Introduction
The Flower Garden Banks National Marine Sanctuary (FGBNMS) is located off the Texas and Louisiana coasts on the continental shelf margin in the north-western Gulf of Mexico, and encompasses just three of the dozens of reefs and banks that occur in this area. Antipatharians are relatively common in some areas of the FGBNMS and 13 species (representing ~5% of the 273 described species) have been recorded from the sanctuary (Opresko et al., Reference Opresko, Nuttall and Hickerson2016), occurring at depths ranging from 50–150 m. The reefs and banks of the north-western Gulf of Mexico, including Elvers Bank, harbour significant mesophotic coral ecosystems and deeper coral communities (ONMS, 2016; Boland et al., Reference Boland, Etnoyer, Fisher, Hickerson, Hourigan, Etnoyer and Cairns2017; Semmler et al., Reference Semmler, Hoot and Reaka2017). Elvers Bank is located 65 km east of the East FGB (Figure 1) at the extreme edge of the continental shelf, extending to a depth of 205 m. Due to its shelf-edge location, surveys during the FGBNMS expeditions suggest a biological community somewhat different from other banks in the region, including assemblages of differing varieties of invertebrates not observed in other locations in the north-western Gulf of Mexico study area. While light measurements at this location are not available, it may be assumed that these deeper environments and associated biota require less light than those at shallower depths. During a National Oceanic and Atmospheric Administration (NOAA) survey cruise (Northwestern Gulf of Mexico Cruise 2016; Cruise DFH-30; Dive 382) conducted in September 2016, a large green, pinnately branched antipatharian colony was photographed at a depth of 172 m at Elvers Bank (Figure 2); however, samples of the colony were not collected at that time due to a malfunction of the manipulator arm on the remotely operated vehicle (ROV). The in-situ photos suggested that the colony represented an undescribed species. In September 2017, during a follow-up cruise to the same area (NW GOMEX Expansion Sites I; Cruise DFH-32; Dive 524), two colonies of what appeared to be the same species were collected at 172 m depth (Figure 3) and preserved in 100% ethanol and RNAlater for morphological and molecular analyses. These two specimens are the subject of this paper.

Fig. 1. The top panel shows the location of the Flower Garden Banks National Marine Sanctuary (FGBNMS), which is located off the Texas (TX) and Louisiana (LA) coasts on the continental shelf margin in the north-western Gulf of Mexico. The bottom panel shows that Elvers Bank is located ~65 km east of West and East FGB. The 200-m depth contour is shown in grey.

Fig. 2. Distichopathes hickersonae sp. nov., colony photographed in situ, at 172 m on Elvers Bank (specimen not collected).

Fig. 3. (A)In-situ photo of holotype of Distichopathes hickersonae sp. nov. (USNM 1517703); (B) laboratory photo of holotype; (C) In-situ photo of paratype (USNM 1548274); (D) laboratory photo of paratype; (E) upper section of two branches of the paratype.
Materials and methods
This report is based on specimens collected during expeditions aboard the RV ‘Manta’ to reefs and banks of the north-western Gulf of Mexico, conducted by NOAA staff of the FGBNMS as well as students and staff of NYC College of Technology (CUNY). The ROV ‘Mohawk’, maintained and operated by the Undersea Vehicle Program at the University of North Carolina at Wilmington, was used to collect the specimens after in-situ photos were taken. Collected specimens were preserved in 100% EtOH and RNAlater and sent to the U.S. National Museum of Natural History (USNMNH), Smithsonian Institution, Washington, DC for further study. Photographs of the skeletal spines were made using a scanning electron microscope (SEM, Zeiss EVO MA 15) housed at the USNMNH. The specimens were coated with a 30–40 nm thick layer of 60% gold: 40% palladium. Analysis of the skeletal spines was conducted from direct examination of the material using a low-power binocular microscope or by examination of photographs taken with the SEM. The size of the polyps, referred to as the transverse diameter, was measured as the distance between the distal edge of the distal lateral tentacles and the proximal edge of the proximal lateral tentacles of the same polyp. The distance between spines was measured from the centre of the base of one spine to the centre of the base of an adjoining spine in the same axial row. The height of a spine was measured as the distance between the apex and the centre of the base of the same spine. Based on convention, the number of axial rows of spines was determined as the number of complete rows (those in which the bases of the spines are visible) that can be counted in one lateral view (also referred to as ‘one aspect’).
