INTRODUCTION
Isolated oceanic islands pose challenges to colonization by marine species while, at the same time, providing evolutionary opportunities for speciation of those few successful colonists (Vermeij, Reference Vermeij, Lomolino and Heaney2004; Dawson, Reference Dawson2016). As a result, oceanic islands are poorer in species richness and often contain more endemic species than continental shelves or islands (Robertson, Reference Robertson2001; Vermeij, Reference Vermeij, Lomolino and Heaney2004; Dawson, Reference Dawson2016). These particularities in isolated communities’ species pools can also foster the emergence of unique behaviours and interactions, as has been documented for marine species at South Atlantic oceanic islands (Lubbock, Reference Lubbock1980; Sazima et al., Reference Sazima, Sazima and Silva2003, Reference Sazima, Krajewski, Bonaldo and Sazima2007; Sazima & Sazima, Reference Sazima and Sazima2004; Gasparini et al., Reference Gasparini, Luiz and Sazima2008). Some of these interactions can have important ecosystem-wide consequences, for example, by enhancing the energetic flux among trophic compartments in these species-depauperate systems (Sazima et al., Reference Sazima, Sazima and Silva2003, Reference Sazima, Sazima and Silva2006; Sazima & Sazima, Reference Sazima and Sazima2004).
Reef fishes engage in a variety of intra- and inter-specific interactions, both among themselves and with invertebrates. Partnerships among fishes include cleaning symbiosis, associations that overwhelm territorial species (mobbing or mob-feeding), hunting by riding, aggressive mimicry and nuclear-follower interactions (Randall & Randall, Reference Randall and Randall1960; Ormond, Reference Ormond1980; Aronson, Reference Aronson1983; Sikkel & Hardison, Reference Sikkel and Hardison1992; Côté, Reference Côté2000; Lukoschek & McCormick, Reference Lukoschek and McCormick2000; Sazima, Reference Sazima2002; Grutter, Reference Grutter and Rohde2005; Randall, Reference Randall2005; Karplus, Reference Karplus2014). Albeit all such interactions occur in isolated oceanic islands (Barlow, Reference Barlow1974; Sazima et al., Reference Sazima, Krajewski, Bonaldo and Sazima2007, Reference Sazima, Grossman and Sazima2010a, Reference Sazima, Guimarães, Reis and Sazima2010b; Quimbayo et al., Reference Quimbayo, Floeter, Noguchi, Rangel, Gasparini, Sampaio, Ferreira and Rocha2012, Reference Quimbayo, Zapata, Floeter, Bessudo and Sazima2014), our work focuses on two of them: cleaning and mob-feeding.
Cleaning behaviour involves cleaner organisms (fishes and shrimps) that pick and eat ectoparasites, damaged tissue and mucus from other animals: the clients (Losey, Reference Losey1972; Grutter, Reference Grutter and Rohde2005). Cleaning interactions have a positive effect on the client's overall health, growth rates and reproductive and recruitment success (Cheney & Côté, Reference Cheney and Côté2003; Grutter, Reference Grutter and Rohde2005; Sun et al., Reference Sun, Cheney, Werminghausen, Meekan, McCormick, Cribb and Grutter2015). Most cleaner fishes are facultative cleaners, while only a handful of species are obligates. Facultative cleaners are most often juveniles that are not reliant upon cleaning as their primary means of obtaining food (‘part-time cleaners’), whereas obligate cleaners, in contrast, clean throughout their life and acquire their food mostly, or entirely, from their clients. We herein use this well-established terminology according to overviews of cleaning symbiosis and a number of often-cited references that propose and/or employ these two categories (Itzkowitz, Reference Itzkowitz1979; Côté, Reference Côté2000; Grutter, Reference Grutter and Rohde2005; Arnal et al., Reference Arnal, Verneau and Desdevises2006; Floeter et al., Reference Floeter, Vázquez and Grutter2007b; Francini-Filho & Sazima, Reference Francini-Filho and Sazima2008; Cheney et al., Reference Cheney, Grutter, Blomberg and Marshall2009; Soares et al., Reference Soares, Bshary, Cardoso, Côté and Oliveira2012).
Besides cleaning, another common multi-species association observed in tropical reef habitats is mobbing in large shoals, a feeding tactic employed to overcome the territorial defences of other fish (Barlow, Reference Barlow1974; Robertson et al., Reference Robertson, Sweatman, Fletcher and Cleland1976; Foster, Reference Foster1985; Reinthal & Lewis, Reference Reinthal and Lewis1986). Feeding mobs decrease per capita suffered attacks from territorial fishes and increase substrate bite-rates and, therefore, food resource acquisitions (Robertson et al., Reference Robertson, Sweatman, Fletcher and Cleland1976; Foster, Reference Foster1985; Reinthal & Lewis, Reference Reinthal and Lewis1986). Several species of surgeonfish, parrotfish, damselfish and wrasse are known to feed in mob shoals within other fish species’ territories, both in the Atlantic and Pacific (Keenleyside, Reference Keenleyside1979; DeLoach, Reference DeLoach1999). Food resources most often sought by these mobbing shoals are algae and eggs (DeLoach, Reference DeLoach1999; Randall, Reference Randall2002; Sazima et al., Reference Sazima, Krajewski, Bonaldo and Sazima2013).
