Introduction
The genus Paramphiascella Lang, 1944, comprises 26 valid species, and has a complex and controversial taxonomic history. It is characterized by two- or thee-segmented antennary exopods, P1 EXP2 without inner seta, P1 EXP3 with four elements, P1 ENP three-segmented, P4 EXP3 occasionally with only two outer spines, and female P5 EXP and baseoendopod with five setae each (Lang, Reference Lang1944). This group of species has interesting ecological interactions. Some have been found between algae of the genera Codium Stackhouse, 1797 and Laminaria Lamouroux, 1813 (Brady, Reference Brady1880; Pallares, Reference Pallares1982); others have been found as associates of ascidians (Vervoort, Reference Vervoort1962) or in sandy bottoms associated with Branchiostoma Costa, 1834 (=Amphioxus Yarrell, 1836) (Guille and Soyer, Reference Guille and Soyer1966), or in wood associated with isopods of the genus Limnoria Leach, 1814 (Rosenfield and Coull, Reference Rosenfield and Coull1974). In addition, Paramphiascella appears to be tolerant to a wide range of environmental conditions and salinity gradients; some have been recorded in Arctic and Antarctic regions (Scott and Scott, Reference Scott and Scott1901; Scott, Reference Scott1903; Pallares, Reference Pallares1982), in the tropics (Dussart, Reference Dussart1984), in brackish water, and in raw sewage effluents (Marcotte, Reference Marcotte1974).
Sediment samples were collected from a shallow estuarine system during a short-term project on the mechanisms and adaptations of harpacticoid copepods to different trophic conditions. Our research led to the discovery of a new species of Paramphiascella, where the interspecific differences are very subtle (Lang, Reference Lang1965; Wells, Reference Wells2007). The new species presented herein, P. aestuarii n. sp., belongs to a group of six species of that genus with a two-segmented A2 EXP, and with the same armature formula of the antennary exopod and P1–P5. Morphological differences were found (i) in the relative length of the apical middle seta of the female and male P5 EXP, (ii) in the inner margin of the distal apophysis and the relative length of the two setiform inner elements of the male P2 ENP2, and (iii) in the spiniform processes on the female second antennulary segment.
Recent studies on several miraciiid species have included molecular phylogenetic analyses to confirm the position of new taxa and their relationships within the family (Yeom and Lee, Reference Yeom and Lee2020, Reference Yeom and Lee2022, Reference Yeom and Lee2023). To advocate for the simultaneous evaluation of molecular data and morphology for improved taxonomic diagnoses, we have incorporated nuclear 18S ribosomal DNA (18S rDNA) and the mitochondrial cytochrome oxidase subunit I (mtCOI) data of the new species and the respective phylogenetic analyses. This current study aims to describe the new species and discuss the relationships amongst the Miraciidae Dana, 1846.
Material and methods
Field and laboratory work
One sediment sample was taken from El Yugo estuary, a shallow estuarine system in the north of Mazatlán, on the Pacific coast of Mexico (23°18′05.3″N 106°29′03.4″W). An accessible site with 0.5 m depth was visited, and the upper 3 cm layer of sediment was collected using a shovel. The sediment was shifted with 500 and 38 μm sieves, and live female copepods were picked up using a Zeiss Stemi 508 stereomicroscope. One oviger female was cultured in seawater for two months and fed with Nannochloropsis oculata Hibberd, 1981, aiming at endogamic lines. After two months, adult specimens were identified through traditional morphological analyses.
Taxonomic and descriptive work
Individuals of the new species described herein were obtained from the cultures described above. The individuals were sorted using an Olympus SZX12 stereomicroscope equipped with DF PLAPO 1X objective and WHS10x eyepieces, and stored in 1 ml vials with 96% ethanol. Illustrations and figures were made from whole individuals and their dissected parts using a Leica DMLB microscope equipped with L PLAN 10X eyepieces, N PLAN 100X oil immersion objective, and a drawing tube. The dissected parts were mounted on separate slides with glycerine as mounting medium, and sealed with Neo-Mount®.
Huys and Boxshall (Reference Huys and Boxshall1991) was followed for general terminology. Willen (Reference Willen2000) was followed for the characterization of the P2 ENP of Thalestridimorpha.
Abbreviations used in the text and tables: A1, antennule; A2, antenna; ae, aesthetasc; apo, apophysis; BENP, baseoendopod; ENP, endopod; EXP, exopod; EXP (ENP)1 (2,3), first (second, third) exopodal (endopodal) segment; P1–P6, first to sixth legs.
DNA extraction, sequencing, and molecular markers (18s rDNA and mtCOI)
DNA extraction and sequencing protocols for genomic characterization followed Llera-Herrera et al. (Reference Llera-Herrera, Yáñez-Rivera, Chávez-Salgado, García-Bernal and Gómezsubmitted). Briefly, only one adult individual was isolated and was food-deprived for two days, then lysed by boiling in a TE buffer and centrifuged, and the clarified supernatant was recovered in a new tube on ice. The genomic DNA was enriched by multiple displacement amplification using a Phi-29 polymerase included in the Repli-G Mini kit (Qiagen, Maryland, USA), and after enrichment, the DNA was purified using Ampure XP beads. Amplified DNA was fragmented using the Nextera DNA Flex Library Preparation Kit with UDI Illumina adapters (Illumina, San Diego, CA, USA) and sequenced in an Illumina NovaSeq instrument using the v1.5 PE-150 chemistry to generate three Gb of nucleotide sequences. Genome assembly was done with MaSuRCa V 4.1.0 and the mitochondrial genome was analysed with MitoFlex (Li et al., Reference Li, Li, Wang and Zhang2021).
The 18S rDNA scaffold (2872 pb) was retrieved using a local database generated with the makeblastdb program included with BLAST 2.12.0 + package and the blastn in the primary genome assembling. The mtCOI gene (1555 pb) was retrieved from mitochondrial genome annotation. Sequence information was deposited in the NCBI data base (18S: PQ505074 and mtCOI: PQ499583).
Phylogenetics
Sequences of Miraciidae from GenBank were downloaded and used in the phylogenetic analyses. The 18S rDNA analyses included 13 species in three subfamilies (Supplementary Table S1), and Parathalestris verrucosa Itô, 1970 and Pseudotachidius bipartitus Montagna, 1980 as external groups. The mtCOI analyses included 40 Miraciidae species and the same two external groups (Supplementary Table S2). The multiple alignment was done with MAFFT (Katoh et al., Reference Katoh, Rozewicki and Yamada2019). ModelTest-NG v0.2.0 (Darriba et al., Reference Darriba, Posada, Kozlov, Stamatakis, Morel and Flouri2020) was used to infer appropriate evolutionary models for each gene 18S: TrN + I + G4 and COI: GTR + I + G4. The alignment was edited with TRIMAL using the Xgap option. The obtained matrix for 18S has 15 taxa and 1730 characters, and 120 taxa and 567 characters for mtCOI.
Bayesian analyses of phylogeny were performed with MrBayes 3.2.7a (Ronquist et al., Reference Ronquist, Teslenko, van der Mark, Ayres, Darling, Höhna, Larget, Liu, Suchard and Huelsenbeck2012). The default prior distribution of parameters was used for MCMCMC analyses, with one cold chain and three heated chains for 1,000,000 generations, and sampled every 100th. Bayesian inference trees were visualized using the Interactive Tree Of Life v6 (iTOL, https://itol.embl.de, accessed on 27 Jun 2024) (Letunic and Bork, Reference Letunic and Bork2021). Average pairwise distances for mtCOI were calculated between the new species and representative Miraciidae species using the K2P model.
Results
Systematics
Family Miraciidae Dana, 1846
Subfamily Diosaccinae Sars G.O., 1906
Genus Paramphiascella Lang, 1944
Type species: Paramphiascella hispida (Brady, 1880).
Other species: Paramphiascella aestuarii n. sp., P. aquaedulcis Dussart, 1984, P. austroatlantica Pallares, 1982, P. bodini Marcotte, 1974, P. brucei (Scott T. and Scott A., 1901), P. bulbifer Guille and Soyer, 1966, P. calcarifer (Sewell, 1940), P. commensalis (Seiwell, 1928), P. coulli Marcotte, 1974, P. curtiseta Chislenko, 1971, P. dahmsi Chullasorn, 2010, P. delamarei Guille and Soyer, 1966, P. faurei Bodin, 1968, P. ferrarii Chullasorn, 2010, P. fulvofasciata Rosenfield and Coull, 1974, P. hyperborea (Scott T., 1903), P. intermedia (Scott T., 1897), P. langi (Monard, 1936), P. mediterranea Lang, 1948, P. pacifica Vervoort, 1962, P. roberti (Monard, 1935), P. robinsonii (Scott A., 1902), P. sirbonica Por, 1973, P. vararensis (Scott T., 1903), P. xiphophora Lang, 1965.
