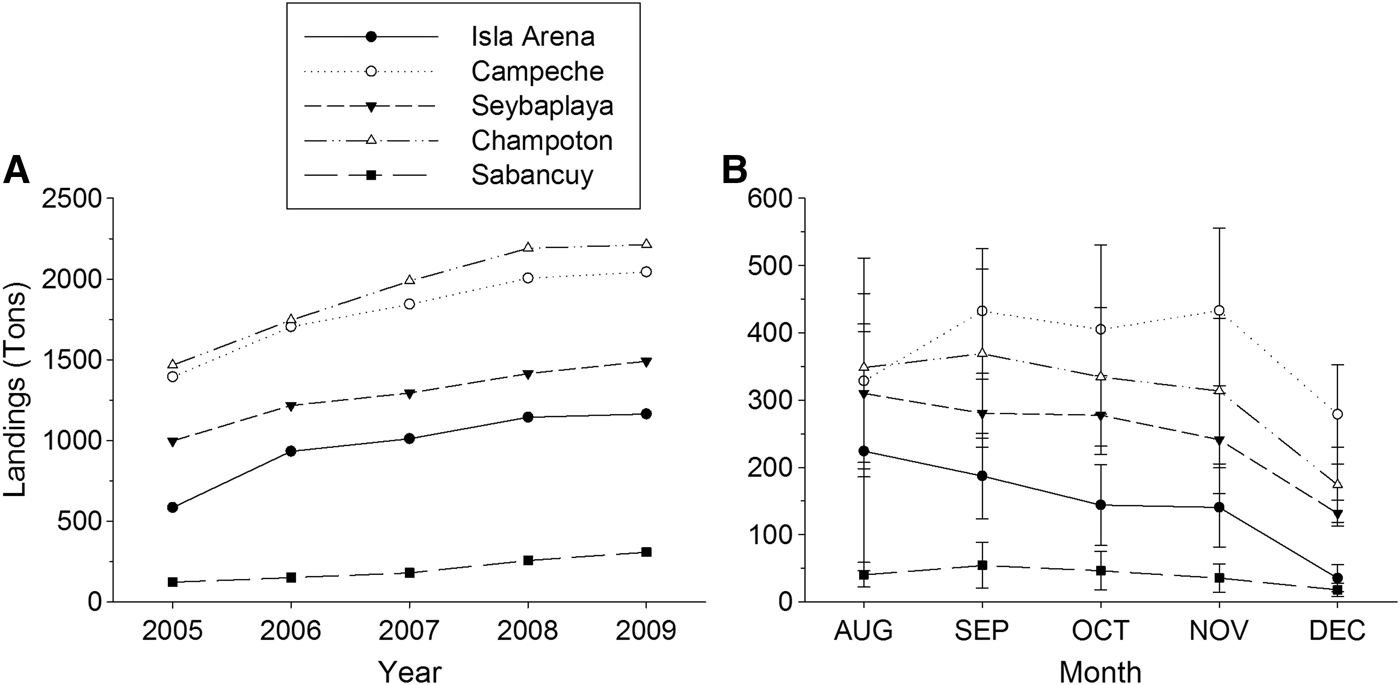
Fig. 2. Octopus maya annual landings (A) and mean monthly catches (B) by locality in Campeche during 2005–2009.
In Campeche state mostly small vessels fish daily for octopus from sunrise to noon or early afternoon. Octopus are landed whole and pooled in factories where they were sampled before eviscerating. In Isla Arena octopus is eviscerated on board, thus sampling for whole octopus was complicated. A monthly sample of 310 individual O. maya were sampled at each landing place during the fishing season (1 August to 15 December) from 2005 to 2009. In 2005 and 2006 some localities were visited twice during the same month (a sample each fortnight). In total 116 monthly samples were analysed (including three samples with ≤21 octopus from Isla Arena) during the five fishing seasons. In addition, octopus taken illegally off season and confiscated by authorities in February 2006 and January–February 2007 (458 individuals) in Campeche and Lerma were sampled. A total of 37,943 octopus were analysed. Isla Arena and Villamadero were irregularly sampled.
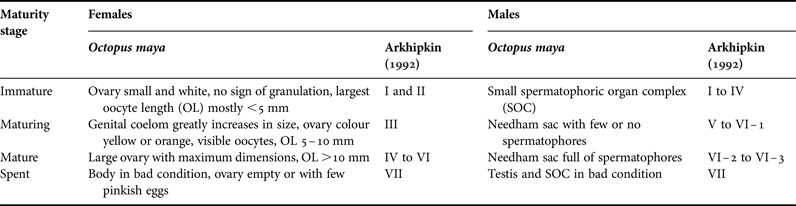
In each octopus, ML to nearest mm was measured. Sex and maturity were assigned upon examination of reproductive organs following a scale of four maturity stages (Mangold, Reference Mangold and Boyle1987; Leporati et al., Reference Leporati, Pecl and Semmens2008; Table 1). Body weight (BW) was also recorded to nearest g in years 2006, 2007 and 2009 (23,344 octopus in 73 monthly samples).
Table 1. Scale of maturity stages for Octopus maya females and males. Equivalences with maturity stages from a more detailed scale by Arkhipkin (Reference Arkhipkin1992) are given.
Sex ratio was calculated for each sample. Significant deviations from the 1:1 proportion were tested performing a χ2 test. Octopus size distributions failed for normality test. Hence, Kruskal–Wallis non-parametric ANOVA and Mann–Whitney U test were performed when comparing size distributions.
ML–BW relationships were fitted to potential model BW = a ML b. Model parameters a and b were compared through an extra-sum-of-squares F test. Octopus size (ML or BW) at maturity (ML50% or BW50%) was estimated for each locality and year after fitting a logistic curve to the relative ML (each 5 mm) or weight (each 100 g) distribution of mature and spent individuals (Leporati et al., Reference Leporati, Pecl and Semmens2008; Lourenço et al., Reference Lourenço, Moreno, Narciso, González and Pereira2012). ML50% or BW50% were compared by season and locality through a two-way ANOVA test.