There have been several studies on cleaning symbiosis, mob-feeding and nuclear-follower feeding interactions (opportunistic fishes that follow substrate-disturbing fishes), but most of these were conducted along coastal reefs, leaving oceanic islands woefully understudied (but see von Eibl-Eibesfeldt, Reference von Eibl-Eibesfeldt1955; Gasparini & Floeter, Reference Gasparini and Floeter2001; Sazima et al., Reference Sazima, Krajewski, Bonaldo and Sazima2007; Gasparini et al., Reference Gasparini, Luiz and Sazima2008; Bertoncini et al., Reference Bertoncini, Machado, Barreiros, Hostim-Silva and Verani2009). At Ascension island, particularly, knowledge on these interactions is restricted to mentions and pictures in Lubbock (Reference Lubbock1980); Bingeman & Bingeman (Reference Bingeman and Bingeman2005) and Wirtz et al. (Reference Wirtz, Bingeman, Bingeman, Fricke, Hook and Young2014). As these isolated environments constitute evolutionary units on their own (Dawson, Reference Dawson2016), they might also be engines for the appearance of novel interactions among species, some with uninvestigated ecosystem consequences (Sazima et al., Reference Sazima, Sazima and Silva2003, Reference Sazima, Krajewski, Bonaldo and Sazima2007; Sazima & Sazima, Reference Sazima and Sazima2004; Gasparini et al., Reference Gasparini, Luiz and Sazima2008). Therefore, our objective is to partially fill the gap that exists in our understanding of isolated islands in the Atlantic. To achieve this, here we present qualitative and quantitative data on reef fish associations in one of the most isolated tropical islands in the world: Ascension Island, South Atlantic. We focused on three key questions about cleaning interactions and mob-feeding behaviour: (1) Which species are the main reef cleaners and clients around the island, what are the relative contributions of both these associates to cleaning interactions, and are these contributions proportional to their abundances? (2) What are the main participants of feeding mobs (mobbers, followers and aggressors) and how strong are the interactions among them? (3) What is the probability of occurrence of cleaning interactions and mob-feeding behaviour per unit of time and area on Ascension's reefs?
METHODS
Cleaning and mob-feeding behaviours were recorded over a 12-day expedition to Ascension Island, South Atlantic Ocean (7°56′S 14°25′W) in August 2015 and from previous and subsequent observations by one of the authors (J. Brown). All observations were made while scuba diving at depths of 5–15 m at four sites around Ascension: English Bay (including One Hook), where most of the diving was conducted, Comfortless Cove, Northeast Point and Boatswain Bird Island (Figure 1).
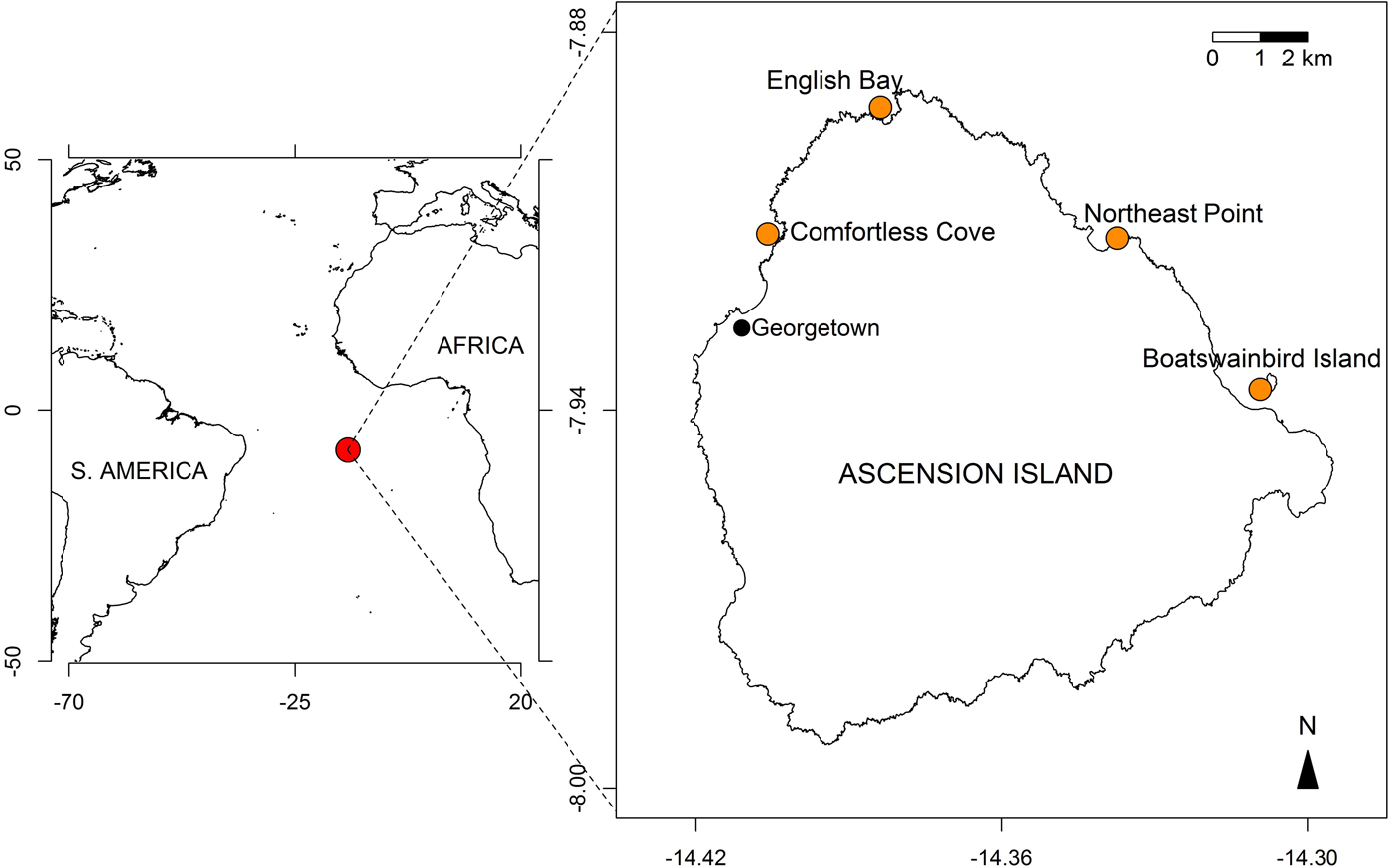
Fig. 1. Maps showing the location of Ascension Island in the South Atlantic Ocean (7°56′S 14°25′W; red circle in left panel) and the sampled sites around the island (orange circles, right panel).