Subsamples of the specimens were subjected to molecular analysis which involved the sequencing of three mitochondrial gene regions (trnW-IGR-nad2, cox3-IGR-cox1 and nad5-IGR-nad1; IGR = intergenic region) and three nuclear gene regions (ITS2, 28S and SRP54). DNA extraction, DNA quantification, PCR primers and reagents, PCR thermocycling profiles, PCR cleanup, cycle sequencing, cycle sequencing cleanup, traditional Sanger sequencing on an ABI-3730xL, multiple sequence alignment, model selection, and Maximum likelihood-based tree building followed the protocol detailed in MacIsaac et al. (Reference MacIsaac, Best, Brugler, Kenchington, Anstey and Jordan2013). Brugler et al. (Reference Brugler, Opresko and France2013) showed that the nad5-IGR-nad1 region was the most variable region within the black coral mitogenome; however, the cox3-IGR-cox1 region revealed the greatest number of unique haplotypes (i.e. unique species). Given that the phylogenetic reconstructions presented in Brugler et al. (Reference Brugler, Opresko and France2013) remain the most comprehensive to date, newly obtained sequence data (corresponding to nad5-IGR-nad1 and cox3-IGR-cox1 only) were added to the multiple sequence alignments from Brugler et al. (Reference Brugler, Opresko and France2013). Maximum likelihood-based phylogenies were built to infer the evolutionary relationship of Distichopathes hickersonae to known taxa within the Order Antipatharia. Currently, datasets for the three nuclear gene regions (ITS2, 28S and SRP54) are not as comprehensive (in terms of representative species) as the mitochondrial gene regions; thus, these regions were simply used to look for genetic differences between the holotype and paratype.
Results
Systematics
Order ANTIPATHARIA Milne-Edwards & Haime, Reference Milne Edwards and Haime1857
Family APHANIPATHIDAE Opresko, Reference Opresko2004
Diagnosis
Corallum sparsely to densely branched (i.e. bramble-like, bushy, broom-like or fan-shaped), or pinnulated with simple pinnules arranged in two or more rows. Spines conical; usually covered to varying degrees with conical tubercles, but may be smooth; apex of spines simple (acute or rounded), occasionally bifurcated. Polyps 0.6–3 mm in transverse diameter.
Remarks
The family was originally established with two subfamilies, the Aphanipathinae (including the genera Aphanipathes, Phanopathes, Tetrapathes, Pteridopathes and Asteriopathes) with subequal polypar spines, and the Acanthopathinae (including the genera Elatopathes, Distichopathes, Rhipidipathes and Acanthopathes) with very unequal (anisomorphic) polypar spines where the circumpolypar spines are very enlarged and the hypostomal spines are reduced or even absent (Opresko, Reference Opresko2004). Recent DNA sequencing studies (e.g. Brugler et al., Reference Brugler, Opresko and France2013) using mitochondrial markers (cox3-IGR-cox1 and nad5-IGR-nad1) have not supported the recognition of these two subfamilies, and in fact the genera Elatopathes and Distichopathes have closer affinities to the family Myriopathidae than to other genera in the Aphanipathidae (Figures 4 and 5). In addition, the genus Acanthopathes grouped with several different families depending on the molecular marker or tree-building algorithm employed, and the genus Rhipidipathes grouped with genera in the Antipathidae (not shown). These results, if confirmed by more extensive molecular studies using higher resolution markers (i.e. Ultra-Conserved Elements; see Quattrini et al., Reference Quattrini, Faircloth, Duenas, Bridge, Brugler, Calixto-Botía, DeLeo, Foret, Herrera, Lee and Miller2018), may necessitate a taxonomic revision of the family.