RESULTS
Octopus maturation during the season
Females were predominantly immature individuals (59%), while 22% were maturing, 16% mature and 3% spent. Females remain immature in most of the fishing season. November accounts for the rapid maturing of females. It is noteworthy that mature and spent females are found, although at low rates, anytime during the fishing season. Mature female frequencies rise from 3% during the first weeks to 46% at the end of the season. Spent females remain low during the season, then rise to 10–24% by December in central localities and they form the bulk in confiscated samples from January and February (Figure 3).
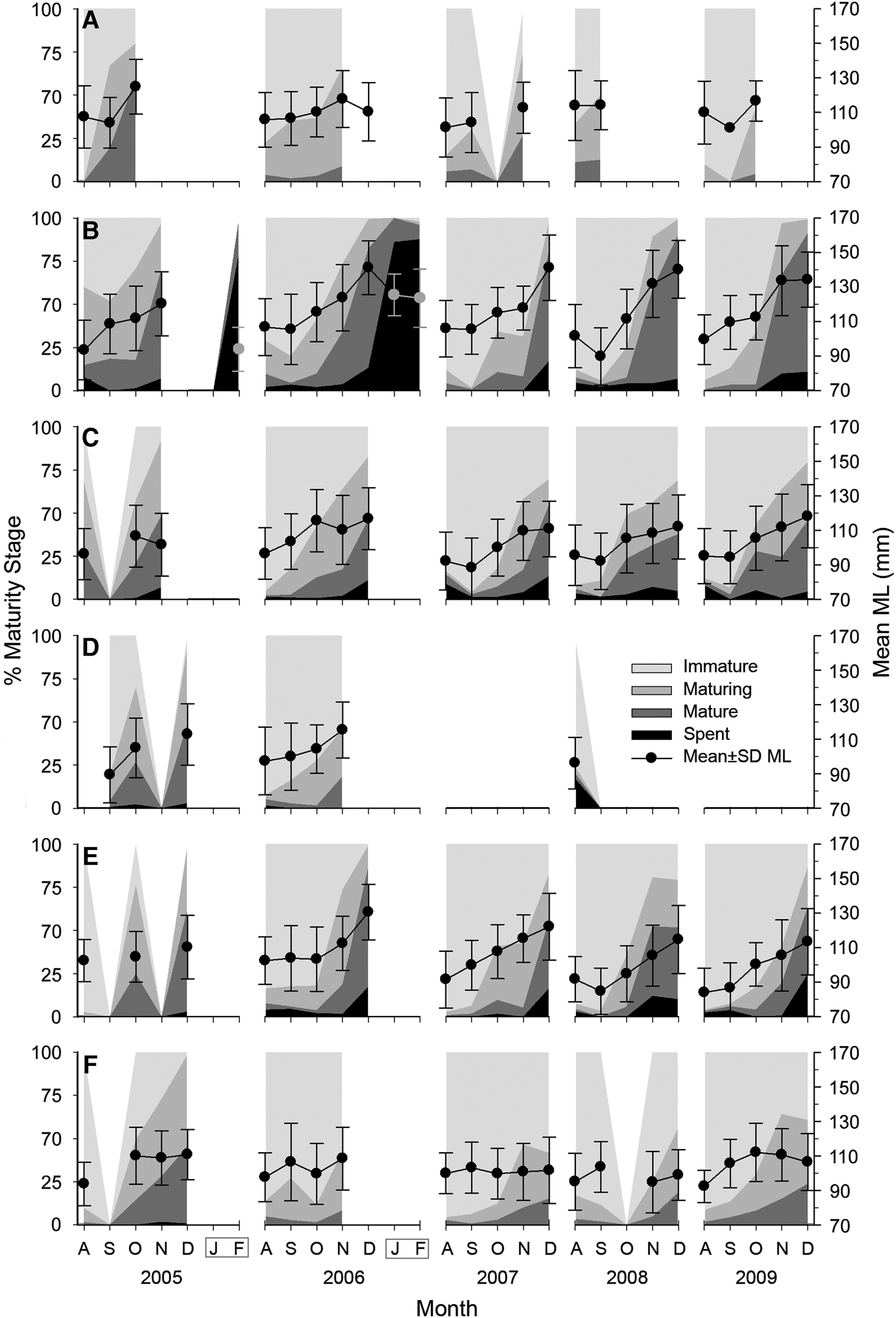
Fig. 3. Monthly relative frequency of maturity stages for female Octopus maya from Campeche by season (August to December) and locality: (A) Isla Arena; (B) Campeche; (C) Seybaplaya; (D) Villamadero; (E) Champoton; (F) Sabancuy. Dots show mean monthly mantle length (ML) and standard deviation (right axis). Note that after season January and February were sampled at Campeche in 2006 and 2007 (grey box).
Maturity stage composition of females varies between localities. Campeche yielded the largest mature and spent percentages in December (ranging 80–90%) followed by Champoton (52–87%), Seybaplaya (37–54%) and Sabancuy (8–45%). Spent females were found only in central localities; three individuals were landed at Sabancuy and none at Isla Arena. Maturity of males did not show such differences (Figure 3).
Males were predominantly mature individuals (65%), while 20% were maturing, 13% immature and 1% spent. They mature earlier in the season than females. At the beginning of the fishing season 40% are already mature and by September most are mature. Most males taken after the season were also spent (Figure 4). Unlike females, male maturity composition evolved quite uniformly along localities.