Cleaning interactions
Cleaning events were recorded opportunistically while diving, with the use of direct observations, photographs and video footages. As no standardized search method was employed (i.e. by observation time), only relative contribution of cleaning by each cleaner could be derived. We calculated it as the number of cleaning events in which this cleaner was involved relative to the total number of recorded cleaning events. Thus, if one cleaner species was observed cleaning the same client species in two distinct events (i.e. separated from each other in time or space), these events were considered as independent and therefore counted as two. The abundance of cleaners and clients was estimated from underwater visual surveys conducted along standard 20 × 2 m strip transects (Krajewski & Floeter, Reference Krajewski and Floeter2011; Pinheiro et al., Reference Pinheiro, Ferreira, Joyeux, Santos and Horta2011). In this sampling method, a diver swims unfolding a tape and recording, counting and estimating the size of large, fast swimming and water column-dwelling fishes. After reaching the end of the 20 m layout, the diver returns retracting the tape and employing the same procedures for small, benthic associated and cryptic fishes (Floeter et al., Reference Floeter, Krohling, Gasparini, Ferreira and Zalmon2007a; Krajewski & Floeter, Reference Krajewski and Floeter2011).
Mob-feeding behaviour
Mob-feeding events were recorded and quantified from video recordings using whole group focal animal sampling (Lehner, Reference Lehner1979). When a mob was detected, the diver started recording it as the fishes swam and/or fed and continued for ~3 min or up to the point at which the mob left the area. We consider mob-feeding events as those in which a group of individuals from a nuclear fish species (i.e. the species which leads the mob) fed over the substrate while being followed by opportunist fish species and chased away from either feeding or reproductive territories by territorial fish species. During mob-feeding events, we identified each species involved and recorded the species’ role (whether nuclear, hereafter referred to as ‘mobber’, follower, or aggressor), its abundance, and the intensity of the interactions. Two types of interactions were identified: following, which intensity was quantified as the sum of the time each individual followed the feeding mob; and aggressive (or agonistic), which intensity was quantified as the sum of the number of chases each individual made towards the feeding mob. We also estimated the substratum bite rate of the entire mob by counting the bite rate of randomly sampled feeding individuals and averaging and multiplying it by the total number (usually 3–5) of individuals feeding on the mob.
Probability of occurrence of interactions
Additionally, to understand how common are cleaning and mob-feeding events, we used remote filming of fixed areas of the reef per unit of time. From this we obtained the probability of occurrence of these interactions per m2 of reef during a 10 min time period. The method employed consisted of positioning portable cameras focused on small areas of the reef and recording over 15 min per sample (Longo & Floeter, Reference Longo and Floeter2012; Longo et al., Reference Longo, Ferreira and Floeter2014). Cleaning interactions were recorded in an area of 2 m2 due to the generally small size of cleaner fishes. Mob-feeding events, however, are highly mobile in nature and therefore were recorded over a larger area of 6 m2. Spatial replicates assured the representativeness of this type of sampling under the work conditions (daytime hours during the winter). Since cameras were generally deployed in such a way as to minimize the occurrence of shadowed ledges, vertical walls and small caves (Longo & Floeter, Reference Longo and Floeter2012), frequency of cleaning interactions was possibly underestimated.
Statistical tests
To answer if cleaners cleaned proportionally to their abundances, we used a Chi-square test. Differences in abundance and bite-rate between mobs were tested through Student's t-tests.
RESULTS
Cleaning interactions
We recorded five cleaner species at Ascension (Figure 2): four reef fishes, the Island hogfish Bodianus insularis – juveniles only (Figure 2A, B), the French angelfish Pomacanthus paru – also juveniles only (Figure 2C–E), the St Helena butterflyfish Chaetodon sanctaehelenae (Figure 2E) and the Ascension wrasse Thalassoma ascensionis – mostly as juveniles (Figure 2F); as well as one crustacean: the white-striped cleaner shrimp Lysmata grabhami (Figure 2G, H). All of them are facultative cleaners, and only the angelfish and the shrimp are widely distributed. The Island hogfish is endemic to the Mid-Atlantic Ridge Islands (Ascension, St Helena and St Paul's Rocks); the butterflyfish is endemic to Ascension and St Helena and the Ascension wrasse is endemic to Ascension.

Fig. 2. Interactions between cleaners and clients at Ascension Island's reefs. (A) At a cleaning station, Island hogfish (Bodianus insularis) juveniles cluster to clean the head of a spotted moray (Gymnothorax moringa). (B) Two juvenile hogfish clean the head of a green sea turtle (Chelonia mydas); the top one is nibbling at a lesion, not visible in the photo. (C) A juvenile French angelfish (Pomacanthus paru) inspects a posing squirrelfish (Holocentrus adscensionis). (D) An older angelfish cleans the jaw of a black jack (Caranx lugubris). (E) A juvenile angelfish and an adult St Helena butterflyfish (Chaetodon sanctaehelenae) clean the mouth of a yellow goatfish (Mulloidichthys martinicus). (F) A juvenile Ascension wrasse (Thalassoma ascensionis) inspects the lower jaw of a whitespotted moray (Muraena pavonina). (G) A white-striped cleaner shrimp (Lysmata grabhami) cleans the jaw of a brown moray (Gymnothorax unicolor) in its cleaning station in a crevice. (H) The same shrimp species cleans the snout of a viper moray (Enchelycore nigricans).
