Introduction
The ampithoid genus Sunamphitoe Bate, 1857 is known to be comprised of large-bodied herbivorous amphipods often associated with brown algae, specifically kelps (Poore et al., Reference Poore, Hill and Sotka2008; Peart, Reference Peart2017). The genus was recently verified to be a senior synonym of Peramphithoe Conlan & Bousfield, Reference Conlan and Bousfield1982, by both morphological and molecular phylogenetic analyses (Peart & Ahyong, Reference Peart and Ahyong2016; Sotka et al., Reference Sotka, Bell, Hughes, Lowry and Poore2016). With the species transferred from Peramphithoe (Peart & Ahyong, Reference Peart and Ahyong2016) and also with subsequently described species (Peart, Reference Peart2017; Griffiths, Reference Griffiths2019), the genus Sunamphitoe is currently represented by 38 species. Among them, four species have been documented from Japan (Ishimaru, Reference Ishimaru1994): S. orientalis (Dana, 1853), S. pelagica (Milne-Edward, 1830), S. plumosa Stephensen, 1944 and S. tea (Barnard, 1965).
An extremely large-bodied Sunamphitoe species (maximum length, more than 40 mm) was found on the surface of a kelp Saccharina longissima which was collected by the second author from Katsurakoi, south-east coast of Hokkaido, Japan. In this paper, we herein describe and illustrate the species as Sunamphitoe gigantea sp. nov. Additionally, nucleotide sequences of mitochondrial cytochrome c oxidase subunit I (COI) obtained from the type specimens were also provided for DNA barcoding. Phylogenetic analysis for COI sequences of Sunamphitoe species was also carried out.
Materials and methods
Sampling
Sampling was carried out at Katsurakoi, south-east coast of Hokkaido, Japan (Figure 1; 42°56′56.1″N 144°26′31.1″E) from April to November in 2017. The specimens were collected from the surface of Saccharina longissima fronds obtained from subtidal kelp beds (<3 m deep) by snorkelling.

Fig. 1. Map of Japan showing the type locality (indicated by the black star) of Sunamphitoe gigantea sp. nov.
Morphological observation
Body length was measured from the tip of rostrum along the dorsal margin to the posterior margin of telson (measurements were done on the curved body). Specimens were dissected under a binocular stereomicroscope, and appendages were fixed on slide mounts with Hoyer's medium. A part of pleopods were stored in 99% ethanol for subsequent DNA analysis. Observations and line drawings were made by using a light microscope and a binocular stereomicroscope with aid of drawing tube. All the specimens examined in this study were deposited in the National Museum of Nature and Science, Tokyo (NSMT).
DNA extraction and COI sequencing
The following DNA extraction and COI sequencing were carried out at the Bioengineering Lab. Co., Ltd (Kanagawa, Japan). Genomic DNA was extracted from a part of pleopods of seven specimens (including the holotype and the allotype) by using Lysis buffer for PCR (TaKaRa). The target sequences of COI were amplified using the method of two-step tailed PCR procedure for library preparations. This two-step tailed PCR method consists of first-stage and second-stage PCRs. In the first-stage PCR, gene specific amplification is performed. In the second-stage PCR, index-sequences (for sample identification) and adapter-sequences (for subsequent sequencing) are added. The first-stage and second-stage PCR primers are shown in Table 1. The first-stage PCR was carried out with two kinds of primer sets (see Table 1): for each, a total volume of 20.0 µl containing 1.0 µl of template DNA (of which the concentration was not standardized), 0.5 µl of each primer (each 10.0 µM), 10.0 µl of 2× Gflex PCR Buffer, 0.4 µl of Tks Gflex (TaKaRa) and 7.6 µl of deuterium depleted water (DDW). The first-stage PCR conditions were as follows: the initial denaturing step was set at 94°C for 1 min; followed by 35 cycles of 10 s at 98°C, 15 s at 52°C and 30 s at 68°C; the final elongation was set for 5 min at 68°C. The second stage of PCR was carried out in a total volume of 10.0 µl containing 2.0 µl of mixture of first-stage PCR products performed with two kinds of primer set, 1.0 µl of 10 × Ex Buffer, 0.8 µl of dNTPs (each 2.5 mM), 0.5 µl of each primer (each 10.0 µM), 0.1 µl of Ex Taq (5 U µl−1) (TaKaRa), and 5.1 µl of DDW. The programme of amplification for the second-stage PCR was 94°C for 2 min, 12 cycles (94°C for 30 s, 60°C for 30 s, 72°C for 30 s) and 72°C for 5 min. Concentration of the prepared amplicon libraries was evaluated using Synergy H1 (BioTek) and QuantiFluor dsDNA System (Promega). Quality of the library was evaluated using Fragment Analyzer and dsDNA 915 Reagent Kit (Advanced Analytical Technologies). The amplified products were then sequenced on an MiSeq (Illumina) 2 × 300 bp platform.