Paramphiascella aestuarii n. sp.
(Figures 1–8)
urn:lsid:zoobank.org:act:D4F0D77D-41BD-4248-A839-D0CEAA343262
Type locality. A small brackish water body of El Yugo estuary located close to the beach (Mazatlán, Sinaloa) (23°18′04.61″N, 106°29′03.23″W), 1 m depth; 04 February 2020; Raúl Llera-Herrera leg.
Material examined. ♀ holotype (ICML-EMUCOP-040220-01) and ♂ allotype (ICML-EMUCOP-040220-02) preserved in alcohol; one ♂ paratype dissected and mounted onto five slides (ICML-EMUCOP-040220-03), and one ♀ paratype dissected and mounted onto seven slides (ICML-EMUCOP-040220-04); the following separate parts preserved in alcohol were also included in the type series (paratypes) (ICML-EMUCOP-040220-05): ♀ urosome, ♀ urosome + P2–P4-bearing somites, ♀ cephalothorax + P2–P4-bearing somites, ♀ cephalothorax + P2–P4-bearing somites.
Etymology. The specific epithet, aestuarii, is the second declension of the Latin noun aestuārium, tidal marsh. It is a noun in the genitive singular.
Description of female. Total body length of holotype measured from tip of rostrum to posterior margin of caudal rami 455 μm; habitus fusiform, widest at posterior end of cephalothorax, tapering posteriad.
Prosome consisting of cephalothorax with fused first pedigerous somite, and second to fourth free pedigerous somites. Cephalothorax length:width ratio, 1.2; posterior hyaline fringe finely striated, with caudal margin finely serrated (plain?). Free pedigerous somites without lateral or dorsal expansions; without spinular ornamentation; posterior hyaline fringe as in cephalothorax; width of second to fourth pedigerous somites decreasing progressively, with few surface sensilla.
Urosome (Figures 1A, B, 2A) consisting of fifth pedigerous somite (first urosomite), genital double-somite (genital—second urosomite—and third urosomites fused), two free urosomites, and anal somite. Fifth pedigerous somite (Figure 2A) visibly narrower than preceding somites; with short dorsolateral spinular row.

Figure 1. Paramphiascella aestuarii n. sp., female; (A) urosome, dorsal (P5-bearing somite omitted); (B) urosome, lateral (P5-bearing somite omitted). Scale bars: A–B, 200 μm.
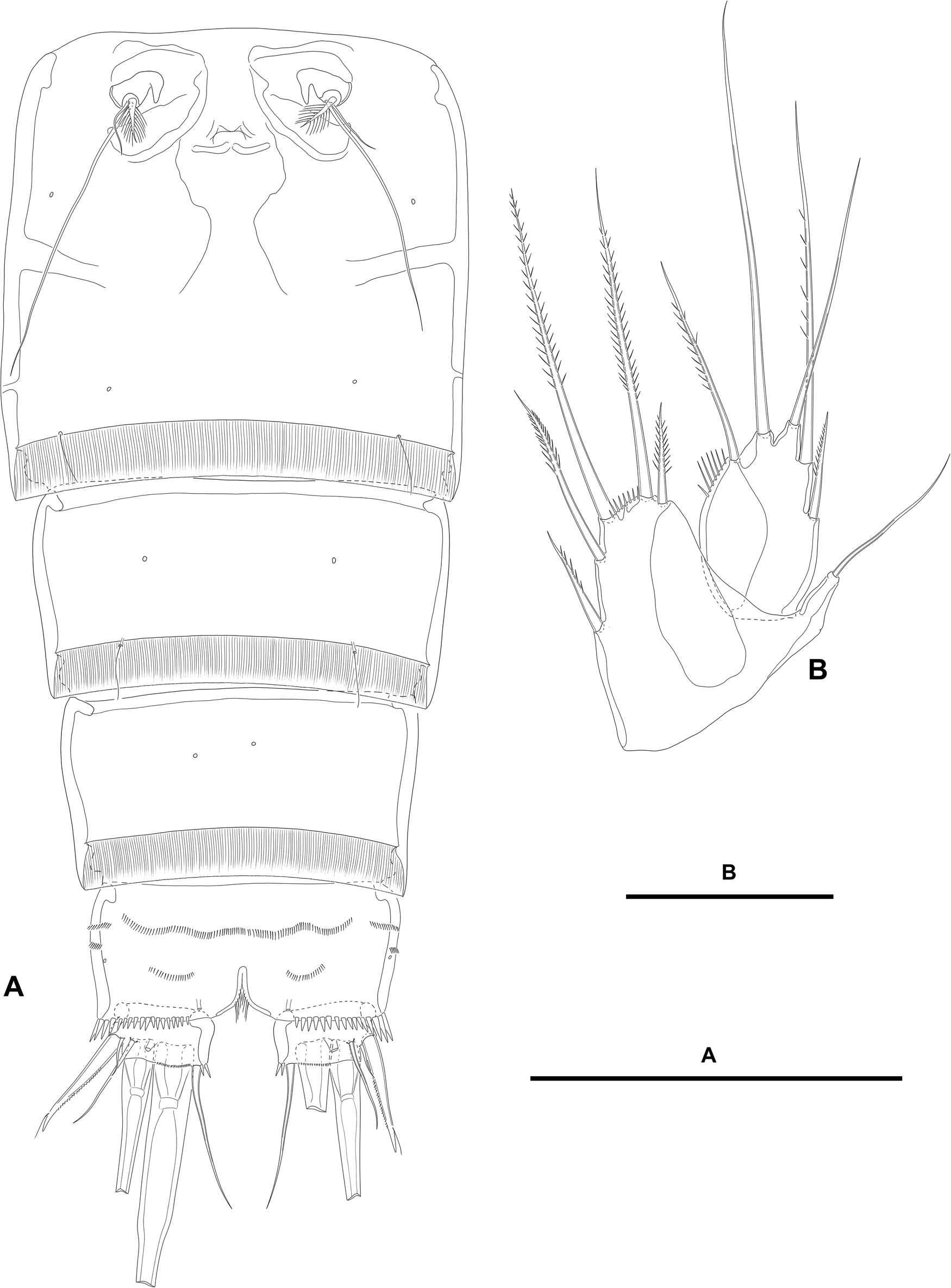
Figure 2. Paramphiascella aestuarii n. sp., female; (A) urosome, ventral (P5-bearing somite omitted); (B) P5, anterior. Scale bars: A, 100 μm; B, 50 μm.
Second and third urosomites separated by dorsolateral internal rib (Figure 1A, B), but fused ventrally forming genital double-somite (Figure 2A); the latter slightly longer than wide, widest part measured in the middle; anterior and posterior halves of genital double-somite with laterodorsal sensilla as shown, without spinular ornamentation; posterior hyaline fringe finely striated, with caudal margin finely serrated (plain?); genital complex as figured, with large copulatory pore; genital apertures covered by P6 (Figure 2A).
Fourth urosomite (Figures 1A, B, 2A) largely as posterior half of genital double-somite.
Fifth urosomite without sensilla or spinules (Figures 1A, B, 2A); posterior hyaline fringe as in previous somites, but dorsomedial part extended forming pseudoperculum (Figure 1A).
Anal somite twice as wide as long (Figures 1A, 2A); with rows of small spinules laterally (Figure 1B) and ventrally (Figure 2A), with larger and stronger spinules close to insertion of caudal rami laterally (Figure 1B) and ventrally (Figure 2A); with medial cleft ventrally; anal operculum without spinular ornamentation, triangular, medial associated sensilla displaced posteriorly on each side (Figure 1A).
Caudal rami short, about 1.5 times as wide as long, visibly shorter than anal somite; with few inner spinules at the base of caudal seta VI (Figures 1A, 2A), each ramus with one ventral pore (Figure 2A); with seven elements (Figures 1A, B, 2A); seta I small, ventral to seta II, the latter spiniform, flagellate, and ornamented as figured; seta III ventral, nearly as long as seta II, aligned to setae I and II; setae IV and V distal, thickened proximally; seta VI issuing at inner distal corner, longer than seta II; dorsal seta VII triarticulate at base, dorsal to seta VI, situated subdistally close to inner margin.