For three of these cleaners, we recorded cleaning stations: the hogfish and the angelfish – habitually near the bottom or in the water column (Figure 2A–D) and the shrimp – only in rock crevices (Figure 2G, H). Cleaning interactions were initiated by the potential clients, which posed or adopted particular colours in front of the cleaner or the cleaning station (Figure 2E). The hogfish was the only species that formed groups while cleaning (up to about 15 juvenile individuals; Figure 2A), whereas other species cleaned mostly individually. We also recorded the angelfish and the butterflyfish sharing the same client (Figure 2E) on one occasion.
In a total of 64 events of cleaning interactions, the highest proportion was led by the Island hogfish (35.9%), followed by the French angelfish (28.1%), the white-striped cleaning shrimp (14.1%), the St Helena butterflyfish and the Ascension wrasse (10.9% each). Both the angelfish and the hogfish had the most diverse pool of clients (10 species including the green sea turtle Chelonia mydas cleaned by the hogfish), followed by the wrasse (six), and the butterflyfish and the shrimp (five species each, Figure 3). Proportion of cleaning interactions did not follow cleaner abundance (χ2 = 16.84, df = 3, P < 0.001). The most abundant cleaner, the Ascension wrasse, had the smaller contribution to overall cleaning events while the less abundant cleaner, the French angelfish, had the second highest contribution (Table 1).
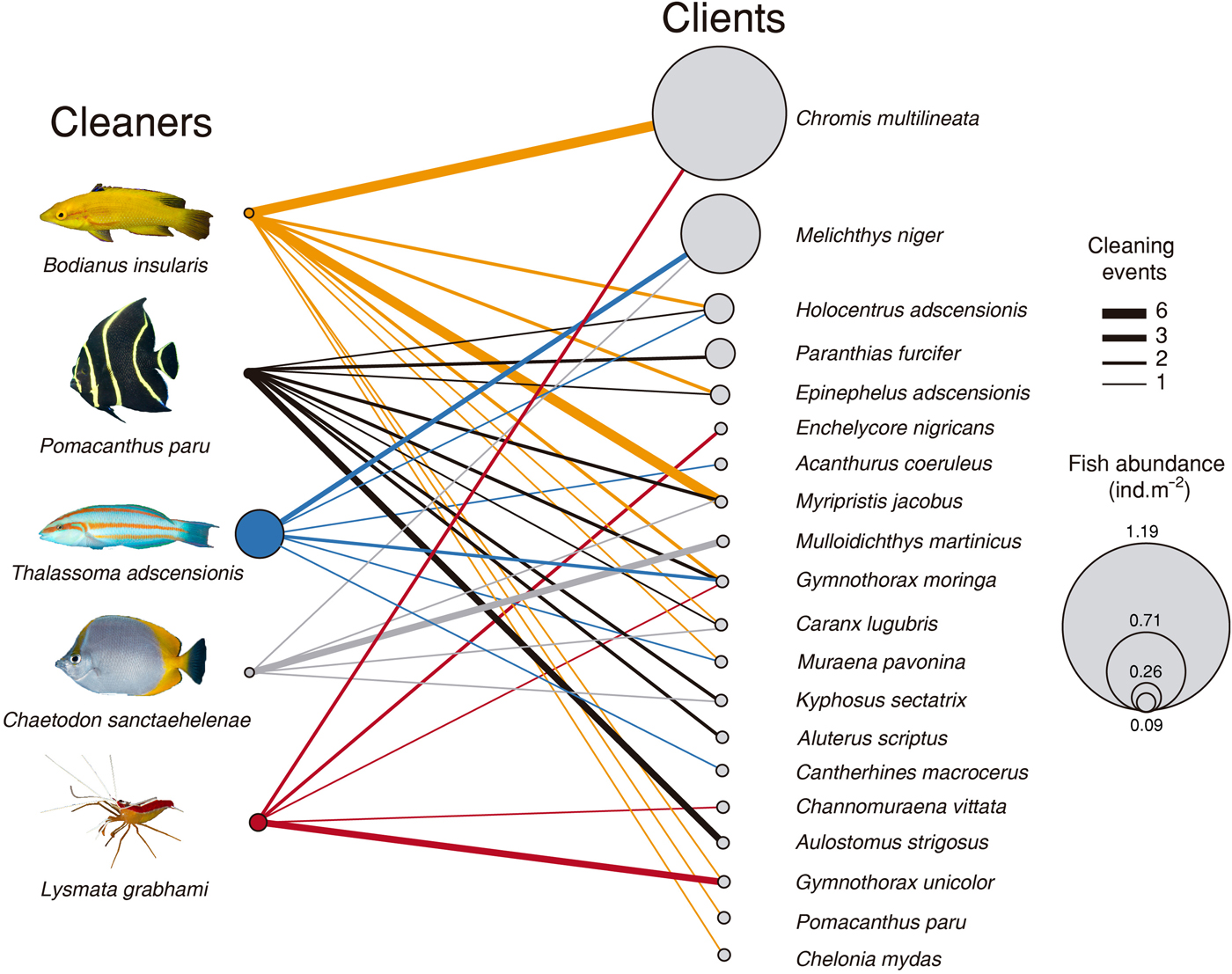
Fig. 3. Cleaner-client interaction network at Ascension Island's reefs. Circle size is proportional to abundance (ind. m−2), except for Lysmata grabhami and Chelonia mydas, whose abundance was not quantified. Lines indicate cleaner-client interactions; line width is proportional to the number of cleaning events.
Table 1. Number of observed cleaning interactions per cleaner and client species at Ascension Island's reefs. Fish taxonomy follows Nelson (Reference Nelson2006) except for Epinephelidae (Craig & Hastings, Reference Craig and Hastings2007). All families are listed in alphabetical sequence, except for the sea turtle.