Table 1. Primer sets used for the first-stage and second-stage PCR. The first-stage PCR was carried out with two kinds of primer sets (LCO1490/HCO2049 and IntF/HCOmR)

After sequencing, the sequences with start regions which completely matched with the primer sequences were extracted, and then the primer sequences were removed by using the fastq_barcode_splitter in the Fastx Toolkit. Low-quality sequences (values <20) were removed. Short sequences (length <40 bp) and their paired sequences were discarded by using the Sickle Tools.
Target sequence of COI was divided into the first and the second half. For both first and second halves of target COI sequences, the processed sequences were merged by using the Paired-end merge script FLASH. The merged sequences having a high frequency were selected for both first and second half of COI sequences, and then these two sequences were combined into one total target sequence of COI by using CAP 3. All the obtained COI sequences were deposited into the International Nucleotide Sequence Database Collaboration (INSDC) through the DNA Data Bank of Japan.
Phylogenetic analysis
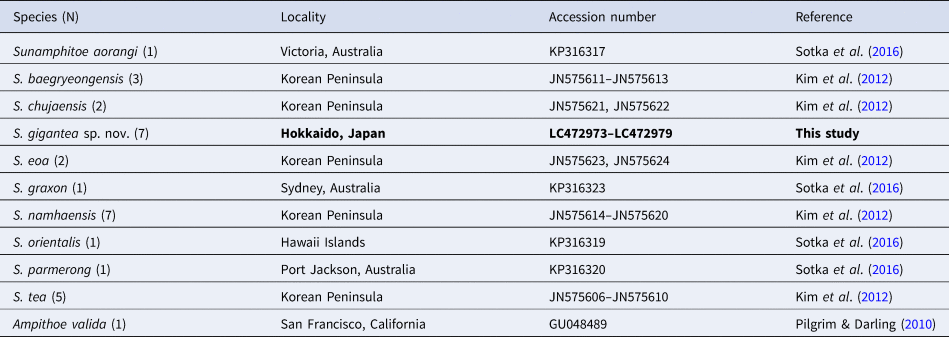
A phylogenetic analysis was conducted by using MEGA 7.0 software (Kumar et al., Reference Kumar, Stecher and Tamura2016). In addition to the COI sequence of our materials, COI sequences of other Sunamphitoe spp. retrieved from INSDC were also used for the analysis (Table 2). All sequences were aligned using Clustal W (Thompson et al., Reference Thompson, Higgins and Gibson1994). Nucleotide sequence divergences within and between species were calculated using Kimura 2-parameter distances. The maximum likelihood method was used to construct a tree. The strength of clade support was assessed with bootstrap resampling with 1000 replicates (Felsenstein, Reference Felsenstein1985). Ampithoe valida COI sequence (INSDC accession number: GU048489) was chosen as an outgroup.
Table 2. List of the species, their localities and INSDC accession numbers used for the genetic analysis
Results
SYSTEMATICS
Order Amphipoda Latreille, 1816 Family Ampithoidae Boeck, 1871 Genus Sunamphitoe Bate, 1857 [Japanese name: Nise-hige-naga-yokoebi-zoku] Species Sunamphitoe gigantea sp. nov. [New Japanese name: Oni-hige-naga]
TYPE MATERIAL
All the specimens were collected from the surface of fronds of Saccharina longissima, which grew naturally in the rocky shore at Katsurakoi, Kushiro, Hokkaido, Japan (42°56′56.1″N 144°26′31.1″E), by T. Onitsuka and A. Ito.