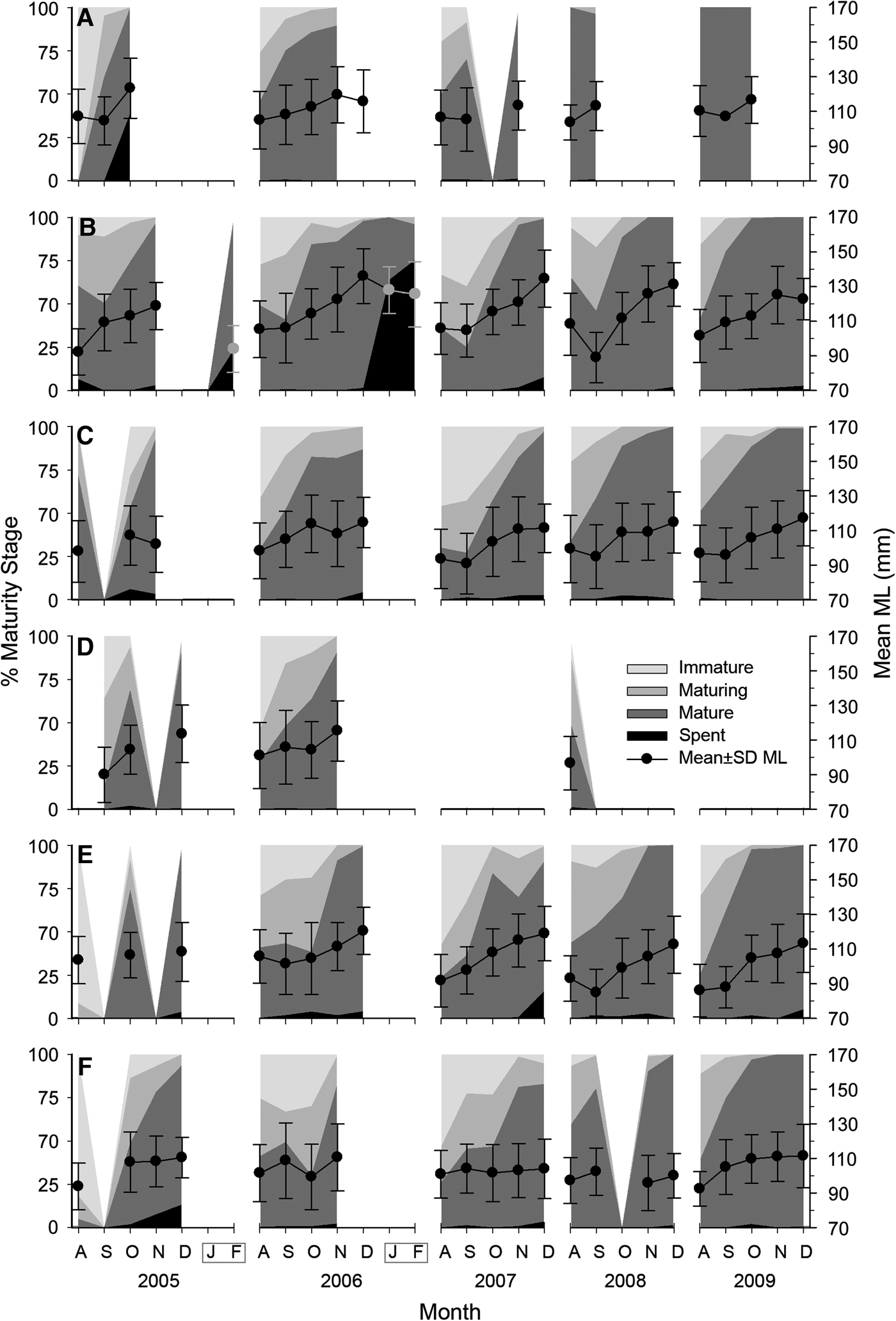
Fig. 4. Monthly relative frequency of maturity stages for male Octopus maya from Campeche by season (August to December) and locality: (A) Isla Arena; (B) Campeche; (C) Seybaplaya; (D) Villamadero; (E) Champoton; (F) Sabancuy. Dots show mean monthly mantle length (ML) and standard deviation (right axis). Note that after season January and February were sampled at Campeche in 2006 and 2007 (grey box).
Sex ratio
Overall female participation was 45% (ranging from 37–55%). In 77 out of 115 monthly samples (67%) sex ratio was not statistically significant from the expected 1:1 (χ2 test, P > 0.05; black triangles, Figure 5). Males dominate in most other samples, particularly in most (70%) of the August months sampled. Females dominated after the fishing season (χ2 test, P < 0.05; white triangles, Figure 5B).