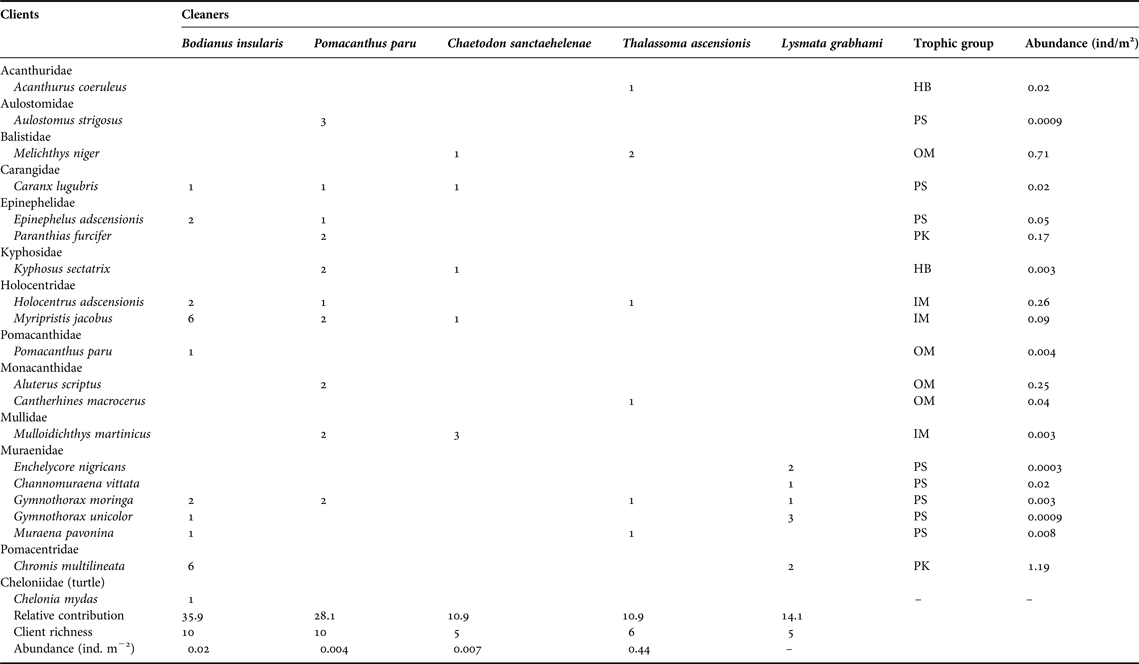
HB, herbivores; IM, mobile invertebrate predators; MC, macrocarnivores; OM, omnivores; PK, planktivores; PS, piscivores.
A total of 19 fish client species in 12 families and one sea turtle were recorded (Table 1). Five species of morays acted as clients, followed by groupers, squirrelfish and filefish (two species each), whereas other fish groups had one client each (Table 1). Eight client species were piscivores, four were omnivores and the remainder trophic groups were planktivores, herbivores and mobile invertebrate predators (two or three species each, Table 1). The abundance of clients, as measured from underwater visual surveys, varied from 0.0003 to 1.19 individuals m-2. The viper moray (Enchelycore nigricans) was the least abundant client, whereas the brown chromis (Chromis multilineata) was the most abundant (Table 1).
Mob-feeding behaviour
We consistently identified two types of feeding mobs: those led by the African sergeant (Abudefduf hoefleri) (Figure 4A); and those led by mixed shoals of the barber surgeonfish (Acanthurus bahianus) and blue tang (Acanthurus coeruleus) (Figure 4B, C). The black triggerfish, or blackfish (Melichthys niger) was also frequently observed feeding in large groups along the bottom (Figure 4D) but, as it neither attracted followers nor was habitually chased by territorial species, its groups were not considered mob-feeders. Additionally, we recorded groups of yellow goatfish (Mulloidichthys martinicus) with some (Figure 4E) or all (Figure 4F) individuals displaying a dark colouration and an active swimming behaviour closely resembling those of the African sergeant mobs. The goatfish, however, were never observed to feed while shoaling.

Fig. 4. Mobs of Ascension Island reef fishes. (A) A large feeding mob led by the African sergeant (Abudefduf hoefleri). (B) and (C) Feeding mobs of surgeonfish Acanthurus bahianus and A. coeruleus. Note darkened colour of A. bahianus. (D) A group of the black triggerfish, or blackfish (Melichthys niger) feeding along the bottom. (E) and (F) Shoaling yellow goatfish (Mulloidichthys martinicus) while darkened. Although the latter species was neither recorded displaying feeding mobs, nor feeding in groups, shoaling individuals sometimes displayed the dark colouration characteristic of other mobs.
Thirteen mob-feeding events ranging 22–436 s (mean = 100 s) were analysed, six of them led by surgeonfish (Acanthurus spp.) and seven by the African sergeant. While surgeonfish mobs were characterized by simultaneously having individuals simultaneously feeding and swimming, and moved a few metres over several minutes; almost all individuals of the African sergeant mobs engaged in the same activity, temporally interspersing feeding and swimming while moving tens of metres over a few minutes. While follower species intermingled with the mobbers, aggressor species chased the mobbers from the vicinities of the mob (Figure 5).

Fig. 5. Participants in reef fish feeding mobs at Ascension Island. (A) and (B): mobs led by the African sergeant (Abudefduf hoefleri). (C) and (D): mobs led by surgeonfish (Acanthurus bahianus and A. coeruleus); note darkened colour of A. bahianus. Black ellipses and circles indicate followers; red ellipses and circles highlight aggressors. Note (in B) a light-coloured sergeant major (Abudefduf saxatilis) intermingling with and following a dark-coloured mob of the African sergeant (the sergeant major was not recorded feeding while within the mob).