Rostrum (Figure 3A) triangular, not fused to cephalothorax, reaching middle of second antennulary segment in lateral view, with tip slightly bifid, with two subdistal sensilla, without dorsal pore.

Figure 3. Paramphiascella aestuarii n. sp., female; (A) rostrum; (B) antennule; (C) antenna; (D) free endopodal segment of the antenna. Scale bars: A–D, 50 μm.
Antennule (Figure 3B) eight-segmented; all segments smooth, except for medial and subdistal spinular rows on first segment. All setae smooth, except for strong pinnate element on second segment; seventh segment with two, eighth segment with four biarticulated setae. Armature formula: 1(1); 2(11); 3(7); 4(3 + [1 + ae]); 5(2); 6(3); 7(4); 8(4 + acro). Acrothek consisting of two setae and one aesthetasc fused basally.
Antenna (Figure 3C, D). Coxa short, with some long outer spinules proximally. Allobasis as long as free endopodal segment; with proximal row of inner short spinules; with two abexopodal setae of which the proximal very reduced, the subdistal well-developed and spinulose. Free endopodal segment elongate; with longitudinal row of strong inner spinules; with subdistal and outer rows of shorter spinules (fringes?) as shown; armature composed of two lateral spines and one slender seta, distally with one outer spine, three geniculate medial setae, one outer slender element, and one spinulose geniculate seta fused basally to small pinnate element. Exopod two-segmented; first segment longer than second, unornamented, with one seta; second segment elongate, unornamented, with one lateral and two distal setae as shown.
Mandible (Figure 4A). Coxa well-developed. Gnathobasis wide; with one strong multicuspidate, and several smaller teeth, set of short spinules, one spine, and one pinnate ventral seta. Palp three-segmented, composed of basis, and one segment endopod and exopod. Basis elongate; with strong spinules at base of exopod, and with slenderer spinules subdistally as depicted; armed with three distal setae, two of which pinnate, one bare. Exopod visibly smaller than endopod, with two long and two short setae. Endopod with one lateral seta proximally, two subdistal lateral elements, and three distal setae.

Figure 4. Paramphiascella aestuarii n. sp., female; (A) mandible; (B) maxillule; (C) maxilla; (D) maxilliped. Scale bars: A–D, 50 μm.
Maxillule (Figure 4B). Praecoxa with two short rows of spinules as shown; arthrite with two surface setae and some dorsal spinules proximally; distal armature composed of eight spines ornamented as shown, and one pinnate seta. Coxal endite with two setae. Basis with two endites; proximal endite with row of small spinules at its base and armed with three setae; distal endite with three setae, one of which with long spinules. Exopod and endopod distinct, one-segmented; endopod larger than exopod, with three setae; exopod small, with two setae.
Maxilla (Figure 4C). Large syncoxa with outer and inner small spinules as shown; with three endites; proximal endite bilobed, each lobe with one seta; middle endite shorter than distal endite, the former with two, the latter with three setae. Basis drawn out into strong claw, with accompanying strong spine and one slender seta. Endopod small, as long as wide; with four setae, one of which lateral.
Maxilliped (Figure 4D) subchelate. Syncoxa visibly shorter than basis, subquadrate; with longitudinal row of spinules as shown; armed with three setae of which proximal shorter. Basis elongate; with longitudinal row of strong spinules; armed with two setae. Endopod one-segmented, with one claw-like element and three setae.
P1 (Figure 5A). Intercoxal sclerite transversely elongate, unornamented. Praecoxa transversely elongate, seemingly unornamented. Coxa massive; with four anterior rows of spinules, and one inner and one outer posterior row of longer ornaments. Basis trapezoid; with slender inner spinules, with stronger spinules between rami and at base of inner spine, and with small spinules at the base of outer element; outer and inner spines bipinnate, the latter flagellate. Exopod three-segmented, barely reaching tip of ENP1; no pores detected on exopodal segments; EXP1 longest, EXP3 shortest; all segments without outer or inner distal processes; EXP1 and EXP2 with outer spine and longitudinal row of outer spinules; EXP1 without, EXP2 with inner setules; EXP3 with outer spinules as shown, with two outer spines and two apical geniculate setae. Endopod three-segmented; segments without inner or outer distal processes; no pores detected on endopodal segments; ENP1 longest, about three times as long as wide, with outer longitudinal row of strong spinules and with slenderer and longer inner ornaments; armed with one inner subdistal spiniform element; ENP2 shorter than ENP3, slightly wider than long, with few outer spinules, armed with one inner slender seta; ENP3 about twice as long as wide, ornamented as in previous segment, with one inner slender seta, and two apical strong spines, one of which geniculate.

Figure 5. Paramphiascella aestuarii n. sp., female; (A) P1, anterior; (B) P2, anterior. Scale bars: A–B, 50 μm.
P2–P4 (Figures 5B, 6A, B). Intercoxal sclerites trapezoid; distal pointed tines decreasing in size and lateral outgrowth of each tine increasing in size from P2 to P4; intercoxal sclerite of P2 and P3 unornamented, of P4 with posterior spinules as shown. Praecoxa transversely elongate, triangular, with transverse row of small spinules. Coxa rectangular, with four (P2) or three (P3 and P4) rows of spinules anteriorly, and with row of outer spinules posteriorly. Basis with outer spine (P2) or slender seta (P3 and P4); with (P2 and P3) of without (P4) strong acute inner process; with spinules at the base of outer element and between rami; P2 seemingly without, P3 and P4 with long slender inner spinules. Exopod three-segmented, longer than endopod; exopodal segments with outer and inner spinules, and distal inner fringe (EXP1 and EXP2) as shown; pore observed on P2 EXP2 and on P4 EXP3; EXP1 with outer spine, without inner armature; EXP2 with outer spine and inner seta; EXP3 with three outer spines and two distal elements, P2 EXP3 without, P3 EXP3 with one, P4 EXP3 with two inner setae. Endopod three-segmented; of P2 and P3 reaching middle of EXP3, of P4 barely reaching tip of EXP2; endopodal segments ornamented with outer spinules and with distal inner ornaments (ENP1 and ENP2) as shown; pore observed on P3 ENP3 and P4 ENP3; ENP1 with inner seta, of which inner seta of P2 ENP1 shortest, stiffer, and strongly ornamented; ENP2 with inner seta; ENP3 with one (P2 and P4) or two (P3) inner setae, two distal elements and one distal outer spine.

Figure 6. Paramphiascella aestuarii n. sp., female; (A) P3, anterior; (B) P4, anterior. Scale bars: A–B, 50 μm.
Setal formula of swimming legs as follows:

P5 (Figure 2B). Baseoendopod trapezoid; with slender outer basal seta. Endopodal lobe well-developed; with five setae of which outermost and innermost shortest; with spinules along wide space between apical longer setae; with hyaline outer area. Exopod oval; with five setae of which outermost shortest, medial longest; with row of inner spinules; with hyaline inner area.
P6 (Figure 2A) a flap covering genital aperture; without surface ornamentation; with one short and one very long slender seta, and one spinulose thick element; each leg accompanied by short inner pointed outgrowth.
Description of male. Total body length of allotype measured from tip of rostrum to posterior margin of caudal rami, 450 μm. General body shape as in female. Sexual dimorphism expressed in urosomal segmentation, ventrolateral spinular ornamentation of urosomites, antennule, basis of P1–P3, P5, and P6.
Urosome (Figure 7A–C) as in female except for genital (P6-bearing) somite and third urosomite separated; third and fourth urosomites as in female except for lateroventral spinular row close to posterior margin; fifth urosomite as in female; anal somite as in female dorsally, laterally with one short row of small spinules, ventrally with one row of spinules with medial ornaments larger than in female; caudal rami as in female.

Figure 7. Paramphiascella aestuarii n. sp., male; (A) urosome, dorsal (P5-bearing somite omitted); (B) urosome, lateral (P5-bearing somite omitted); (C) urosome, ventral (P5-bearing somite omitted). Scale bars: A–C, 100 μm.