Holotype: 1 male 39.3 mm (NSMT-Cr 26733, INSDC LC472973), 19 October 2017.
Allotype: 1 female, 33.6 mm, NSMT-Cr 26734, INSDC LC472974, same date as holotype (note: 13 juveniles were also collected from the same nest).
Paratypes: 1 male, 25.5 mm, NSMT-Cr 26735, INSDC LC472976; 1 ovigerous female, 38.6 mm, NSMT-Cr 26736, INSDC LC472975 (note: NSMT-Cr 26735 and 26736 were collected from a single nest); 1 ovigerous female, 36.3 mm, NSMT-Cr 26737, 24 April 2017. – 1 male, 34.6 mm, NSMT-Cr 26738, INSDC LC472978; 1 ovigerous female, 42.6 mm, NSMT-Cr 26739, INSDC LC472979 (note: NSMT-Cr 26738 and 26739 were collected from a single nest); 1 female, 38.9 mm, NSMT-Cr 26740 (note: 82 juveniles were also collected from the same nest); 1 ovigerous female, 40.6 mm, NSMT-Cr 26741, 29 May 2017. – 1 male, 25.3 mm, NSMT-Cr 26742, INSDC LC472977, 17 November 2017.
DIAGNOSIS
Body very large, maximum length of more than 40 mm. Antenna 1 flagellum less than 2 times length of peduncle. Antenna 2 slender; flagellum without dense plumose setae on ventral margin. Mandibular palp present, with 3 articles. Maxilla 1 inner plate with 2 slender setae. Maxilla 2 inner plate narrow, outer plate broader than inner plate. Male gnathopod 2 enlarged; propodus, palm straight, not clearly defined, without distinct protrusion, with dense setae on posteroproximal corner; dactylus reaching to posterodistal angle of propodus, not beyond carpus. Female gnathopod 2 much smaller than that of male; propodus palm well defined, with spine. Pereopods 3 and 4 bases, anterior margin with group of long setae subproximally. Pereopods 5–7 not enlarged; basis, posterodistal lobe absent or indistinct, not reaching ischium; merus, carpus not expanded. Uropod 3 peduncle about 3 times as long as rami. Telson, posterior margin rounded, not acute.
DESCRIPTION OF MALE
Based on holotype male, 39.3 mm (NSMT-Cr26733). Body (Figure 2A) very large, laterally compressed, smooth on surface.
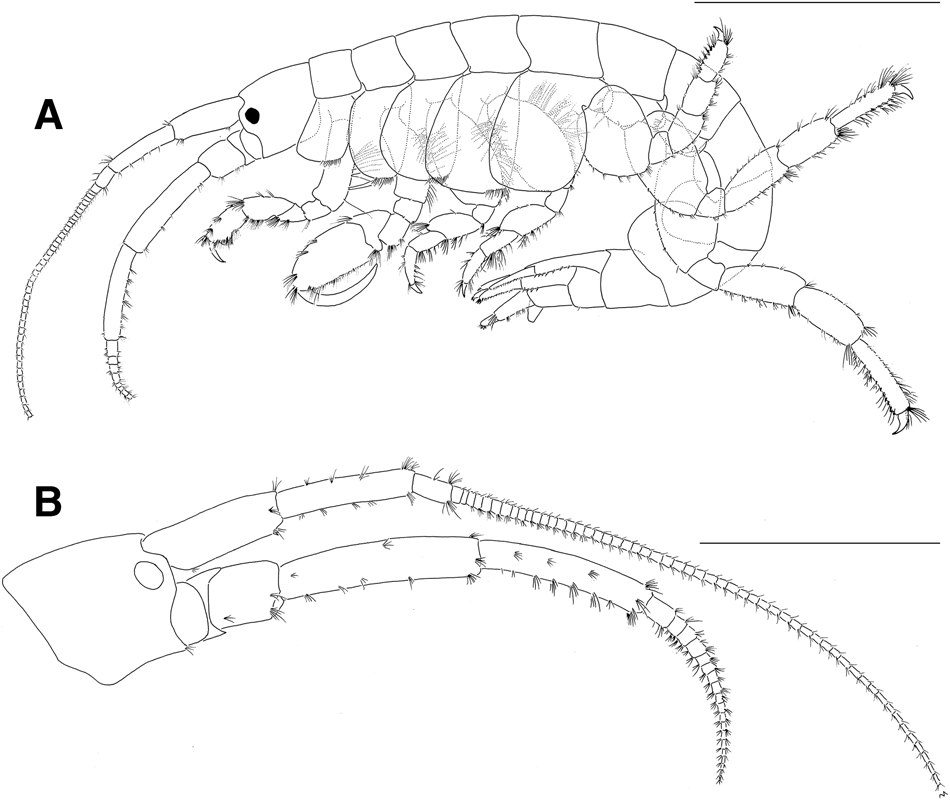
Fig. 2. Sunamphitoe gigantea sp. nov., holotype male, 39.3 mm (NSMT-Cr 26733). (A) habitus (setae partly omitted; coxal gills and pleopods omitted), lateral view; (B) head, lateral view. Scale bar: A, 10.0 mm; B, 5.0 mm.