African sergeant mobs were smaller than surgeonfish mobs (100.6 ± 12.7 against 175.8 ± 13.2 individuals; t = −4.11, df = 10.8, P = 0.0018). Mobbers represented, respectively 84% (only the African sergeant) and 87% (71% the barber surgeonfish and 16% the blue tang) of individuals, the rest being followers (Figure 6). Aggressive events derived predominantly from territorial damselfishes and targeted more frequently mobbers than followers (Figure 6). The main follower of both mob types was the opportunist blackfish (Figure 6), but the main aggressor differed: the Lubbock's damselfish (Stegastes lubbocki), a highly territorial herbivore, was the main aggressor of surgeonfish mobs (Figure 6B); and the brown chromis, another territorial fish while engaged in egg parental care, was the principal aggressor towards African sergeant mobs (Figure 6A).
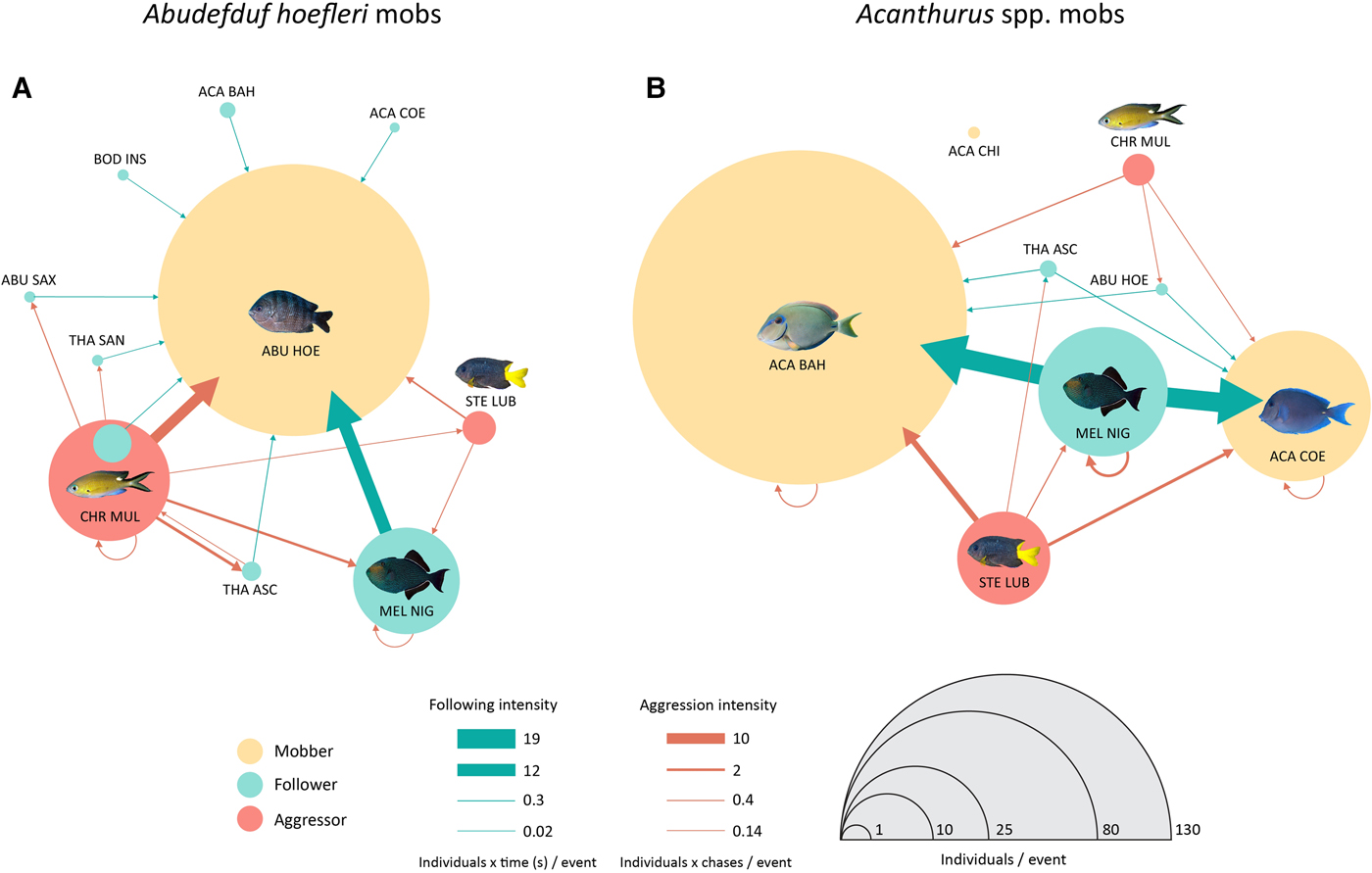
Fig. 6. Interaction networks during events of nuclear-follower mobs led by (A) the African sergeant (Abudefduf hoefleri) and (B) surgeonfish (Acanthurus spp.). Colours indicate behaviour types recorded during the mobbing events (circles) or the interaction types (arrows). Size of circles indicates the abundance of each species during the events and arrows' thickness indicates the intensity of the interactions. Acronyms are abbreviated species names.
Feeding rate also differed between mob types: African sergeant mobs delivered on average 6 ± 2.4 bites s−1 at substrate, whereas Acanthurus spp. mobs delivered almost 11 times more, 70.4 ± 10.7 bites s−1 (t = −5.86, df = 5.5, P = 0.0015). Most of these bites were made by the mobbers (91.4 and 99.8% of total bites in Abudefduf and Acanthurus mobs, respectively), with a smaller proportion opportunistically delivered by followers.