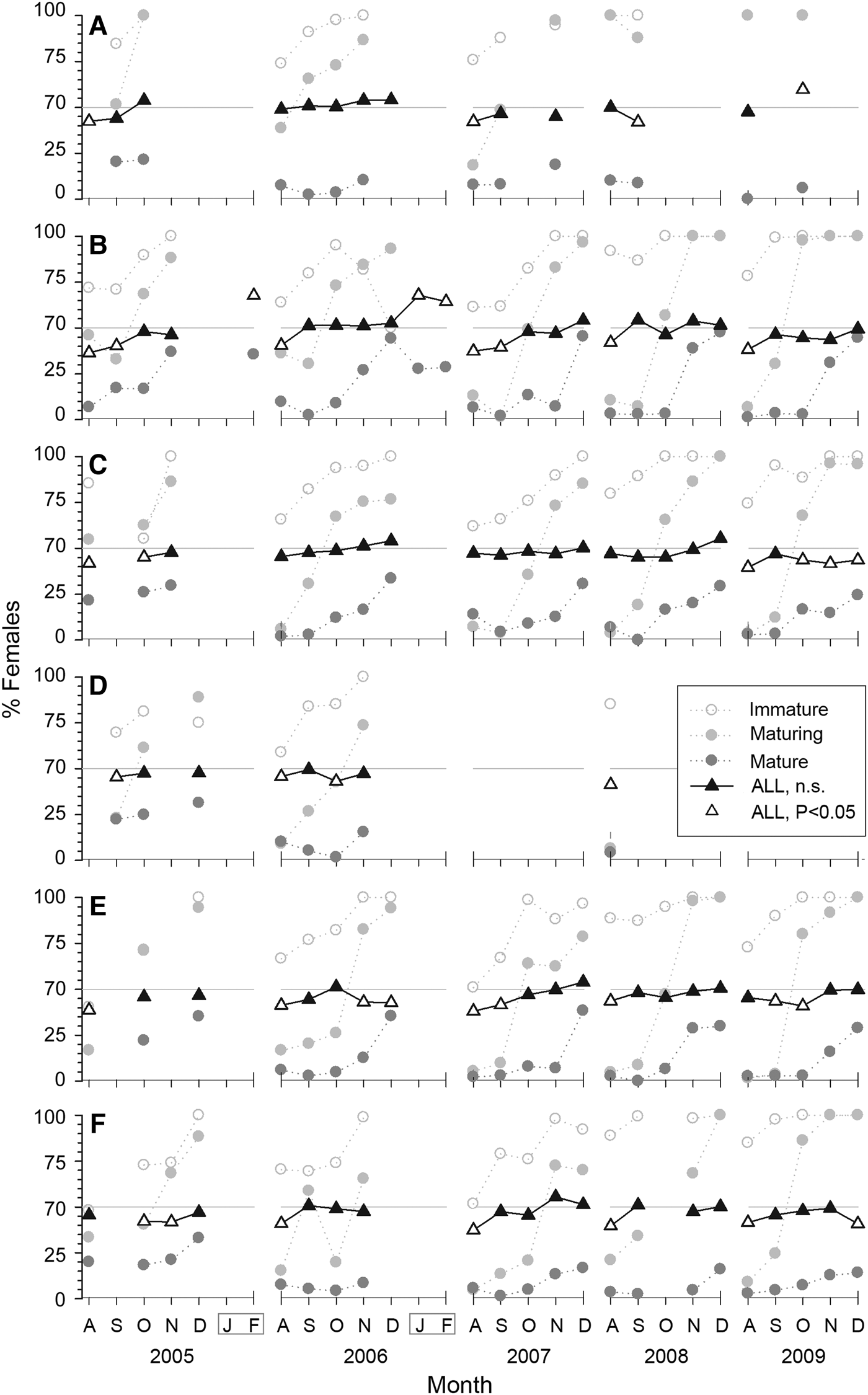
Fig. 5. Monthly sex ratio of Octopus maya by locality and season expressed as percentage of females for each maturity stage and for all octopus (triangles): (A) Isla Arena; (B) Campeche; (C) Seybaplaya; (D) Villamadero; (E) Champoton; (F) Sabancuy. Black triangles denote not significant deviation from the expected 1:1 ratio (shown as a grey horizontal line). White triangles denote significant deviation (P < 0.05).
Sex ratio in octopus varied with size. Octopus >800 g BW were significantly dominated by males (P < 0.05) as female participation in samples progressively decreased with weight. Sex ratio by ML however revealed the opposite trend. It was skewed towards males in the 95–135 mm ML range, while females dominated in octopus > 145 mm ML (P < 0.05) (Figure 6).
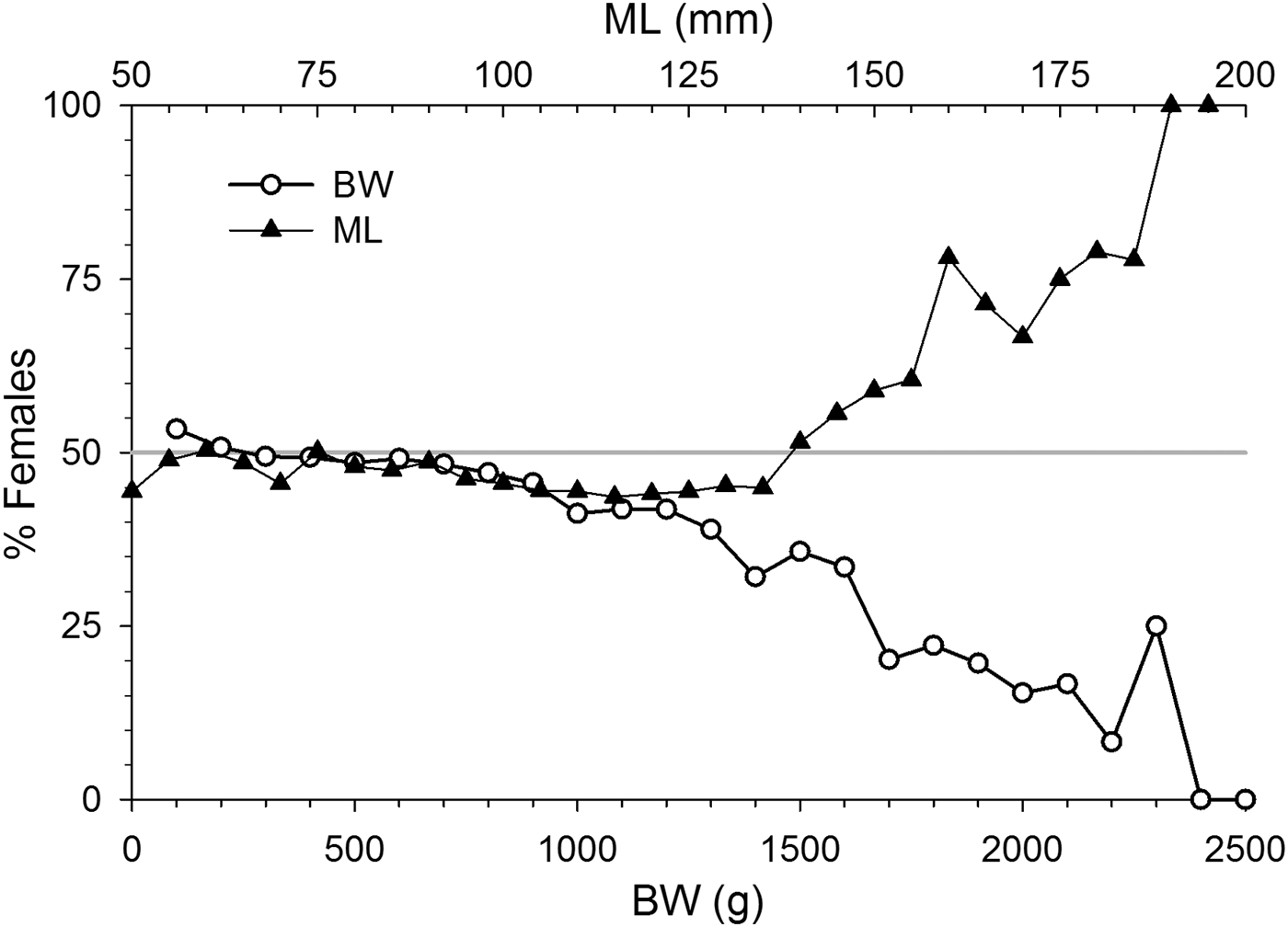
Fig. 6. Octopus maya sex ratio as female % by 100 g of body weight (BW, lower axis) and by 5 mm mantle length (ML, upper axis) intervals.