Antennule (Figure 8A). Haplocer, nine-segmented. First and second segment as in female; third–seventh segments modified; eighth segment as in female; ninth segment modified. Armature formula: 1(1); 2(11); 3(6); 4(2); 5(7 + [1 + ae]); 6(1); 7(1 + 2 modified); 8(3); 9(5 + acro). Acrothek consisting of two setae and one aesthetasc fused basally.

Figure 8. Paramphiascella aestuarii n. sp., male; (A) antennule; (B) P1 basis, anterior; (C) P2 basis and endopod, anterior; (D) P3 basis, anterior; (E) P5, anterior. Scale bars: A–E, 50 μm.
Antenna, mandible, maxillule, maxilla, and maxilliped (not shown) as in female.
P1 as in female except for basis (Figure 8B) with inner striated knob, rather setiform inner element, and lack of spinules at the base of the latter.
P2 as in female except for inner subdistal acute projection of basis with a hyaline membrane (or more sclerotized than in female?), and for sexually dimorphic two-segmented endopod (Figure 8C); ENP1 largely as in female except for inner seta visibly shorter; ENP2 modified into a long segment ornamented with long inner setules proximally, with an outer long apophysis, one subdistal inner element with bifurcated tip, two medial inner pinnate setae, and one proximal blunt inner outgrowth.
P3 as in female except for basis (Figure 8D) with inner acute projection with hyaline membrane (or more sclerotized than in female?).
P4 (not shown) as in female.
P5 (Figure 8E) with both baseoendopods fused medially forming a continuous plate, with outer basal seta arising from long setophore. Endopodal lobe with two strong elements and ornamented with inner and outer spinules as shown. Exopod ornamented with few outer spinules as shown; with five setae of which two outermost shortest, medial seta naked, slender and long, two inner setae bipinnate and thick of which innermost slightly shorter.
P6 (Figure 7B, C) not functional, fused to somite; with one inner spine, one medial long naked seta, and one slightly setulose seta visibly shorter than medial element.
Molecular and phylogenetics
Whole genome draft of P. aestuarii n. sp. was recovered and details will be presented elsewhere (Llera-Herrera et al., Reference Llera-Herrera, Yáñez-Rivera, Chávez-Salgado, García-Bernal and Gómezsubmitted). Mitochondrial cytochrome oxidase subunit I (mtCOI) gene and the nuclear 18S rDNA scaffold were used for phylogenetic analyses with additional miraciid species available from GenBank database (Supplementary Table S1 from 18S rDNA, and Supplementary Table S2 from mtCOI).
The new species described here is well supported according to the 18S rDNA phylogenetic topology within the main group composed of members of Diosaccinae (Figure 9). Paramphiascella aestuarii n. sp. is relatively close to P. fulvofasciata Rosenfield and Coull, 1974 and Amphiascoides sp., and Robertgurneya jejuensis Yeom and Lee, 2022. Interspecific relationships between Amphiascoides Nicholls, 1941 and Paramphiascella were poorly resolved. More sequences are needed to resolve the delimitation between Amphiascoides and Paramphiascella, and to assess the shape of the male P2 ENP2 in these two genera as a useful character for morphological distinction. On the other hand, the pairwise mtCOI genetic distance between both genera also supports their separation (22.42% for Amphiascoides atopus Lotufo and Fleeger, 1995, and 25.41% for Amphiascoides sp., Table 1). The data available so far point toward a close relationship between Amphiascoides and Paramphiascella.

Figure 9. Phylogenetic hypothesis of Miraciidae showing Paramphiascella aestuarii n. sp., placement based on Bayesian inference with nuclear 18s rDNA. *Nodes of high support >0.95 posterior probabilities.
Table 1. Pairwise distance of mtCOI sequences of Paramphiascella aestuarii n. sp. and representatives of some genera of Miraciidae using the K2P model

From Figures 9 and 10, it is clear that more 18S sequences of more representatives of the three miraciid subfamilies are required to revaluate their monophyletic supports. Sarsamphiascus Huys, 2009 appears to bear a well-defined sister group relationship with representatives of the Diosaccinae, Miraciinae, and Stenheliinae Brady, 1880.

Figure 10. Phylogenetic hypothesis of Miraciidae showing Paramphiascella aestuarii n. sp., placement based on Bayesian inference with mtCOI. *Nodes of high support >0.95 posterior probabilities. The number of sequences by taxon is indicated between brackets. All collapsed clades have high support (not shown). According to our phylogenetic analyses, a question mark (?) was added when the sequence did not match the placement in the expected genus.
The mtCOI topology showed unresolved nodes between genera of Miraciidae. However, the support of P. aestuarii n. sp. and its close relationship with Amphiascoides sp. and A. atopus is consistent (Figure 10). The apparent relationship of the clade Paramphiascella-Amphiascoides with representatives of Haloschizopera Lang, 1944 is not well supported. The phylogenetic structure of the genera with two or more species (Sarsamphiascus, Schizopera Sars G.O., 1905, Diosaccus Boeck, 1873, Stenhelia Boeck, 1865, Wellstenhelia Karanovic and Kim, 2014, and Itostenhelia Karanovic and Kim, 2014) is well supported and delimited.
Discussion
Historical background
The taxonomic history of the genus Paramphiascella is complicated and controversial, and it has to do with the erection and definition of other genera. Sars (Reference Sars1905) proposed the genus Amphiascus Sars G.O., 1905 for Dactylopus longirostris Claus, 1863, D. minutus Claus, 1863, D. debilis Giesbrecht, 1881, and A. pacificus Sars G.O., 1905, and in his rediagnosis of the genus, Monard (Reference Monard1928b: 369–370) commented on the variability of P1, segmentation of the A2 EXP (two- or three-segmented), and setation of P2–P4 ENP2 (with one or two inner elements), and noted that the spinular ornamentation of the urosomite, which had been seldom described, could be a reliable character for species separation. Based on the above characters, Monard (Reference Monard1928b: 370–371) subdivided the individuals of the genus found in his samples from Banyuls into seven species-groups, viz., nasutus-, varicolor-, similis-, cinctus-, parvus-, debilis-, and dictydiophorus-group. Later, Monard (Reference Monard1928a) gave a list of 13 species-groups of Amphiascus, viz., nasutus-, varicolor-, giesbrechti-, tenuiremis-, cinctus-, bulbifer-, denticulatus-, blanchardi-, varians-, exiguus-, longicaudatus-, typhlops-, and debilis-group. In his study on the harpacticoids from Argel and Castiglione (Bou Ismaïl District) (Algeria), Monard (Reference Monard1937) commented on some flaws of his 1928 review of the genus Amphiascus, but argued in favour of his subdivision. In his revision of the former family Diosaccidae, Nicholls (Reference Nicholls1941a) proposed the subfamily Amphiascinae Nicholls, 1941 for some genera of the former Diosaccidae (Amphiascopsis Gurney, 1927, Amphiascus s. str. sensu Nicholls (Reference Nicholls1941a), Mesamphiascus Nicholls, 1941, Amphiascoides, Robertsonia Brady, 1880, and Schizopera) and argued on a close relationship between his newly erected subfamily and Dactylopusia Norman, 1903. Also, following Nicholls (Reference Nicholls1941a), Monard's (Reference Monard1928a) nasutus-, varicolor-, giesbrechti-, tenuiremis-, and cinctus-group correspond to Amphiascopsis and to his Amphiascus s. str., and the bulbifer-, denticulatus-, blanchardi-, varians-, and exiguus-groups are a mixture of species of Mesamphiascus and Robertsonia, while the last three groups—longicaudatus-, typhlops-, and debilis-groups—are a mixture of Amphiascoides and Schizopera. Nicholls (Reference Nicholls1941a) also argued that (i) Amphiascopsis and Amphiascoides are well-defined, (ii) Amphiascus s. str. is composed of species allied to the type species of the genus, Dactylopus longirostris ( = Amphiascus longirostris [Claus, 1863]), that cannot be included in Amphiascopsis, and (iii) Mesamphiascus is an amalgam of species intermediate between his Amphiascus s. str. and Amphiascoides. In his temporary notification, Lang (Reference Lang1944) apparently disposed of Nicholls' (Reference Nicholls1941a) Amphiascinae and the subfamily was not mentioned by subsequent authors. He (Lang, Reference Lang1944) divided the genus Amphiascus into several genera (Paradiosaccus Lang, 1944, Antiboreodiosaccus Lang, 1944, and Pseudodiosaccopsis Lang, 1944, of which Paradiosaccus was sunk into synonymy with Diosaccus by himself [Lang, Reference Lang1965]). He (Lang, Reference Lang1944, Reference Lang1948) also rediagnosed the genus Amphiascus (without fixing the type species) and proposed to subdivide that genus into four species-groups (minutus-, varians-, pacificus-, amblyops-group), rediagnosed the genus Amphiascopsis, and erected the genera Dactylopodamphiascopsis Lang, 1944, Amonardia Lang, 1944, Pseudamphiascopsis Lang, 1944, Metamphiascopsis Lang, 1944, Paramphiascopsis Lang, 1944 (for which D. longirostris was also fixed as the type species), Pararobertsonia Lang, 1944, Bulbamphiascus Lang, 1944, Robertgurneya Lang, 1944 with two species-groups (similis-, and spinulosus-group), Typhlamphiascus Lang, 1944, Rhyncholagena Lang, 1944, Amphiascella Lang, 1944, Paramphiascella and Haloschizopera. Lang (Reference Lang1965) commented on the taxonomic chaos of the genus Amphiascus and on the inherent difficulty of making a key to its species. More recently, Huys (Reference Huys2009) noted on the unavailability of the genus Mesamphiascus, and on the orphanhood of Lang's (Reference Lang1944, Reference Lang1948) Amphiascus caused by the fixation of D. longirostris as the type species of Amphiascus by Nicholls (Reference Nicholls1941a) and Paramphiascopsis by Lang (Reference Lang1944), and erected the genus Sarsamphiascus, with its type species Dactylopus minutus, for all the species included in Amphiascus by Lang (Reference Lang1948).