Fig. 4. Partial results of a Maximum likelihood-based phylogenetic reconstruction using mitochondrial nad5-IGR-nad1 sequence data focusing specifically on the families Aphanipathidae and Myriopathidae. The MAFFT L-INS-i v7 based alignment consisted of 49 sequences and 686 sites. jModelTest v2.1.1 selected the TPM3uf + G model of sequence evolution. PhyML v3.1 utilized a BioNJ starting tree, best of NNI and SPR tree topology search options, and 1000 non-parametric bootstrap replicates. The tree was rooted internally to the Leiopathidae. Node support for Distichopathes hickersonae grouping with Elatopathes abietina is 99.9. Distichopathes hickersonae and Elatopathes abietina are genetically identical across 318 comparable base pairs. Node support for the clade grouping sister to Distichopathes + Elatopathes (which includes Stylopathes, Antipathes, Tanacetipathes, Plumapathes and Myriopathes) is 78.

Fig. 5. Partial results of a Maximum likelihood-based phylogenetic reconstruction using mitochondrial cox3-cox1 sequence data focusing specifically on the families Aphanipathidae and Myriopathidae. The MAFFT L-INS-i v7 based alignment consisted of 59 sequences and 1111 sites. jModelTest v2.1.1 selected the GTR + I + G model of sequence evolution. PhyML v3.1 utilized a BioNJ starting tree, best of NNI and SPR tree topology search options, and 1000 non-parametric bootstrap replicates. The tree was rooted to sea anemones (Metridium senile and Nematostella vectensis) and zoanthids (Palythoa sp. and Savalia savaglia). Node support for Distichopathes hickersonae grouping sister to Elatopathes abietina is 99.8. Distichopathes hickersonae and Elatopathes abietina can be genetically distinguished by a single base substitution (C/T) and a 38 bp insertion (in Elatopathes) across 826 comparable base pairs.
Genus Distichopathes Opresko, Reference Opresko2004
Antipathes, de Pourtalès, Reference de Pourtalès1867: 112, Reference de Pourtalès1871: 54, Reference de Pourtalès1880: 118 (in part); van Pesch, Reference van Pesch1914: 85 (in part, as subgenus Aphanipathes).
Aphanipathes Brook, Reference Brook1889: 121 (in part); Opresko, Reference Opresko1972: 993 (in part).
Distichopathes Opresko, Reference Opresko2004: 235–237.
Diagnosis
Corallum monopodial, unbranched, or sparsely to densely branched; tending to be planar with overlapping branches. Stem and branches pinnulate. Pinnules simple, not subpinnulate; arranged primarily in two lateral rows, but with simple short pinnules occurring very rarely on the abpolypar side of the axis. Pinnules also arranged alternately along the stem and branches.
Type species
Distichopathes disticha Opresko, Reference Opresko2004.
Remarks
The genus Distichopathes is distinguished from the other genera in the family Aphanipathidae by the simple, bilateral pinnules and distinctly anisomorphic spines. Species of the genus resemble those of Pteridopathes which also have two rows of simple bilateral pinnules; however, in Pteridopathes the polypar spines are subequal in size or, in some cases, the circumpolypar spines are slightly taller than the other polypar spines. The anisomorphic spines of Distichopathes are similar to those of Elatopathes, the colonies of which also have simple pinnules, but in the latter case the pinnules are arranged in four to six axial rows.
Species assigned to Distichopathes
Three species are currently assigned to the genus, D. filix (de Pourtalès, Reference de Pourtalès1867), D. disticha Opresko, Reference Opresko2004, and D. hickersonae sp. nov.
Distribution
Species of Distichopathes are known only from the North-western Atlantic.
Distichopathes hickersonae sp. nov. Opresko & Brugler
(Figures 2, 3, 6, 7)
Aphanipathes filix, Opresko, Reference Opresko1974: 1003 (in part).

Fig. 6. Distichopathes hickersonae sp. nov., holotype, USNM 1517703: (A) corallum; (B) section of pinnule; (C) close-up view of polypar spines (B and C from SEM stub 481).