ML–BW relationships
Morphometric relationships between ML and BW are:
For females: BW = 0.033 ML2.11, r 2 = 0.74, N = 10,877
For males: BW = 0.0074 ML2.45, r 2 = 0.73, N = 12,399
Relationships between sexes showed statistical differences in a and b parameters (F 2,23270 = 1077, P < 0.001). Growth is allometricaly negative (b < 3) for both sexes (females, F 1,10874 = 5404; males F 1,12396 = 1590; P < 0.001).
Size structure
Octopus maya size in catches ranged from 50 to 200 mm in ML and 80 to >2300 g in BW. Largest female was 2330 g BW and five males were larger, up to 2625 g BW (all mature). Overall mean ML was 106 mm and no differences were found between sexes (Mann–Whitney U test, P > 0.05). Only 20 out of 123 monthly samples showed differences (Figures 3 and 4). Regarding BW, males were overall heavier than females (mean BW: 739 vs 675 g, Mann–Whitney U test, P < 0.01). Most of the samples (75%) from November and December showed this difference (Figure 7).
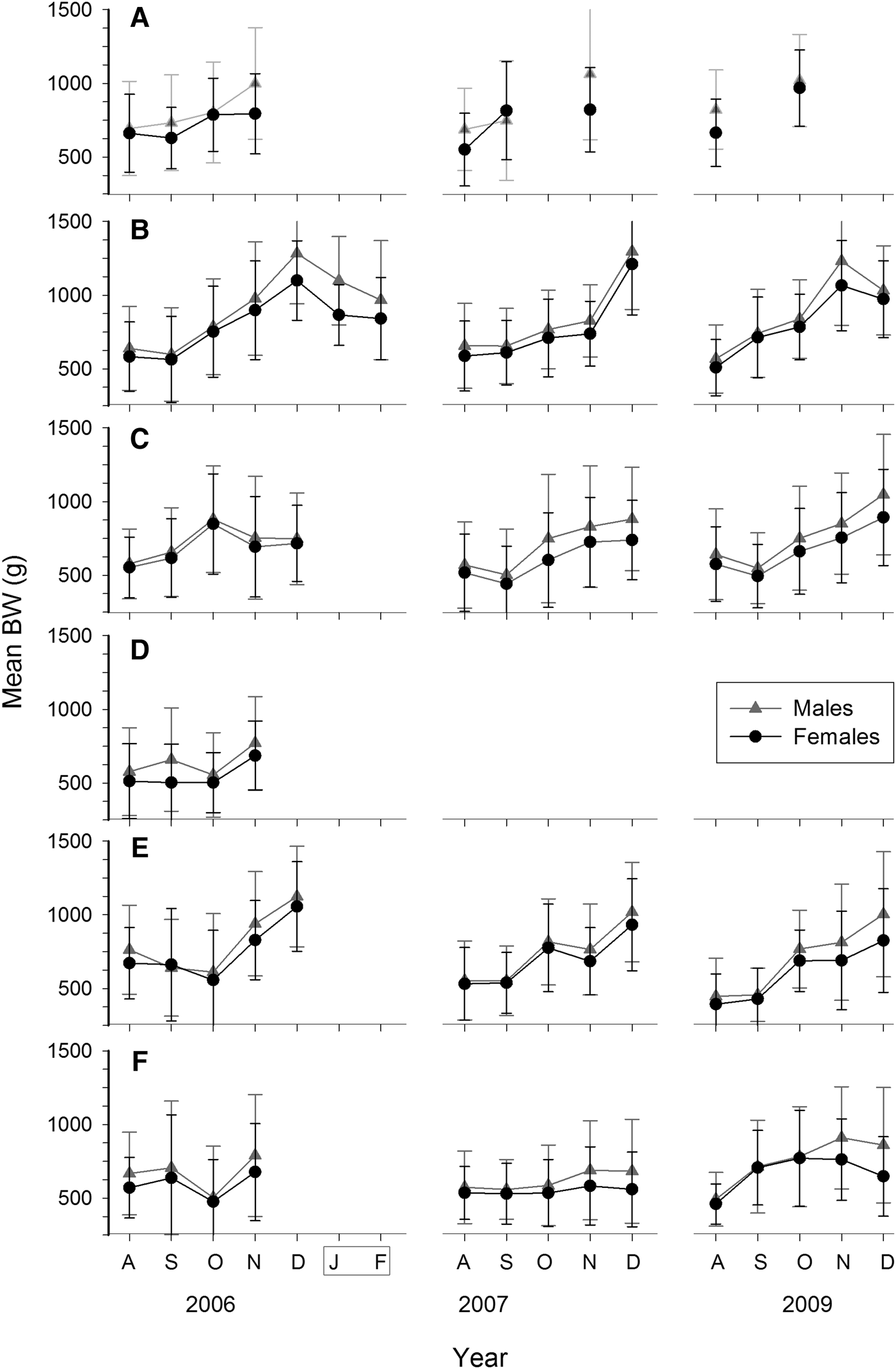
Fig. 7. Monthly mean body weight (BW) ± SD for Octopus maya by sex, season (August to December) and locality: (A) Isla Arena; (B) Campeche; (C) Seybaplaya; (D) Villamadero; (E) Champoton; (F) Sabancuy. Note that after season January and February were sampled at Campeche in 2007 (grey box).