The genus Amphiascoides
The genus name Amphiascoides was coined by Nicholls (Reference Nicholls1941b: 415) for the species included in the debilis-group of Amphiascus plus some other species, but it was Nicholls (Reference Nicholls1941a: 81) who explicitly fixed Dactylopus debilis as the type species. Initially, Nicholls (Reference Nicholls1941b: 415) defined the genus upon the presence of one inner seta on P2–P3 ENP2, P1 EXP2 without inner armature and P1 EXP3 with four elements, but in his revision, Nicholls (Reference Nicholls1941a: 81) rediagnosed the genus and added three additional character states to define it (P2–P4 EXP1 without inner armature; P2–P4 ENP1–3 with inner armature formulae 1,1,1; 1,1,2; and 1,1,1, respectively; reduction in the armature complement of the female and male P5 EXP to five setae only), gave a list of species of Amphiascoides, and presented a key to the species based on the females only. Nicholls (Reference Nicholls1941a) included Stenhelia pygmaea Norman and Scott T., 1905 in his list of species of Amphiascoides. Norman and Scott's (Reference Norman and Scott1905) description did not include any illustration of the species, and their written description was so brief that the identity of the species was difficult to confirm. Fortunately, Norman and Scott (Reference Norman and Scott1906) redescribed the species and presented a full set of figures from which the transfer of the species to Amphiascoides was justified. However, Norman and Scott (Reference Norman and Scott1906) reversed the antennary exopod and mandibular palp in their figures. What they showed as the antennary exopod (Norman and Scott [Reference Norman and Scott1906, plate X, figure 2]) seems to be the mandibular palp with a one-segmented endopod and a two-segmented exopod; the mandibular palp in Norman and Scott (Reference Norman and Scott1906, plate X, figure 3) seems to be the antennary exopod (see below). The antennary exopod is three-segmented and is armed with one seta on the proximal and middle segments, and one lateral and one distal seta on the last segment, which departs from the general scheme of Amphiascoides, casting doubts on its identity. Lang (Reference Lang1944) proposed the genera Amphiascella and Paramphiascella, with Amphiascus linearis Sars G.O., 1906 and Stenhelia hispida Brady, 1880 (=Paramphiascella hispida) as their type species, respectively. Lang (Reference Lang1948) included seven species in Amphiascella, A. brevifurca (Czerniavski, 1868), A. neglecta (Norman and Scott T., 1905), A. subdebilis (Willey, 1935), A. debilis (Giesbrecht, 1881), A. dispar (Scott and Scott, 1894), A. limicola (Brady, 1900), and A. littoralis (Scott T., 1903), and subdivided the genus into three species-groups. However, in a postscript of his monograph, he (Lang, Reference Lang1948: 1619) argued in favour of the synonymy of Amphiascella with Amphiascoides, and some other authors (e.g. Noodt, Reference Noodt1955: 47; Vervoort, Reference Vervoort1964: 193), followed his view (Lang, Reference Lang1965: 547–548). Walter and Boxshall (Reference Walter and Boxshall2023) gave a list of 24 valid species of Amphiascoides, but P. choi is transferred to Amphiascoides, and A. pygmaea (Norman and Scott T., 1905) is provisionally transferred to Amphiascus (see below).
The genus Paramphiascella
To the best of our knowledge, the last diagnosis of Paramphiascella is that of Lang (Reference Lang1944, Reference Lang1948), who diagnosed the genus with two- or thee-segmented antennary exopods, P1 EXP2 without inner seta, P1 EXP3 with four elements, P1 ENP three-segmented, and armature formulae of P2–P4 ‘as in Amphiascella’ (Lang, Reference Lang1944: 22) but P4 EXP3 occasionally with only two outer spines, and female P5 EXP and baseoendopod with five setae each. The genus Paramphiascella is currently composed of 26 valid species (see the list of species above).
The genera Amphiascoides, Paramphiascella, and Paramphiascoides
Wells (Reference Wells1967) found some Amphiascoides-like specimens in sediment samples from Saco da Inhaca (Inhaca island, Mozambique). Wells (Reference Wells1967) noticed that the armature formulae of the swimming legs of his material largely corresponds to that of Amphiascoides and Paramphiascella but, given the unusual architecture of the male P2 ENP (see below), he decided to create a new genus, Paramphiascoides Wells, 1967 for P. mixtus Wells, 1967, and hypothesized on a close relationship between his newly found material, Amphiascoides, and Paramphiascella (see below).
The armature formulae of swimming legs and structure and setation of mouth appendages have proven useless in the distinction of the females of Amphiascoides and Paramphiascella, and also Paramphiascoides, and the separation of these three genera has always been controversial (see also Wells, Reference Wells1967). On the other hand, the structure of the male P2 ENP2 seems to be the only character for the separation of these three genera (see also Vervoort, Reference Vervoort1962; Wells, Reference Wells1967).
The family Parastenheliidae Lang, 1936 possesses the simplest sexual modification in the male P2 ENP within Thalestridimorpha sensu Willen (Reference Willen2000) (see also Huys and Mu, Reference Huys, Mu and Huys2021). Willen (Reference Willen2000) regarded the unmodified male P2 ENP of some species of Parastenhelia Thompson I.C. and Scott A., 1903 and Thalestrella Monard, 1935 (=Karllangia Noodt, 1964), as secondary reversal. Following Willen (Reference Willen2000: 163), the modified male P2 ENP of Amphiascoides/Paramphiascella (but also Paramphiascoides, see below) is a relatively more derived modification of her type 4 of the male P2 ENP of the Thalestrioidea sensu Willen (Reference Willen2000). She described this type of modification as the fusion of the second and third endopodal segments, and the thickening and migration of the distal outer spine and the distal medial seta (elements 1 and 2 in Willen [Reference Willen2000: 167, figure 69]) to the outer margin of the composite segment. This basic pattern can be observed in Amphiascus, Bulbamphiascus, Haloschizopera, Miscegenus Wells, Hicks and Coull, 1982, and, with some slight differences, in Neomiscegenus Karanovic and Ranga Reddy, 2004, and several modified forms occur in other Diosaccinae (Willen, Reference Willen2000).