Head
Head (Figure 2B) about 1.7 times as long as pereonite 1; rostrum indistinct; lateral cephalic lobes weakly angular, truncated, not rounded distally; eyes small, rounded or oval. Antenna 1 slender, weakly setose; length ratio of peduncular articles 1–3 about 34:33:10, article 1 with 2 small spine distoventrally; flagellum longer than peduncle, but less than 2 times length of peduncle; accessory flagellum absent. Antenna 2 slender, setose, shorter than antenna 1; length ratio of peduncular articles 3–5 about 7:20:17; flagellum subequal to peduncular article 5 in length, with 8 articles in left antenna 2 (broken distally), with 19 articles in right antenna 2 (intact), without dense plumose setae on ventral margin.
Mouth parts. Upper lip (Figure 3A) normal, setulose ventrally, posteriorly. Lower lip (Figure 3B) normal, setulose; outer plate deeply notched, lateral lobe longer than medial lobe, mandibular process developed, curved. Mandible (Figures 2 & 3C1): palp 3-articulated, article 1 longer than wide, unarmed, article 2 slightly longer than article 3, with several slender setae distomedially, article 3 tapering on distal half, with dense setae on distomedial margin; left, right incisors 8-, 9-dentate, respectively; left laciniae mobiles 8-dentate, right laciniae mobiles many dentate, dentation on right laciniae mobiles smaller than that of left laciniae mobiles; left, right accessory setal rows including 17, 16 setae, respectively; molar well developed. Maxilla 1 (Figure 3D): palp 2-articulated, article 1 short, unarmed, article 2 incurved, beyond outer plate, with 13 spines apically, ventral surface with several slender setae subdistally; outer plate truncated, with several simple spines, pectinate spines on distal margin; inner plate small, with 2 serrulate spines on subapically medial margin. Maxilla 2 (Figure 3E): outer plate broader than inner plate, slightly longer than inner plate, with dense setae distolaterally, distally, distomedially; inner plate with dense setae medially to distally. Maxilliped (Figure 3F): palp 4-articulate, article 4 medially covered with spinules, unguis acute, well developed; outer plate subovate, extending beyond distal end of palp article 2, with row of teethed spines medially to distally, serrulate setae on distal half of lateral margin; inner plate developed, with serrulate setae medially to distally, 3 spines mediodistally.

Fig. 3. Sunamphitoe gigantea sp. nov., holotype male, 39.3 mm (NSMT-Cr 26733). (A) upper lip, posterior view; (B) lower lip (setules omitted), ventral view; (C1) left mandible, medial view; (C2) incisor, laciniae mobilis and accessory setal row of right mandible, medial view; (D) left maxilla 1 and pectinate spine on the outer plate, dorsal view; (E) left maxilla 2 (setae and setules partly omitted), dorsal view; (F) maxilliped (setae partly omitted), dorsal view. Scale bars: 0.5 mm.