Fig. 7. Distichopathes hickersonae sp. nov., holotype, USNM 1517703: sections of pinnules (from SEM stub 481).
Aphanipathes filix de Pourtalès, Reference de Pourtalès1867: 112 (in part)
?Antipathes dissecta Duchassaing & Michelotti, Reference Duchassaing and Michelotti1864: 48.
Type material
Holotype: USNM 1517703 (SEM 481). North-western Gulf of Mexico, Elvers Bank, 27.8434°N 92.9384°W, RV ‘Manta’, Stn DFH32-524A, Site E1-S, 172 m, NOAA, 25 Sept 2017.
Paratype: USNM 1548274, North-western Gulf of Mexico, Elvers Bank, 27.8433°N 92.9386°W, RV ‘Manta’, Stn DFH32-524B, Site E1-S, 172 m, NOAA, 25 Sept 2017.
Diagnosis
Corallum densely branched, branches tending to lie in one plane. Stem and branches with simple bilateral pinnules. Pinnules up to 2 cm long and 0.3 mm in diameter at their base; arranged in two very regular bilateral rows, with pinnules in each row alternating with those in opposite row. Small simple pinnules occurring very rarely on the abpolypar side of the axis. Spines anisomorphic: circumpolypar spines 0.26–0.35 mm tall, interpolypar spines 0.2–0.25 mm, the hypostomal spines 0.1–0.14 mm, and the abpolypar spines 0.1–0.18 mm. Polyps small, 0.65–0.8 mm in transverse diameter and are placed in a single series on one side of the pinnules, with 8–10 polyps per cm.
Description of the holotype
The holotype (USNM 1517703) is a moderately sized complete colony about 21 cm tall and 17 cm wide (Figures 3A, B & 6A). The colony is branched to the fifth order, and the branching is planar with overlapping adjacent branches. The diameter at the base of the stem just above the holdfast is 3.7 mm. Several large first-order branches originate just above the holdfast, one of which extends to the top of the colony. The stem and branches possess simple, filiform pinnules up to about 2 cm long and 0.3 mm in diameter near the base. The pinnules vary slightly in size from branch to branch, and can also be shorter on the lower parts of the branch compared with the more distal segment. Newly forming pinnules at the branch tips gradually increase in size proximally until they reach a consistent length. The pinnules are arranged in two lateral rows with members of each row spaced 1–1.4 mm apart (from the middle of pinnule at the base to the middle of the base of adjacent pinnule in the same row), resulting in 16–18 pinnules per cm total for both rows. Most of the pinnules are inclined distally, but some extend out at nearly right angles to the axis of the stem or branch. The interior angle formed by the two rows of pinnules is close to 180°. Isolated pinnules occur on the abpolypar side of the stem and branches.
The pinnular spines are distinctly anisomorphic (Figures 6B and 7); the circumpolypar spines are up to 0.35 mm tall, although most are 0.26–0.30 mm. The interpolypar spines are 0.2–0.25 mm, the hypostomal spines 0.1–0.14 mm, and the abpolypar spines 0.1–0.16 mm. The spines are arranged in axial rows, six rows of which can be seen in one lateral view. The density of the polypar spines is mostly 6–7 per mm in each row, whereas the abpolypar spines are much more crowded together with a density of 10–11 per mm. The polypar spines are slightly inclined distally (distal angle 60–80°), and the abpolypar spines are much more distally inclined with a distal angle of about 30°. The spines on the branches and stem are more acicular than those on the pinnules; they are mostly 0.16–0.2 mm in height. Almost all the pinnular spines, both polypar and abpolypar, are covered over the upper (distal) half of their surface with very distinct and very numerous conical tubercles (Figure 6C). The hypostomal spines, however, are either smooth or have only a few tubercles near the apex. In the samples examined, two hypostomal spines are reduced in size in each polyp area.