In their study, Rosenfield and Coull (Reference Rosenfield and Coull1974) argued on the origin of the modified elements on the male P2 ENP2 of P. fulvofasciata and suggested that the modified distal outer spine of the P2 ENP2 in the male CV (element 20, 21 in Rosenfield and Coull [Reference Rosenfield and Coull1974: 311, figure 79]) is the result of the fusion of the distal outer spine and the distal medial seta present in the P2 ENP2 of CIV (elements 20 and 21 in Rosenfield and Coull [Reference Rosenfield and Coull1974: 311, figure 77]). However, fusion of the distal outer spine and the distal medial seta of the male P2 ENP2 during the moult from CIV to CV as the origin of the thickened distal outer spine of the male P2 ENP2 in CV seems unlikely. The two-segmented condition of the male P2 ENP is the result of the fusion of the middle and distal endopodal segments during the last moult from CV to the adult stage (Rosenfield and Coull, Reference Rosenfield and Coull1974). The pronounced rounded blunt, inner outgrowth located proximally on the second segment of the adult male P2 ENP2 of all the species of Paramphiascella and in Amphiascoides atopus, is probably the remains of the former original division between ENP2 and ENP3. The enlargement of this projection in A. atopus is regarded here as apomorphic within that genus and apomorphic for Paramphiascella (see below). A similar, but far less pronounced—and probable homologue—expansion from which an inner proximal seta arises is present in the male P2 ENP2 of many other diosaccins (e.g. Robertsonia glomerata Fiers, 1996, R. adduensis [Sewell, 1940], Robertgurneya hopkinsi [Lang, 1965], R. diversa [Lang, 1965], Amphiascoides petkovskii Lang, 1965, Miscegenus Wells, Hicks and Coull, 1982, Neomiscegenus). The two setiform inner elements present on the adult male P2 ENP2 of most species of Paramphiascella probably correspond to the inner seta of ENP2 and to the inner proximal seta of ENP3 present in the three-segmented ramus of CIV and in the male CV (elements 13 and 15 in Rosenfield and Coull [Reference Rosenfield and Coull1974: 311, figures 77, 79]). The distal inner seta on P2 ENP2 of CIV (element 19 in Rosenfield and Coull [Reference Rosenfield and Coull1974: 311, figure 77]) is the serial homologue of the distal inner element 19 of the male P2 ENP3 of the male CV shown in Rosenfield and Coull (Reference Rosenfield and Coull1974: 311, figure 79). This seta undergoes further modification during the last moult from CV to the adult stage, resulting in the—autapomorphic—stiff element with bi-lobed tip in the adult male P2 ENP2 of Paramphiascella. The distal medial seta present in the last segment of the two-segmented P2 ENP of CIV (seta 20 in Rosenfield and Coull [Reference Rosenfield and Coull1974: 311, figure 77]) is lost in the male during the moult from CIV to CV. The distal outer spine on the second segment of the two-segmented P2 ENP of CIV (element 21 in Rosenfield and Coull [Reference Rosenfield and Coull1974: 311, figure 77]) undergoes thickening and enlargement in the male during the moult from CIV to CV (element 20, 21 in Rosenfield and Coull [Reference Rosenfield and Coull1974: 311, figure 79]), and further modification into a large, strong distal apophysis in the adult male. The same pattern can be observed in P. vararensis (see Kim et al., Reference Kim, Jung and Lee2000). The male of Paramphiascella hyperborea underwent secondary loss of one inner proximal seta of P2 ENP2. Paramphiascella robinsonii and P. sirbonica underwent complete loss of the inner proximal setae. The setation pattern of the male P2 ENP2 of Amphiascoides follows the same pattern except for the unmodified—plesiomorphic—distal setiform inner element.
In his description of Paramphiascoides mixtus, Wells (Reference Wells1967) hypothesized on a close relationship between—the monotypic—Paramphiascoides, Amphiascoides, and Paramphiascella. These three genera share the armature formulae of P1 EXP, and P2–P5. The armature formula of P1 ENP of P. mixtus (1,1,020) is different from most species of Paramphiascella and Amphiascoides (1,1,111). However, some species of the latter two genera, viz., Paramphiascella delamarei, P. pacifica, and Amphiascoides coreanus Lee, Soh and Suh, 2007, and A. nichollsi Lang, 1965, possess also only two elements on P1 ENP3. Another species of Amphiascoides, A. sterilis Monard, 1926, also shares the bisetose distal segment of P1 ENP with the above species, but this species lacks also the inner seta of P1 ENP2. The inner seta of P1 ENP2 is also missing in other species of Paramphiascella and Amphiascoides (Paramphiascella xiphophora, and Amphiascoides koltuni Chislenko, 1977, A. lancisetiger Lang, 1965, A. paradebilis Chislenko, 1978, A. proximus [Scott, 1914], A. sterilis, and A. walteri Suárez-Morales and Avilés-Torres, 2003). As noted above, Wells (Reference Wells1967) could not attribute his newly found Amphiascoides-like specimen neither to Amphiascoides or Paramphiascella because of the unusual architecture of the male P2 ENP and decided to erect a new genus, Paramphiascoides. Wells (Reference Wells1967) commented on a possible common ancestor of Paramphiascella and Amphiascoides, and that Paramphiascoides might have evolved from Amphiascoides. If this is correct, the distal inner, setiform element of the male P2 ENP2 of Amphiascoides, undergoes further radical modification into a strong—probably autapomorphic—apophysis in Paramphiascoides. If this is the case, the transformation into a strong apophysis in Paramphiascoides but into a stiff element with bilobed tip in Paramphiascella is not phylogenetically related but is the result of homoplasy. This scenario seems to be reinforced by the—plesiomorphic—lack of the pronounced rounded blunt, inner proximal outgrowth on the male P2 ENP2 in most species of Amphiascoides (except for A. atopus) and Paramphiascoides. The pronounced, inner outgrowth on the male P2 ENP2 of A. atopus is regarded here as apomorphic within that genus and for Paramphiascella, for whom it is probably homoplasic. The alternative—less parsimonious—scenario assumes an Amphiascoides-like common ancestor for Amphiascoides and Paramphiascella whose distal inner, setiform element of the male P2 ENP2 undergoes modification into the stiff element of Paramphiascella which also develops the pronounced rounded blunt, inner proximal outgrowth on the male P2 ENP2; the distal inner stiff element of the male P2 ENP of Paramphiascella is transformed into a strong apophysis in Paramphiascoides which undergoes secondary loss of the pronounced rounded blunt, inner proximal outgrowth of that segment. In this case, the stiff element of Paramphiascella is homologous to the inner distal apophysis of Paramphiascoides, and the lack of the pronounced rounded blunt, inner proximal outgrowth on the male P2 ENP2 in Amphiascoides (except for A. atopus) and the secondary loss of that structure in Paramphiascoides is the result of homoplasy, as is its presence in A. atopus and Paramphiascella.
In their description of the, so far, monotypic genus Miscegenus, Wells et al. (Reference Wells, Hicks and Coull1982) noted that the female of M. heretaunga could be identified with Paramphiascella, and could be separated from Amphiascoides only by the short P1 ENP1, and from Paramphiascoides by a recognizable trace of the three-segmented A2 EXP. They (Wells et al., Reference Wells, Hicks and Coull1982) also noted that the male of their newly described species could be separated from the other three genera by the P2 ENP only. In their phylogenetic reconstruction, Wells et al. (Reference Wells, Hicks and Coull1982: 168–170) noted (i) that the male P2 ENP of Paramphiascella, Paramphiascoides, and Amphiascoides derived from that of Amphiascus, (ii) that Haloschizopera and Miscegenus constitute a clade with a sister group relationship with Bulbamphiascus, and (iii) that the structure and setation of the male P2 ENP of M. heretaunga indicates that Miscegenus is relatively more primitive than the Paramphiascella-Amphiascoides-Paramphiascella group.
Karanovic and Ranga Reddy (Reference Karanovic and Ranga Reddy2004) proposed the genus Neomiscegenus for N. indicus Karanovic and Ranga Reddy, 2004. They noted that Neomiscegenus belongs to the Amphiascus group as previously defined by Wells et al. (Reference Wells, Hicks and Coull1982), and that N. indicus could be identified with Paramphiascella using Lang's (Reference Lang1965) key. Karanovic and Ranga Reddy (Reference Karanovic and Ranga Reddy2004) suggested a close relationship between Neomiscegenus, and Paramphiascella, Amphiascoides, and Paramphiascoides, and noted that the male P2 ENP is the only reliable character to separate them. They concluded that Neomiscegenus is most closely related to Miscegenus and, contrary to what Wells et al. (Reference Wells, Hicks and Coull1982) thought, they hypothesized on a common ancestor for Amphiascoides, Paramphiascella, Paramphiascoides, Miscegenus, and Neomiscegenus, and argued that the Miscegenus-Neomiscegenus clade could have diverged relatively early from the other branch that gave birth to the Amphiascoides-Paramphiascella-Paramphiascoides clade (see above). Also, judging by the evidence, Neomiscegenus seems to have diverged from a Miscegenus-like common ancestor of Miscegenus and Neomiscegenus. If this is the case, the male P2 ENP of the ancestor invoked by Karanovic and Ranga Reddy (Reference Karanovic and Ranga Reddy2004) should have been similar to Willen's (Reference Willen2000: 163; 167, figure 69) type 4.