Pereon
Gnathopod 1 (Figure 4A1) subchelate, smaller than gnathopod 2, length ratio of basis to dactylus about 17:4:5:9:9:5; coxa subquadrate, deeper than broad, slightly broadened ventrally, with tuft of short setae posteroventrally; basis longer than coxa, posterior margin with dense long setae, medial surface with several groups of long setae, anterior margin with several short setae, anterodistal lobe very small; carpus with dense setae posteriorly to medially; propodus with dense setae posteriorly to medially, palm (Figure 4A2) transverse, defined by spine, posterodistal corner with small spine; dactylus longer than palm, with anteroproximal seta. Gnathopod 2 (Figure 4B) subchelate, large, length ratio of basis to dactylus about 9:2:3:3:9:10; coxa subquadrate, rounded anteriorly to anteroventorally, deeper than broad, with tuft of short setae posteroventrally; basis subequal to coxa in length, posterior margin with dense long setae, anterior margin with several short setae, anterodistal lobe very small; carpus subtriangular, with dense setae posteriorly; propodus enlarged, palm straight without distinct protrusion, not clearly defined, with dense setae posteriorly, setae on posterodistal corner denser, lacking palm defining spines; dactylus curved, long, reaching to posteroproximal angle of propodus, not beyond carpus, lacking anteroproximal seta.

Fig. 4. Sunamphitoe gigantea sp. nov., holotype male, 39.3 mm (NSMT-Cr 26733). (A1) left gnathopod 1, lateral view; (A2) propodus palm and dactylus of left gnathopod 1 (setae omitted), lateral view; (B) left gnathopod 2, lateral view. Scale bars: A1, B, 3.0 mm; A2, 1.0 mm.
Pereopod 3 (Figure 5A) simple, length ratio of basis to dactylus about 17:3:6:5:5:2; coxa deeper than broad, with tuft of short setae posteroventrally, anterior margin roundly convex; basis expanded, anterior margin with group of long setae subproximally, posterior margin with dense long setae; merus expanded distoanteriorly; propodus slightly tapering distally. Pereopod 4 (Figure 5B) similar to pereopod 3, but coxa slightly larger, broadened ventrally, anterior margin rather straight. Pereopod 5 (Figure 5C) slightly shorter than pereopod 4, length ratio of basis to dactylus about 6:2:3:3:4:2; coxa bilobate, anterior lobe strongly enlarged, roundly quadrate, with scattered setae on medial surface, posterior lobe small, rounded, unarmed; basis expanded, with several small spines on anterior margin, group of long setae on medial surface, unarmed on posterior margin, without distinct posterodistal lobe; carpus broader than propodus; propodus with row of spines on flexor margin; dactylus falcate. Pereopod 6 (Figure 5D) longer than pereopod 5, length ratio of basis to dactylus about 11:3:7:7:8:2; coxa bilobate, each lobe rounded, anterior lobe larger than posterior lobe with several setae anteriorly, posterior lobe small, unarmed; basis with row of small spines on anterior margin, group of setae on medial surface, unarmed posteriorly, posteroproximal corner rounded; propodus slightly expanded distally, with row of spines on flexor margin; dactylus falcate. Pleopod 7 (Figure 5E) similar to pereopod 6 but slightly longer, coxa semicircular. Coxal gills present on coxae 2–6.

Fig. 5. Sunamphitoe gigantea sp. nov., holotype male, 39.3 mm (NSMT-Cr 26733). (A–E) left pereopods 3–7, lateral views. Scale bars: 3.0 mm.
Pleon
Epimeral plates (Figure 6A) normally rounded, without distinct teeth or setae. Pleopods normal, similar to each other.

Fig. 6. Sunamphitoe gigantea sp. nov., holotype male, 39.3 mm (NSMT-Cr 26733). (A) epimeral plates 1–3, lateral view; (B) left uropod 1, dorsal view; (C) left uropod 2, dorsal view; (D1) right uropod 3 (spinulation on outer ramus omitted), dorsal view; (D2) spinulation on right uropod 3 outer ramus, dorsal view; (E) telson, dorsal view. Scale bars: A, 3.0 mm; B–D1, E, 1.0 mm; D2, 0.5 mm.