Probability of occurrence of interactions
In 72 remote videos (totalling 720 min analysed), we recorded only five mob-feeding and one cleaning event, resulting in probabilities of occurrence of respectively 1.16 and 0.69% of these interactions per m2 during 10 min. Three of the mobs detected were the more mobile African sergeant mobs and two were the less mobile surgeonfish mobs. The only video-recorded cleaning instance was a juvenile Ascension wrasse Thalassoma ascensionis repeatedly cleaning a blue tang Acanthurus coeruleus.
DISCUSSION
Our study increases the knowledge of reef fish associations at isolated oceanic islands by focusing on one of the most isolated of tropical islands in the world: Ascension Island in the South Atlantic Ocean. The existence of unreported and possibly unique behaviours, such as the mob-feeding behaviour reported here by the African sergeant (Abudefduf hoefleri), indicates that isolated islands might generate not only endemic species, but also distinct behaviours and interactions (Lubbock, Reference Lubbock1980; Price & John, Reference Price and John1980; Bingeman & Bingeman, Reference Bingeman and Bingeman2005; Wirtz et al., Reference Wirtz, Bingeman, Bingeman, Fricke, Hook and Young2014). Additionally we add to the few scattered comments on Ascension Island's fish associations by presenting new records and quantifying both cleaning and mob-feeding interactions.
Cleaning interactions
We found only facultative cleaners at Ascension (sensu Côté, Reference Côté2000; Grutter, Reference Grutter and Rohde2005; Francini-Filho & Sazima, Reference Francini-Filho and Sazima2008). The only obligate fish cleaners in the Atlantic are gobies of the genus Elacatinus, which occur in four oceanic islands in the South-western Atlantic (Fernando de Noronha and Atol das Rocas, Sazima et al., Reference Sazima, Carvalho-Filho and Sazima2008; Batista et al., Reference Batista, Veras, Oliveira, Oliveira, Tolotti, Marins, Zill, Pereira, Hazin and Silva2012; and Trindade and Martim Vaz, Gasparini & Floeter, Reference Gasparini and Floeter2001; Guimarães et al., Reference Guimarães, Gasparini and Rocha2004), but are absent in both the Mid-Atlantic Ridge Islands (Lubbock, Reference Lubbock1980; Lubbock & Edwards, Reference Lubbock and Edwards1981; Edwards & Glass, Reference Edwards and Glass1987; Feitoza et al., Reference Feitoza, Rocha, Luiz-Júnior, Floeter and Gasparini2003) and the Tropical Eastern Atlantic (Wirtz et al., Reference Wirtz, Ferreira, Floeter, Fricke, Gasparini, Iwamoto, Rocha, Sampaio and Schliewen2007; Quimbayo et al., Reference Quimbayo, Floeter, Noguchi, Rangel, Gasparini, Sampaio, Ferreira and Rocha2012). It is possible that due to the absence of obligate cleaners in some oceanic islands, the cleaner role is taken over by facultative cleaners, some of which are endemic to these islands. For instance, eight facultative cleaner species, including two endemics are recorded at St Tomé and Príncipe in the Eastern Atlantic (Quimbayo et al., Reference Quimbayo, Floeter, Noguchi, Rangel, Gasparini, Sampaio, Ferreira and Rocha2012) and two facultative cleaners, including one endemic, are recorded at Azores in the North Atlantic (Bertoncini et al., Reference Bertoncini, Machado, Barreiros, Hostim-Silva and Verani2009). In the more isolated Ascension Island, we observed that three of the five cleaners are endemic to the Mid-Atlantic Ridge, Ascension and St Helena or to Ascension only. As far as we know, there is no clear evidence of obligate cleaning habitats for the widely distributed white-striped cleaner shrimp. The available data suggest that its colour pattern is similar to obligate cleaner fishes (Limbaugh et al., Reference Limbaugh, Pederson and Chace1961) and that it has high cleaning efficiency (Jonasson, Reference Janasson1987). We therefore cautiously opted to consider this shrimp as a facultative cleaner until detailed observational and diet studies are available about its cleaning role.
In our study, contribution to total cleaning events was unrelated to cleaner abundance. Juvenile Island hogfish and French angelfish contributed mostly to cleaning events but had low abundance. This might be an outcome of these species' contrasting colour pattern (e.g. Cheney et al., Reference Cheney, Grutter, Blomberg and Marshall2009), capable of drawing more attention from client fishes and from divers compared with the butterflyfish and the wrasse. Juveniles of other Bodianus species and the French angelfish are active cleaners in many places, even in some where obligate lineages do occur (e.g. Johnson & Ruben, Reference Johnson and Ruben1988; Wicksten, Reference Wicksten1998; Sazima et al., Reference Sazima, Moura and Sazima1999, Reference Sazima, Grossman and Sazima2010c; Luiz et al., Reference Luiz, Carvalho-Filho, Ferreira, Floeter and Sazima2008). For instance, at the coastal Abrolhos Archipelago, in Brazil, the French angelfish has a cleaning role similar to that of the obligate cleaner barber goby (Elacatinus figaro), both of them tending cleaning stations and cleaning a species-rich assemblage of clients (Sazima et al., Reference Sazima, Guimarães, Reis and Sazima2010b).
Most of the client types we recorded at Ascension, namely damselfish, triggerfish, goatfish, sea chub, squirrelfish, groupers and morays, conform to the types (even if not always the same species) habitually recorded at other oceanic islands in the Atlantic (Gasparini & Floeter, Reference Gasparini and Floeter2001; Sazima et al., Reference Sazima, Bonaldo, Krajewski and Sazima2005; Francini-Filho & Sazima, Reference Francini-Filho and Sazima2008). On the other hand, the green sea turtle is rarely recorded as a client at oceanic islands. This turtle is cleaned by a wrasse at Trindade Island (Gasparini & Floeter, Reference Gasparini and Floeter2001) and by two damselfish and two surgeonfish at the Fernando de Noronha Archipelago (Sazima et al., Reference Sazima, Grossman and Sazima2010a).