The male P2 ENP2 of Paramphiascella aquaedulcis was described by Dussart (Reference Dussart1984: 52, figure 27: 60) with two inner setae and two unequal spiniform processes similar to that of P. mixtus. The presence of the blunt inner proximal projection on the second segment of the adult male P2 ENP2 of P. aquaedulcis seems to indicate his belonging to Paramphiascella but the nature of its distal inner short apophysis needs to be verified. Paramphiascella mediterranea was created by Lang (Reference Lang1948) for Amphiascus affinis sensu Monard (Reference Monard1926, Reference Monard1928b) and Amphiascus vararensis sensu Monard (Reference Monard1928a, Reference Monard1935, Reference Monard1937), and the male P2 ENP2 was consistently described with a blunt inner proximal projection, two setiform elements, and one distal apophysis. The loss of the stiff element with bilobed tip seems to have occurred secondarily in P. mediterranea. Chullasorn et al. (Reference Chullasorn, Anansatitporn, Kangtia, Klangsin and Jullawateelert2011) described the adult female and male, and nauplii of Paramphiascella choi Chullasorn, Anansatitporn, Kangtia, Klangsin and Jullawateelert, 2011. The male P2 ENP2 was described with a blunt inner proximal projection, with three inner setiform elements, and one distal strong apophysis (see Chullasorn et al., Reference Chullasorn, Anansatitporn, Kangtia, Klangsin and Jullawateelert2011: 32, figure 9b: 35). Chullasorn et al. (Reference Chullasorn, Anansatitporn, Kangtia, Klangsin and Jullawateelert2011: 41) gave a list of character and character states to justify the allocation of their species to Paramphiascella, commented on other characteristics in which their species differ from other congeners, and argued on a closer relationship between P. choi and P. fulvofasciata. In our opinion, the male P2 ENP of P. choi does not correspond to Paramphiascella, but to Amphiascoides, and seems to be closely related to A. atopus. Here we remove P. choi from that genus and reallocate the species into Amphiascoides as Amphiascoides choi (Chullasorn, Anansatitporn, Kangtia, Klangsin and Jullawateelert, 2011).
As noted above, several authors commented on the difficulty of separating the females of Amphiascoides and Paramphiascella, but also Paramphiascoides. The separation of these three genera can be successfully and accurately done through careful inspection of the structure and setation of the male P2 ENP. Several species of Paramphiascella (P. brucei, P. bulbifer, P. curtiseta, P. delamarei, P. faurei, P. langi, P. roberti, P. vararensis) and Amphiascoides (A. brevifurca, A. debilis, A. dispar, A. golikovi Chislenko, 1977, A. intermixtus [Willey, 1935], A. koltuni, A. littoralis, A. nanoides [Sars G.O., 1911], A. nichollsi, A. proximus, A. pygmaea, A. sterilis, A. walteri) are known from the female only or the male has not been described completely. The male of these species still awaits discovery and full re-description to justify their belonging to their respective genera.
The mandible, maxillule, maxilla, and maxilliped of Amphiascoides and Paramphiascella have received little attention and have been seldom described in detail. Judging by the available published illustrations of the mouthparts of the different species of both genera, it seems that their structure and setation is rather conservative, with minor differences in armature complement and/or segmentation. On the other hand, contrary to the above appendages, and given its size and ease of observation, the segmentation of the antennary exopod of the different species of Amphiascoides and Paramphiascella might give some clues on their interspecific relationships. A reduction in the segmentation of the antennary exopod from three to two segments occurs in both genera and two main lineages seem to have evolved independently within them. Most species of Paramphiascella (P. aquaedulcis, P. austroatlantica, P. bodini, P. bulbifer, P. coulli, P. curtiseta, P. delamarei, P. faurei, P. fulvofasciata, P. hispida, P. hyperborea, P. intermedia, P. mediterranea, P. pacifica, P. roberti, P. vararensis) possess three-segmented antennary exopods, and only few species (P. aestuarii n. sp., P. calcarifer, P. commensalis, P. dahmsi, P. ferrarii, P. langi, and P. sirbonica) display two-segmented antennary exopods. The antennary exopods of P. xiphophora, P. brucei, and P. robinsonii remain undescribed. The antennary exopod of P. mixtus is two-segmented. Within Amphiascoides, only four species (A. brevifurca, A. koltuni, A. proximus, and A. walteri) possess two-segmented antennary exopods. Amphiascoides atopus, A. breviarticulatus Kunz, 1983, A. bulbiseta Pallares, 1975, A. choi, A. coreanus, A. dimorphus Lang, 1965, A. dispar, A. lancisetiger, A. nanoides, A. nanus (Sars G.O., 1906), A. neglectus (Norman and Scott T., 1905), A. nichollsi, A. paradebilis, and A. petkovskii, possess three segmented A2 exopods. The antennary exopods of A. debilis, A. golikovi, A. intermixtus, A. littoralis, A. sterilis, and A. subdebilis, remain unknown. When three-segmented, the first segment of A2 EXP of Paramphiascella, Amphiascoides, and Paramphiascoides, bears one seta; the third segment possesses one lateral seta (except for P. intermedia whose A2 EXP3 lacks lateral armature), and some variation from four (A. bulbiseta), to three (A. breviarticulatus, A. lancisetiger, A. nanus), two (most species), and one seta (P. intermedia) may occur in the last segment. The middle segment invariably lacks armature. In his written description, Norman and Scott (Reference Norman and Scott1905) described the antennary exopod of A. pygmaea as three-segmented but they did not give any illustration of this appendage. Norman and Scott (Reference Norman and Scott1906, Pl. X, figure 3, not figure 2, see above) showed that the armature formula of the three-segmented A2 EXP of A. pygmaea is 1,1,110. Pending redescription of both sexes of the species, we propose to temporarily remove A. pygmaea from Amphiascoides and to reallocate the species into Amphiascus as Amphiascus pygmaeus (Norman and Scott T., 1905).
The interspecific differences between the species of Paramphiascella are very subtle (Lang, Reference Lang1965; Wells, Reference Wells2007), and some species are so similar that one has to pay special attention to the microcharacters of the male P2 ENP for species separation (Marcotte, Reference Marcotte1974). Making and using keys for species separation is not an easy task either, and the results of the identification should not be taken as phylogenetically correct without an in-depth analysis. Wells (Reference Wells2007) relied on the presence/absence of different characters and character states available in the literature. Some of these characters might have been overlooked by the original authors or are the result of some kind of misinterpretation. For example, in separating P. calcarifer from P. langi, P. robinsonii, and P. sirbonica, Wells (Reference Wells2007: 553) invoked some differences in the shape of the caudal setae, length ratio of P1 ENP1:EXP, length:width ratio of P1 ENP1, and architecture of the fourth segment of the male antennule. If Sewell's (Reference Sewell1940) drawing of the P1 is correct and precise, P. calcarifer could be unique by the elongation of P1 ENP1, the latter reaching beyond the tip of EXP. Interestingly, the A2 EXP and A2 ENP, and the female and male antennulary segments are also—suspiciously—enlarged. Wells (Reference Wells2007: 553) also expressed doubts about the identity of several descriptions and records of P. robinsonii, and about the identity of the male of that species and P. sirbonica. Wells (Reference Wells2007: 553) used the presence/absence of a ‘long blade shaped extension’ of the distal margin of the fourth segment of the male antennule that extends well into the sixth segment to separate P. robinsonii sensu Pallares (Reference Pallares1968) and P. sirbonica from other records of P. robinsonii, and from P. calcarifer, and P. langi. The blade-like structure shown for P. robinsonii in Pallares (Reference Pallares1968: 74, Pl. XXIV-2) and for P. sirbonica in Por (Reference Por1973: 101, Pl. VII, figure 40), is actually an inner muscular or chitinized structure of the globose modified segment of the male antennule reaching well beyond the proximal margin of the next segment. This inner structure is present also in the distal part of the fifth segment of the male antennule of P. aestuarii n. sp. and reaches beyond the middle of the sixth segment. This structure is also present in the globose modified segment of the male antennule of some other diosaccins like Robertsonia sp. (Gómez, pers. obs.), and is probably involved in the flexibility and strength of the geniculation of the male antennule during attachment to the female.