Temporal structure
A gradual increase in octopus size is evident during the fishing season (Figure 7; Mann–Whitney U test between consecutive months, P < 0.05). However, growth was not evident during the first two months in most seasons (Mann–Whitney U test, P > 0.05). Growth is also not evident in females from Sabancuy in 2007 (Figure 7F), where no differences were found for mean BW among the five months (K–W non parametric ANOVA, N = 720, χ2 = 3.0, d.f. = 4, P > 0.05) as a result of the low presence of mature, large females in catches. Samples confiscated after the season show substantial body reduction as they are dominated by spent individuals (Figure 7B).
Inter-annual variability in octopus dimensions (ML or weight) was high, with significant differences between years of any month in the same locality and sex (K–W non-parametric ANOVA, P < 0.05). Exceptions were few. Both sexes from Sabancuy during September showed no inter-annual differences in ML among the five years (K–W non-parametric ANOVA, P > 0.05; Figures 3 and 4).
Spatial structure
Significant differences in mean BW were found between localities in each month of the three seasons (K–W non-parametric ANOVA, P < 0.05; Figure 7). The pattern is similar for both sexes, although different in each season. Isla Arena yielded the largest means in BW in any month it was sampled. Campeche followed next, with larger means than the remaining localities in most months, mainly in December. No consistent pattern was found among the southern localities. Octopus from Sabancuy showed the lowest monthly means except in 2009, when they were larger than both Seybaplaya and Champoton (Figure 7). Exclusion of mature females to remove the effect of the fishing gear when comparing these monthly mean BWs did not alter that pattern (not shown).
Most (58%) octopus measured in catches were under the legal size (110 mm ML). The sublegal size octopus share changed between localities. Isla Arena and Campeche showed 51 and 49% respectively. At Seybaplaya, Villamadero and Champoton they ranged 61–65% and at Sabancuy they raised to 67%.
Size at maturity
Octopus maya matures at a wide range of body size. Mature and spent individuals can be found along the size interval taken in commercial catches, 45–60 to 200 mm ML or 140–180 g to 2500 g BW (Figure 8). Almost all females (90%) were mature at 160 mm ML and 2000 g BW. Males (95%) were all mature at 130 mm ML and 1000 g BW (Figure 9).
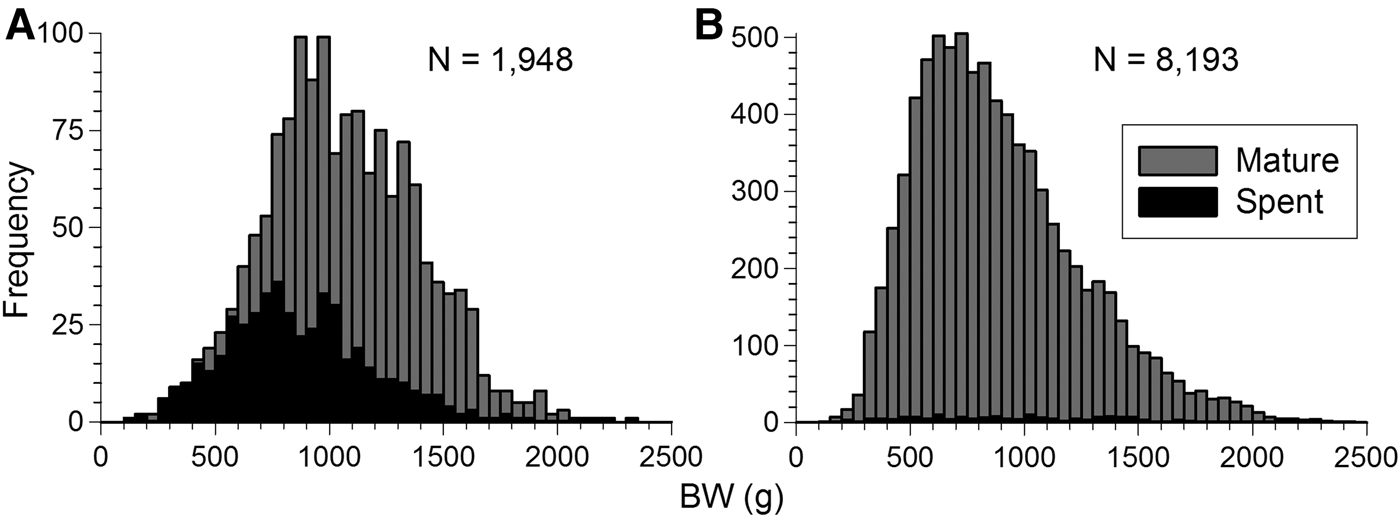
Fig. 8. Body weight (BW) distribution of mature and spent Octopus maya from Campeche in 2006, 2007 and 2009 for (A) females and (B) males.