The polyps are 0.65–0.8 mm in transverse diameter and are placed in a single series on one side of the pinnules, with 8–10 polyps per cm. The distance between centres of adjacent polyps is 1–1.2 mm.
Intraspecies variation
The paratype (USNM 1548274, Figure 3C–E) agrees with the holotype in all skeletal and polypar characters. The pinnules are mostly 1.5 cm long, but can be as much as 2 cm. The density of the pinnules is 16–18 per cm total for both lateral rows. The pinnules at the tips of the branches increase in length proximally, but the rate of increase varies from branch to branch (Figure 3E). On sections of pinnules 0.20–0.24 mm in diameter, the circumpolypar spines range in size from 0.26–0.32 mm, the interpolypar spines are about 0.24 mm, the hypostomal spines 0.11–0.13 mm and the abpolypar spines 0.15–0.18 mm. The polyps are similar to those of the holotype in the transverse diameter, with a resulting similar density of 9 per cm.
Comparisons
The two other nominal species in the genus are D. filix (de Pourtalès, Reference de Pourtalès1867) and D. disticha Opresko, Reference Opresko2004. Specimens that conform to Pourtalès' original description of D. filix characteristically are monopodial, unbranched, and have very short pinnules 0.6–1 cm long (Figure 8A). Colonies of D. disticha are sparsely branched, with up to three orders of branching, and have pinnules that are mostly 3–7 cm long (maximum about 12 cm) (Figure 9A). In contrast, colonies of D. hickersonae can be densely branched (up to five orders of branching) and have pinnules that are up to 2 cm in length. The fact that the examined colonies of D. disticha and D. hickersonae are of comparable size would support the supposition that the differences in the length of pinnules and the density of the branching are not age-related intraspecific phenomena.

Fig. 8. Distichopathes filix Pourtales, specimen from R/V Gerda sta. 1125: (A) corallum; (B) section of pinnule.

Fig. 9. Distichopathes disticha, holotype, MCZ 38476: (A) corallum: (B) section of pinnule; (C) close-up view of polypar spines.
The pinnular spines of D. hickersonae are morphologically more similar to those of D. disticha (Figure 9B) than to those of D. filix (Figure 8B). In D. filix the spines usually have a clavate shape in that some are slightly swollen towards the apex, and the apolypar spines can be distinctly flattened and bent upwards at the apex. In contrast, the spines of D. disticha and D. hickersonae are more regularly cylindrical. In terms of the size of the spines, D. hickersonae is also more similar to D. disticha. The circumpolypar spines are mostly 0.26–0.30 mm tall in D. hickersonae (maximum about 0.35 mm); up to 0.4 mm in D. disticha, and up to 0.55 mm in D. filix. In the samples examined, the interpolypar spines, hypostomal spines and abpolypar spines in D. hickersonae are similar to or slightly larger than the corresponding ones in the other two species (Table 1). Although the number of observations is limited, one difference seen between D. hickersonae and D. disticha is in the number of hypostomal spines that are reduced in size; in D. hickersonae only two such hypostomal spines are found in each polyp area, whereas in D. disticha three are present.
Table 1. Morphometric comparison of the three species of Distichopathes

a Based on a limited number of specimens.
The maximum height of the spines, especially the polypar spines, has in the past been a key character in separating closely related antipatharian species. The differences seen here in the size of the circumpolypar spines between D. disticha and D. hickersonae, although not great, are suggestive that as larger suites of specimens are examined, the population distribution of this character will be different for each of these species.
Remarks
As in most antipatharian species complexes, the morphological boundaries between the three nominal species of Distichopathes are likely to be difficult to clearly define because of the inherent variability in most taxonomic characters. Overlaps in characters such as the length of the pinnules and size of the spines between closely related species is to be expected. The information presented in Table 1 is based on small sample sizes, especially for D. hickersonae and D. disticha; therefore, the values shown, such as the typical length of the pinnules and height of the spines will most likely change as more colonies are analysed. In addition, some specimens previously assigned to D. filix (see Table 4 in Opresko, Reference Opresko1972), particularly those that have intermediate pinnule lengths of 1–2 cm, will have to be re-evaluated to determine if the morphology of the spines provides a better indication of whether they are more closely related to D. filix or to D. hickersonae.