Urosome
Uropod 1 (Figure 6B) peduncle with dorsolateral row of spines on distal 0.6, dorsomedial row of spines on distal 0.7, ventrolateral row of slender setae on proximal 0.8, distal end with long ventral spur; outer ramus about 0.7 times as long as peduncle (distoventral spur excluded), inner ramus longer and more slender than outer ramus, both outer and inner rami bearing rows of spines on both lateral, mesial margins, lacking slender setae. Uropod 2 (Figure 6C) peduncle with dorsolateral and dorsomedial rows of spines on distal half, without slender setae, distal end with ventral spur; outer ramus about 0.8 times as long as peduncle (distoventral spur excluded), inner ramus longer and more slender than outer ramus, both outer and inner rami bearing rows of spines on both lateral and mesial margins, lacking slender setae. Uropod 3 (Figure 6D1) peduncle cylindrical, reaching beyond posterior margin on telson, with several tufts of slender setae laterally, medially, several spines dorsodistally, distolaterally, lateral half of distoventral margin slightly extended, concealing base of outer ramus, fringed with slender setae; outer ramus slightly tapering, about 0.4 times as long as peduncle, finely spinulate dorsolaterally to ventrolaterally (Figure 6D2), with several short setae laterally, 2 recurved spines distally; inner ramus subquadrate, slightly shorter than outer ramus, with several small spines dorsodistally, dense slender setae ventrodistally. Telson (Figure 6E), roundly trapezoid, wider than long, posterior margin roundly convex, both lateral margins with 3–5 slender setae, short pappose seta, small telsonic cusp, dorsal surface with 1–2 short simple seta(e), long simple seta on both left, right side.
DESCRIPTION OF FEMALE
Generally similar to males (Figure 7A); gnathopod 1 (Figure 7B) similar to that of males, but carpus slightly stouter; gnathopod 2 (Figure 7C) smaller than that of males, propodus much smaller than that of males, palm well defined, oblique, bearing spine; coxae 2–5 with oostegites (Figure 7D), each oostegite similar, tapering distally, marginally setose on about distal half, some of setae curled distally.

Fig. 7. Sunamphitoe gigantea sp. nov., allotype female 33.6 mm (NSMT-Cr 26733). (A) habitus (setae partly omitted; coxal gills, oostegites and pleopods omitted), lateral view; (B) merus to dactylus of left gnathopod 1 (setae omitted), lateral view; (C) merus to dactylus of left gnathopod 2 (setae omitted), lateral view; (D) oostegite of left pereopod 3, lateral view. Scale bar: A, 10.0 mm; B–D, 1.0 mm.
VARIATIONS
Peduncular article 1 of antenna 1 bears 1 or 2 small spines distoventrally. Flagellar articles of antennae 1 and 2 increase numbers in large individuals. Coxae are sometimes notched on the ventral margin. Spines and setae on pereopods and uropods vary in numbers individually.
Male gnathopod 2 shows ontogenetic morphological change: in small males, palm well defined bearing a spine, and dactylus is much shorter than propodus (Figure 8A, B), while in large males, posterior margin of propodus is straight and the palm is obscure without spine, and dactylus is elongate reaching proximal end of propodus (Figure 8C).

Fig. 8. Ontogenetic morphological change in male gnathopod 2. (A–C) merus to dactylus of left gnathopod 2 (setae omitted), lateral view: (A) paratype male, 25.3 mm (NSMT-Cr 26742); (B) paratype male, 34.6 mm (NSMT-Cr 26737); (C) holotype male, 39.3 mm (NSMT-Cr 26733). Scale bars: 1.0 mm.
COLOURATION IN LIFE
Body (Figure 9) generally yellowish (or sometimes greenish) light brown without mottling, without sexual dimorphism; eyes red; antennae 1, 2 flagellae white or whitish yellow, with dark brown band subdistally.

Fig. 9. Sunamphitoe gigantea sp. nov. (A) holotype male, 39.3 mm (NSMT-Cr 26733), lateral view; (B) allotype female 33.6 mm (NSMT-Cr 26734), lateral view. Scale bar: 10.0 mm.