Fig. 9. Octopus maya maturity stage cumulative relative frequencies by 5 mm mantle length (ML) class (left) and by 100 g body weight (BW) class (right) for females (A–D) and males (E–H) from Campeche (A–B, E–F) and Sabancuy (C–D, G–H) during 2007. White logistic curves were fitted to mature plus spent frequencies. White arrows show size at maturity. Blank areas denote no data.
No reliable fitting could be done to calculate size at maturity for females from Isla Arena, Villamadero and Sabancuy due to lack of mature females in catches. Figure 9 shows an example of curve fitting for Campeche and Sabancuy in 2007. Males mature at a smaller size than females. ML50% ranged 117–133 mm for females and 77–100 mm for males. BW50% ranged 931–1238 g in females and 331–613 g in males (Table 2). Inter-annual differences for both parameters were significant in males, while differences between localities were not. For females no differences were found in any case. In order to provide single figures of size at maturity by sex and dimension, we suggest the arithmetic means shown in Table 2.
Table 2. Octopus maya size at maturity for mantle length (ML 50% ) in mm and body weight (BW 50% ) in grams by sex, landing localities and year in Campeche.
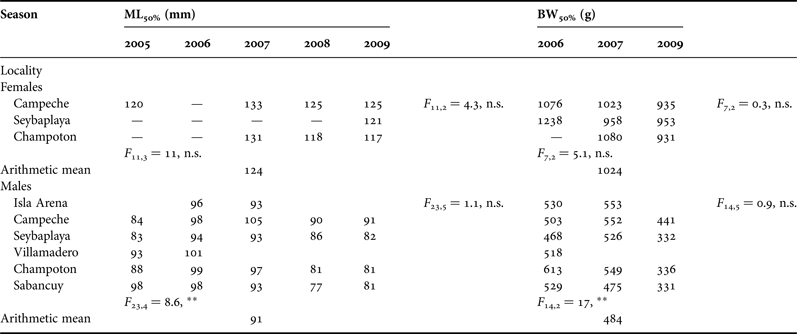
In several cases including females from Isla Arena, Villamadero and Sabancuy no ogives could be fitted (filled with a bar). Results of ANOVA test between fishing seasons and localities are shown for each sex and dimension.
**Very significant (P < 0.01).
n.s., not significant (P > 0.05).
DISCUSSION
Octopus maya grows and gradually matures during the fishing season from August to December. Spawning peak starts in the first half of December, as suggested by the incremental share of spent females. They totally dominated in January and February. Solís-Ramírez (Reference Solís-Ramírez, Lang and Hochberg1997, Reference Solís-Ramírez1998) described a similar seasonal life cycle for O. maya in which mating occurs in September and October, spawning in November and December, and hatching in January and February. This pattern would correspond to a year-long life cycle, as is commonly found in octopods (Mangold, Reference Mangold and Boyle1987).
Decreasing landings along the fishing season (Figure 2B) coupled with an increasing mean octopus size (Figures 3, 4 & 7) support this pattern. Although this study was limited to the fishing season, abundance and biomass of octopus off fishing season is known to be quite low, precluding any profitable fishery. Landing data for 1965–1969, before the current seasonal closure, reveal that only 2.5% of the annual catch was made from January to June (Solís-Ramírez, Reference Solís-Ramírez1962, Reference Solís-Ramírez1998). A recent study revealed negligible CPUEs during the closed season of 2013 (Gamboa-Álvarez et al., Reference Gamboa-Álvarez, López-Rocha and Poot-López2015). Our interpretation is that most of the octopus population during the closed season are juveniles or pre-recruits which follow the main reproductive peak. Thus the seasonal ban established for O. maya since 1984 just follows the fishing period settled long before (July to December), but it is important since it protects breeding females and juveniles that follow the main, seasonal cycle described above. Information currently lacking on O. maya juveniles' abundance and distribution is needed to complement this inference.
Superimposed to this seasonal, discrete reproductive pattern (or optimum spawning period; Leporati et al., Reference Leporati, Pecl and Semmens2008), is an extended low intensity trend in all months sampled. Presence of a few mature and spawning females during all the fishing season suggest that a small portion of the population does not follow the reproductive seasonality, and might be spawning throughout the year. Octopus size distribution from April to July show individuals 90–150 mm ML (Arreguín-Sánchez et al., Reference Arreguín-Sánchez, Solís-Ramírez and González de la Rosa2000) and divers claim that sizeable octopus is available all year around, although their smaller abundances off season do not sustain a fishery. Spawning along the year is a common feature in other octopus, as restriction of spawning to a short period could have disastrous consequences for the population (Boyle & Rodhouse, Reference Boyle and Rodhouse2005; Cortez et al., Reference Cortez, Castro and Guerra1995). Thus the O. maya spawning pattern would be reclassified from a strictly seasonal (Mangold, Reference Mangold and Boyle1987) to an extended seasonality, probably year round, with a dominant peak of spawning activity which sustains the fishery. The timing of the spawning peak in December deserves further attention on environmental variables or species constrains.
Mature and spent females cease feeding and shelter to spawn, thus they are less likely to be caught in traps and trawls (Wells & Wells, Reference Wells, Wells, Giese and Pearse1977). For that reason, octopus taken using lines baited with crabs would not be representative of the O. maya population during the spawning season (Van Heukelem, Reference Van Heukelem1976, Reference Van Heukelem and Boyle1983). In peripheral localities as Isla Arena and Sabancuy, where fishing effort and catches are smaller, absence of other fishing practices account for few mature females and a lack of spent females in catches. This fact precluded to estimate female size at maturity. This also causes fishermen to stop fishing octopus by December (Figure 2B) as they switch to other marine resources, so the season is not completely sampled at those places.