Gómez et al. (Reference Gómez, Corgosinho and Rivera-Sánchez2021), based on morphology and parsimony analyses, supported the close relationships between Amphiascoides, Paramphiascella, and Robertgurneya. This lineage has been supported with molecular evidence from 18S rRNA by Yeom and Lee (Reference Yeom and Lee2020, Reference Yeom and Lee2022, Reference Yeom and Lee2023) and in this study. However, more evidence is required to evaluate the monophyly of each genus and to provide support for their evolutionary affinities to corroborate the relevance of the morphological structure from male P2 ENP2.
The mtCOI topology, as expected by the evolutionary rate, provided unresolved relationships. However, all genera with two or more species for which the mtCOI sequence is available, are well-structured, pointing out the need of incorporating molecular sequences of more species of Amphiascoides, Paramphiascoides, and Paramphiascella to better understand their relationships. The lack of correspondence amongst the available sequences of 18S rDNA and mtCOI makes multilocus analyses difficult. Yeom and Lee (Reference Yeom and Lee2020) provided recently a detailed table with the genetic distance between almost all species included in their study. Here we give only the genetic distance between the new species and the other available sequences for other miraciids. The genetic distance between the new species and Amphiascoides atopus (22%), and Amphiascoides sp. (25%) are slightly higher than 20% as reported by Yeom and Lee (Reference Yeom and Lee2023) between Rhyncholagena and the other species considered in their study.
Position and relationships of P. aestuarii n. sp.
The new species presented herein, P. aestuarii n. sp., belongs to the group of species of that genus with a two-segmented A2 EXP (P. calcarifer from the Maldivian Archipelago [Sewell, Reference Sewell1940], P. commensalis from the Woods Hole Region [Massachusetts, USA] [Seiwell, Reference Seiwell1928; Wilson, Reference Wilson1932], P. dahmsi and P. ferrarii from Thailand [Chullasorn, Reference Chullasorn2010], P. langi from Argelia [Monard, Reference Monard1936], and P. sirbonica from Lake Bardawil [Sinai Peninsula; Por (Reference Por1973)]). The armature formula of the antennary exopod and P1–P5 in both the female and male are identical in all the species of this group. In his figure of P3 of P. sirbonica, Por (Reference Por1973: Table VIII, figure 50) omitted the inner seta of P3 EXP2, but its place is indicated by a deep inner subdistal groove on that segment. Also, the female antennule, and the male and female postantennal mouthparts of the species above have not been described properly, or they show very conservative segmentation patterns and setal complements. All the above makes it difficult to separate the species and to hypothesize on their phylogenetical relationships. The full picture becomes blurred also because the male of P. langi remains undescribed and nothing can be said regarding the sexual dimorphism of the male P2 of that species. The P1 ENP1 is as long as the entire P1 EXP in P. aestuarii n. sp., P. calcarifer, P. commensalis, P. dahmsi, P. ferrarii, P. langi, and P. sirbonica, but some slight differences were observed in the relative length of the apical middle seta of the female and male P5 EXP. These differences could be useful for species separation, but their phylogenetic values seem questionable. The middle apical seta of the female P5 EXP is shorter than its inner neighbour in P. langi, P. sirbonica, and P. commensalis. Paramphiascella langi can be separated from the other two species by the shape of caudal setae IV and V. Paramphiascella commensalis and P. sirbonica can be separated by the inner armature of the male P2 ENP2 (with two setiform elements and the typical stiff element in P. commensalis, but only with the typical stiff element in P. sirbonica). The new Mexican species is similar to P. ferrarii, P. dahmsi, and P. calcarifer in that the middle apical seta of the female and male P5 EXP is as long or longer than its neighbour inner seta. Paramphiascella dahmsi can be separated from P. ferrarii, P. calcarifer, and P. aestuarii n. sp. by the irregular inner margin of the apical apophysis of the male P2 ENP2, by the cylindrical shape of the inner modified stiff element, and the subequal inner setiform elements in the same segment. The inner margin of the distal apophysis of the male P2 ENP2 is plain in P. ferrarii, P. calcarifer, and P. aestuarii n. sp., the inner modified stiff element tapers distally, and the proximal inner setiform element is shorter than its distal neighbour. However, P. ferrarii differs from P. calcarifer and the new species in the relative length of the two setiform inner elements of the male P2 ENP2. The proximal seta is less than half the length of the other seta in P. ferrarii, but more than half the length in the other two species. The only record of P. calcarifer is the original description of Sewell (Reference Sewell1940). He described two forms of P. calcarifer, P. calcarifer f. minor, and P. calcarifer f. major, with ‘no appreciable difference between’ them (Sewell, Reference Sewell1940). He noticed some slight differences in the general shape of the female P5 but this was attributed to intraspecific variability, and he thought that there was no valid reason for the separation of these two forms as distinct species. Some differences were observed between P. calcarifer and the new Mexican species. These are outlined below. Sewell (Reference Sewell1940) described and showed the P4-bearing somite with a ‘claw-like spine’ on the distal posterior corner on each side of the somite. This is not but the pointed expansion of the somite that can be observed dorsally in many other copepods and is of no taxonomic value. The first segment of the female antennule was described with only one subdistal spinular row; other species display one proximal and one distal spinular row, and Sewell (Reference Sewell1940) might just have overlooked the proximal one. Sewell (Reference Sewell1940) described the antenna as three-segmented assuming the presence of a basis and a two-segmented endopod. This is most probably erroneous, and the integumental line at the base of the exopod could represent the remains of the former division between the basis and the first endopodal segment. He described the second endopodal segment of the antenna with two lateral spines, one distal inner spine, four distal geniculate setae, and one outer slender element. We believe that Sewell (Reference Sewell1940) missed the slender seta close to the distal lateral spine and the small seta fused basally to the distal outer spinulose element. Some differences were also observed in the setation pattern of the mandibular palp and rami, but Sewell (Reference Sewell1940) might just have overlooked some setae (the setation pattern of A1 and postantennal mouthparts of—at least—this group of species with a two-segmented A2 EXP is rather conservative). We are not certain about the accuracy of Sewell's (Reference Sewell1940) drawings regarding the relative length of the setae of the female P5 (e.g. the outermost element of the female P5 EXP is spiniform in P. calcarifer, but setiform in the other species). The most important difference between P. calcarifer and P. aestuarii n. sp. is the presence, in P. calcarifer, of one proximal and one subdistal outer spiniform processes on the female second antennulary segment. These processes are absent in P. aestuarii n. sp.
A comprehensive revision with more molecular data of representatives of Amphiascoides, Paramphiascoides, and Paramphiascella is needed. Also, an integrative approach is required to shed more light on the relationships of these genera towards the clarification of the systematics of the Diosaccinae.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0025315424001097.
Acknowledgements
This is a contribution of project A204320 ‘Comparative genomics in harpacticoid copepods: mechanisms and adaptation to different trophic conditions’ financed by the Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica of the Dirección General de Asuntos del Personal Académico of the Universidad Nacional Autónoma de México (UNAM-DGAPA-PAPIIT). The authors thank Giselle A. Chávez-Salgado for her assistance. The authors acknowledge The Nippon Foundation-Nekton Ocean Census Programme (https://oceancensus.org/) for supporting the discovery and description of this species. This is Ocean Census Species Number 41.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article. Additional information is available from the corresponding author upon reasonable request.
Author contributions
S. G., B. Y.-R., and R. L.-H. designed the study. B. Y.-R. and R. L.-H. contributed to field collection. R. L.-H. cultured and obtained genomic sequences and mitochondrial assemblies. S. G. studied morphology, prepared the illustrations, and wrote the first draft of the manuscript. B. Y.-R. performed phylogenetic analyses. All authors reviewed the manuscript and approved the final version.
Financial support
This work was supported by the Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica of the Dirección General de Asuntos del Personal Académico of the Universidad Nacional Autónoma de México (UNAM-DGAPA-PAPIIT) (grant number A204320). The authors acknowledge The Nippon Foundation-Nekton Ocean Census Programme (https://oceancensus.org/) for supporting the discovery and description of this species. This is Ocean Census Species Number 41.
Competing interest
None.
Ethical standards
All applicable institutional, national, and international guidelines for the care and use of animals were followed.