DISTRIBUTION
Known only from type locality, Katsurakoi, Kushiro, Hokkaido, Japan (Figure 1).
HABITAT
Found on the surface of fronds of a kelp Saccharina longissima inhabiting subtidal rocky shore.
ECOLOGICAL NOTE
Specimens of the new species were found occupying a nest which was constructed by the apical part of kelp blade being rolled up (Figure 10) as reported in some other congeners (e.g. Cerda et al., Reference Cerda, Hinojosa and Thiel2010). The following four patterns were observed as inhabitant(s) of single nest: (1) single male only, (2) single female only, (3) single female with many juveniles and (4) a pair of male and ovigerous female.

Fig. 10. Nests of Sunamphitoe gigantea sp. nov. and their inhabitants. (A1) nest occupied by the holotype male; (A2) holotype male (NSMT-Cr 26733) in the nest; (B) allotype female (NSMT-Cr 26734) and its juveniles in a nest.
Male gnathopod 2 shows ontogenetic morphological changes (see VARIATIONS). Furthermore, it was found that a male and an ovigerous female coexisted in a nest, though the male gnathopod 2 was not fully developed (NSMT-Cr 26735 and 26736; NSMT-Cr 26738 and 26739). This suggests that male sexual maturity may occur prior to morphological maturity of their gnathopod 2. Males were smaller than coexisting ovigerous females in these two cases.
GENETIC ANALYSIS
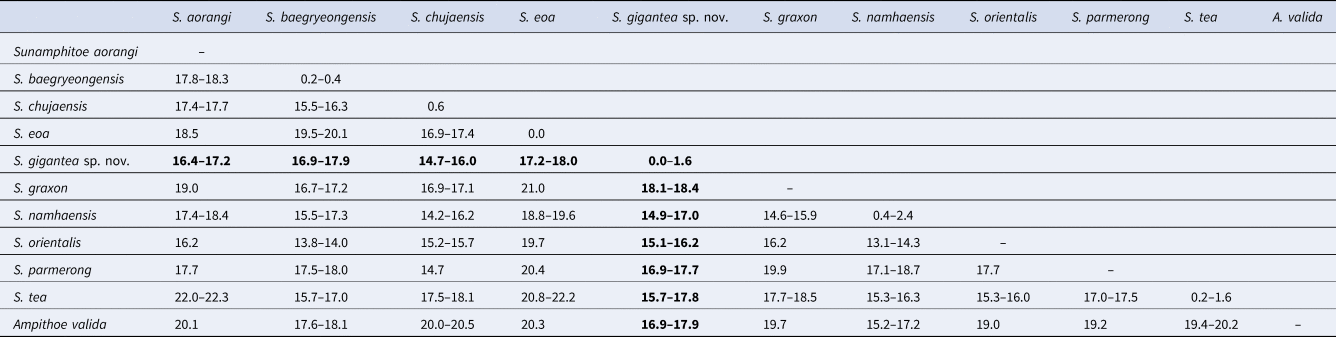
In total, 643–658 bp of COI sequences were obtained from seven specimens including the holotype and the allotype (INSDC accession numbers, LC472973–LC472979 for finally obtained COI sequences, DRA008087 for raw fastq data). During the alignment procedure, all positions containing gaps or missing data were eliminated, and then, a total of 506 bp was used for the analysis. Intra-specific divergence of COI sequences within the new species was less than 2%, while inter-specific divergences between the new species and congeners were greater than 14% (Table 3).
Table 3. Kimura 2-parameter distances of COI sequences (%) among Sunamphitoe species
In the phylogenetic tree of the COI sequences (Figure 11), all the sequences of S. gigantea sp. nov. formed a monophyletic clade that was supported by 100% bootstrap value. The sister clade of S. gigantea sp. nov. comprised of S. eoa and S. aorangi, though it was supported by a low bootstrap value. Species-level clade was supported by a high bootstrap value for each Sunamphitoe species, indicating the usefulness of COI sequences for DNA barcoding. However, all the higher-level clades (higher than species level) were supported by low bootstrap values, indicating that using only COI sequences may be insufficient for determining the phylogenetic relationship among Sunamphitoe species and other kinds of sequencing may be necessary for further validation.