Illegal practices in the central localities from Campeche to Champoton such as spear diving or pots is readily revealed by the presence of mature and spent octopus in catches. These practices also fish a larger share of sublegal size octopus (Cabrera et al., Reference Cabrera, Ramos-Miranda, Salas, Flores-Hernández and Sosa-López2012), as crabs used as bait in the legal fishery are large enough to prevent smaller octopus from attacking them. In these localities with largest catches and fishing effort the octopus season is completely fished. Thus catches taken by diving are less selective and they would better represent the population than those taken with baited lines alone. Nowadays, it is difficult to assess gear selectivity as catches taken by different gears are pooled together at landing facilities.
It is remarkable that no shifts from the expected 1:1 sex ratio were found in most O. maya samples. Surprisingly, samples from August were mainly dominated by males, when no fishing gear bias is expected to occur as mature females are scarce early in the season. The equal sex ratio was maintained even in localities without illegal fishing, where it was expected to shift toward males. In other octopus species, skewed sex ratios in catches are common and they may reflect gender differential conduct as migration, feeding, female brooding behaviour or postspawning mortality, all depending on the fishing gear (Mangold, Reference Mangold and Boyle1983; Hartwick et al., Reference Hartwick, Ambrose and Robinson1984; Smith et al., Reference Smith, Groeneveld and Maharaj2006; Raberinary & Benbow, Reference Raberinary and Benbow2012). All the same, equal sex ratios are not uncommon in catches taken non-selectively with spears (Raberinary & Benbow, Reference Raberinary and Benbow2012). Our unusual finding could be due to the fact that the life cycle of this shallow-water octopus is completely undertaken inside fishing grounds. Mature and spent octopus are readily available to divers, unlike other octopus fisheries where spawning females migrate out of fishing grounds (Hatanaka, Reference Hatanaka1979; Raberinary & Benbow, Reference Raberinary and Benbow2012).
Reproductive features of O. maya follow those known for other octopods. The maturation process is clearly distinct between sexes, as is generally known for other species (Mangold, Reference Mangold and Boyle1987; Smith et al., Reference Smith, Groeneveld and Maharaj2006; Leporati et al., Reference Leporati, Pecl and Semmens2008). Octopus males mature earlier and remain mature over a larger part of their life (Grubert & Wadley, Reference Grubert and Wadley2000; Smith et al., Reference Smith, Groeneveld and Maharaj2006; Avila-Poveda et al., Reference Avila-Poveda, Colin-Flores and Rosas2009), dominating over immature and maturing individuals in catches and being ready to mate during all the season. Immature females dominate in landings since they mature later, although they may mate (Mangold, Reference Mangold and Boyle1983, Reference Mangold and Boyle1987). Both sex are subequal in size, as males reach larger sizes and dominate between larger octopus, probably because they grow faster or live longer (Hatanaka, Reference Hatanaka1979; Mangold, Reference Mangold and Boyle1983; Smith et al., Reference Smith, Groeneveld and Maharaj2006). Larger MLs reached by females, however, are attributed by us to the fact that hypertrophy of reproductive organs in large, mature females distended their mantle. Females otherwise face considerable BW reduction at the end of their life due to spawning and loss of muscle in the absence of feeding (Mangold, Reference Mangold and Boyle1983; Leporati et al., Reference Leporati, Pecl and Semmens2008).
Important variability in O. maya size was found between different years for the same month and locality, as well as between localities. Octopus raised in captivity showed that temperature and food availability control final sizes (Van Heukelem, Reference Van Heukelem1976), so environmental variables could be responsible for geographic and inter-annual differences. It has recently been suggested that cephalopod populations are affected by environmental conditions rather than by fishing (Quetglas et al., Reference Quetglas, Rueda, Alvarez-Berastegui, Guijarro and Massutí2016).
A large range in size at maturity is a common feature in octopus, even among O. maya raised in captivity under similar conditions (Van Heukelem, Reference Van Heukelem1976). Although no statistical differences were observed for female size at maturity, difference in BW50% between years for the same locality may be as large as 26% (Table 2). Geographic differences have been observed for size at maturity in other octopus species (Carvalho & Sousa Reis, Reference Carvalho and Sousa Reis2003; Lourenço et al., Reference Lourenço, Moreno, Narciso, González and Pereira2012; Storero et al., Reference Storero, Narvarte and González2012). Inter-annual differences in this trait however had never been tested before to our knowledge. Our finding that size at maturity may vary more between seasons than between localities raise concerns about conclusions from many other octopus studies. Recognition of this variability emphasizes the need to avoid small sample sizes based on a single season and/or locality or pooling data from different seasons as it is customary in most octopus fishery biology studies in order to establish fishing regulations.
If management is intended to protect spawning females (Fernández-Rueda & García-Flórez, Reference Fernández-Rueda and García-Flórez2007), actual minimum legal size of 110 mm ML falls 14 mm short of our arithmetic mean ML50% estimated for females, 124 mm ML (Table 2). All the same, our five years of sampling show that most catches fall under the current legal size anyway. On the other hand, customary commercial minimum gutted BW for O. maya exports to the European Union is 450 g (OJ, 2005), which according to Solís-Ramírez (Reference Solís-Ramírez1988) matches the BW corresponding to the current minimum legal ML. However, this commercial gutted BW is actually 50% smaller than our estimation (non-gutted) of 1024 g for female BW50% (Table 2), raising concern on established regulations and uses.
ACKNOWLEDGEMENTS
We are greatly indebted to numerous octopus fishermen and octopus facilities who kindly allowed us to sample their catch, especially Pololo, Uribe, Chicopanes, Pancho Maco, Sanchez family, Ganzo, Arceo and Narvaez, among others. Students from Universidad Autónoma de Campeche, Instituto Tecnológico de Lerma and ECOSUR are acknowledged for their enthusiastic participation during sampling.
FINANCIAL SUPPORT
This study was funded by CONACyT (Consejo Nacional de Ciencia y Tecnología) FOMIX grants CAMP-2005-C01-040, CAMP-2007-C01-71844 and YUC-2008-C06-108675.