Molecular analysis
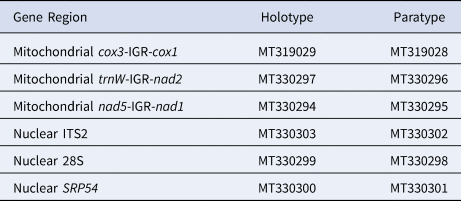
We compared the DNA sequence of the holotype (USNM 1517703) and paratype (USNM 1548274) using a combination of three mitochondrial and three nuclear gene regions. The holotype and paratype were genetically identical across all six gene regions (total number of base pairs compared: 2878 bp). We sequenced 1701 bp of mitochondrial DNA, which consisted of 492 bp from trnW-IGR-nad2, 880 bp from cox3-IGR-cox1 and 329 bp from nad5-IGR-nad1 (IGR: intergenic region). We also sequenced 1177 bp of nuclear DNA, which consisted of 603 bp from ITS2, 394 bp from 28S and 180 bp from SRP54 (Table 2). We were unable to obtain tissue from representatives of D. disticha and D. filix and corresponding sequence data is not available on GenBank; thus, we could not include either congeneric in our molecular analysis.
Table 2. GenBank accession numbers for the holotype (USNM 1517703) and paratype (USNM 1548274) of Distichopathes hickersonae sp. nov.
Etymology
Named in recognition of Emma L. Hickerson (NOAA's FGBNMS Research Coordinator). Since 2005, Emma has generously been inviting co-authors DMO and MRB, as well as underrepresented minority undergraduates from CUNY, to the FGBNMS to survey and collect black corals. These research cruises aboard the MV ‘Spree’ and RV ‘Manta’ have resulted in the discovery of a number of new species of antipatharian corals.
Distribution
Known only from the north-western Gulf of Mexico at 172 m depth.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S002531542000051X.
Data deposition information
All newly obtained sequence data have been uploaded to NCBI's GenBank (see Table 2 for individual accession numbers).
Acknowledgements
Special thanks to the shipboard crew of the RV ‘Manta’ and to the members of the scientific party (Nadia Alomari [CUNY], Erin Easton [UTRGV] and Robert McGuinn [DSCRTP]) for their efforts in helping collect the material on which this paper is based. Thanks to Sheila Moaleman (CUNY) and Nicole Bellaflores-Mejia (CUNY) for providing the first in-situ photos. MRB gratefully acknowledges Estefania Rodriguez (Curator of Marine Invertebrates at the AMNH) for allowing Team Black Coral to utilize her molecular lab. Specimens were collected using the National Marine Sanctuary Foundation's ROV ‘Mohawk’ operated by the Undersea Vehicle Program at the University of North Carolina at Wilmington, and the efforts of the ROV operators Lance Horn, Jason White and Eric Glidden are greatly appreciated. The specimens were examined in the SEM lab at the USNMNH, and assistance was generously provided by S. Whitaker. DMO and MRB are Research Associates at the USNMNH and gratefully acknowledge that affiliation. MRB is also a Research Associate at the American Museum of Natural History and gratefully acknowledges that affiliation as well.
Author contribution
Conceptualization: DMO, MRB; Methodology: DMO, MRB; Formal analysis and investigation: DMO, SLG, RJ, KP, MN, MRB; Writing – original draft preparation: DMO, MRB; Writing – review and editing: DMO, SLG, RJ, KP, MN, GPS, MRB; Funding acquisition: DMO, GPS, MRB; Resources: DMO, MN, GPS, MRB; Supervision: MRB.
Financial support
Funding for cruises was provided, in part, by NOAA's Deep-Sea Coral Research and Technology Program to GPS. Funding for this project was provided in part by the City University of New York, the Flower Garden Banks National Marine Sanctuary, and a U.S. Department of Justice grant to the Smithsonian Institution.