Fig. 11. Maximum likelihood tree of Sunamphitoe species and an outgroup Ampithoe valida, based on 506 base pairs of COI sequences. Values at nodes indicate bootstrap values above 70%.
ETYMOLOGY
The species name ‘gigantea’ is derived from their large body size.
REMARKS
Among congeners, Sunamphitoe gigantea sp. nov. most resembles S. eoa (von der Brüggen, Reference von der Brüggen1907) in the conspicuously large body (maximum body length is 42.6 mm in S. gigantea sp. nov. and 38 mm in S. eoa according to Tzvetkova, Reference Tzvetkova1967), well developed mandibular palp, slender antenna 2, undefined male gnathopod 2 palm with the straight margin, unenlarged pereopods 5–7. However, the new species can be distinguished from S. eoa by the following points: (1) antenna 1 flagellum is much shorter than that of S. eoa; (2) maxilla 1 inner plate bears 2 slender setae in the new species, while that of S. eoa bears 3 setae; (3) gnathopod 2 dactylus of large male just reaches to posteroproximal end of propodus, whereas that of S. eoa reaches beyond carpus; (4) pereopods 3 and 4 bases bear a group of long setae on the sub-proximal area of anterior margin, while those of S. eoa lack long setae on its anterior margin. Moreover, S. gigantea sp. nov. and S. eoa also differ genetically in COI (17.2–18.0%) greater than the threshold distances (3.5–4%) proposed for amphipod species discrimination (Witt et al., Reference Witt, Threloff and Hebert2006; Rock et al., Reference Rock, Ironside, Potter, Whiteley and Lunt2007; Hou et al., Reference Hou, Li and Li2009). Therefore, we concluded that S. gigantea sp. nov. undoubtedly represents a novel species. The morphological comparisons among the new species and closely related species were summarized in Table 4.
Table 4. Morphological comparison among Sunamphitoe gigantea sp. nov., S. baegryeongensis and S. eoa

Immature males of this new species are also close to Barnard's (Reference Barnard1954) description of S. eoa from Oregon (note: Conlan & Bousfield (Reference Conlan and Bousfield1982) suggested that Barnard's (Reference Barnard1954) material might be another species rather than S. eoa). However, immature males of the new species differ from Barnard's material in the following characters: (1) gnathopod 2 palm is well defined with a small spine; (2) gnathopod 2 propodus lacks a large process on posterodistal corner; (3) pereopod 3 basis has a group of long slender setae on anterior margin; (4) distoventral spur on uropod 2 peduncle is longer; and (5) uropod 3 peduncle is longer.
Sunamphitoe gigantea sp. nov. also resembles S. baegryeongensis (Kim & Kim, Reference Kim and Kim1988), however, is easily distinguished from it by the following points: (1) S. gigantea sp. nov. is much larger (maximum body length more than 40 mm) than S. baegryeongensis (maximum body length 9 mm); (2) in large males, propodus palm of gnathopod 2 is rather straight in S. gigantea sp. nov., while strongly concave in S. baegryeongensis; (3) pereopod 7 basis of S. gigantea sp. nov. is unarmed on posterior margin, while that of S. baegryeongensis bears a small spine on the posterodistal corner; (4) uropod 3 peduncle of S. gigantea sp. nov. is much longer than that of S. baegryeongensis; (5) they also differ genetically (COI, 16.9–17.9%).
Acknowledgements
We gratefully acknowledge Akira Ito (Hokkaido National Fisheries Research Institute, Japan Fisheries Research and Education Agency) for supporting field sampling. We are grateful to the members of Bioengineering Lab. Co., Ltd, for their contributions to DNA extraction and COI sequencing. We deeply thank Christopher Norman (Japan Scientific Texts) for improving the English in the manuscript. We thank anonymous reviewers for their helpful comments on our manuscript.
Financial support
This study was partly supported by Grant-in-Aid for JSPS fellows (No. 18J13185).

