Mob-feeding behaviour
We recorded several instances of mobbing at Ascension. Surgeonfishes mobbed while feeding over algal turfs and sandy patches on and around Lubbock's damselfish territories. The barber surgeonfish (Acanthurus bahianus) occurs at Ascension, St Helena and on the Brazilian coastal shelf and oceanic islands (Bernal & Rocha, Reference Bernal and Rocha2011). Albeit abundant and usually performing feeding mobs at some coastal sites in Brazil (e.g. Arraial do Cabo, Ferreira et al., Reference Ferreira, Floeter, Gasparini, Ferreira and Joyeux2004), the only South-western Atlantic oceanic island where this species is common is Trindade Island, where it does not form such feeding groups or mobs (Gasparini & Floeter, Reference Gasparini and Floeter2001). The low abundance of aggressive territorial pomacentrids at Trindade (Pinheiro et al., Reference Pinheiro, Ferreira, Joyeux, Santos and Horta2011) could be a factor influencing the absence of mobs despite the great numbers of this surgeonfish. At Ascension, the high abundance of the endemic Lubbock's damselfish (our personal communication) might force surgeonfish to group into mobs to maximize feeding rate per individual. Single species mobs of the barber surgeonfish have also been observed at St Helena, where the St Helena damselfish (Stegastes sanctaehelenae) occurs in high densities (J. Brown personal communication).
Contrary to surgeonfish mobs, feeding mobs of sergeant fish (genus Abudefduf) have not been reported to occur naturally to date at least in the Atlantic. Reproductive males of the sergeant major (Abudefduf saxatilis) guarding nests and driven away by passing scuba divers had their nests attacked by groups of opportunistic conspecifics (Cheney, Reference Cheney2008). The same species displays group-feeding while clearing sites for nesting at St Paul's Rocks (Francini-Filho et al., Reference Francini-Filho, Coni, Ferreira, Alves, Rodrigues and Amado-Filho2012). Both these events involve blue coloured, reproductive males feeding constantly at the same flat or vertical surface for many minutes prior to egg-laying by females (Francini-Filho et al., Reference Francini-Filho, Coni, Ferreira, Alves, Rodrigues and Amado-Filho2012). These behaviours, although superficially similar, are fundamentally distinct from the one we report here for the African sergeant A. hoefleri: (1) the mobs we recorded were unrelated to divers’ presence, as shown by their occurrence on remote filming; (2) they did not feed continually over a particular area, but rather for 10–20 s and then moving away; and (3) the darkened, not bluish, colour and vigorous swimming movements with both the pectoral and caudal fins were conspicuous. The dark colour of mobbing individuals sharply contrasts with the intense blue colouration of African sergeants observed alone (Vella et al., Reference Vella, Vella and Darmanin2016).
The African sergeant (Abudefduf hoefleri) was formerly known only from the Eastern Atlantic (Loris & Rucabado, Reference Loris, Rucabado, Quéro, Hureau, Karrer, Post and Saldanha1990; Wirtz et al., Reference Wirtz, Ferreira, Floeter, Fricke, Gasparini, Iwamoto, Rocha, Sampaio and Schliewen2007; Espino et al., Reference Espino, Tuya and Brito2015). Therefore, we extend its range to Ascension and also report its mob-feeding behaviour for the first time.
Sergeant fishes are omnivorous generalists, switching between plankton and benthic feeding depending on resource availability (Randall, Reference Randall1967; Sazima et al., Reference Sazima, Sazima and Silva2006). During the mob-feeding events, the African sergeant was chased much more by the brown chromis (Chromis multilineata) than by the Lubbock's damselfish (Stegastes lubbocki) (Figure 6). The brown chromis is a seasonally territorial species (sensu Ridley, Reference Ridley1978) during its reproductive period when males defend their brood vigorously (Myrberg et al., Reference Myrberg, Brahy and Emery1967; Ridley, Reference Ridley1978). Therefore, we believe that the African sergeants are opportunistically exploring algal turfs from Lubbock's damselfish territories but apparently concentrate on the brown chromis's egg clutches, a highly energetic resource. Although spawns could not be observed from the analysed videos, African sergeants' foraging bouts were concentrated along near-vertical surfaces, consistent with micro-habitats preferred for spawning by pomacentrids in general (Montgomery, Reference Montgomery1981). Moreover, eggs of the brown chromis are minute and not organized in compact masses such as those from other pomacentrids (Myrberg et al., Reference Myrberg, Brahy and Emery1967) and therefore hardly visible to the human eye.
We also suggest that the darkened colouration of the African sergeant could help it avoid aggressions from the brown chromis while in their territories. This could occur by resembling harmless species (from a reproductive male brown chromis’ point of view) such as the detritivorous barber surgeonfish or by resembling the numerically dominant blackfish (Melichthys niger). This type of similarity, called aggressive mimicry, has been described for a variety of fish species (Ormond, Reference Ormond1980; Sazima, Reference Sazima2002; Randall, Reference Randall2005), always in the form of a mimic predating on adult individuals. If the suspected egg predation is confirmed, it would add a previously undocumented facet to aggressive mimics. The blackfish is the most abundant species in Ascension's reefs and around other oceanic islands in the Atlantic, such as Trindade and St Paul's Rocks, attaining swarming abundances (Lubbock, Reference Lubbock1980; Price & John, Reference Price and John1980; Gasparini & Floeter, Reference Gasparini and Floeter2001; Brewin et al., Reference Brewin, Brown and Brickle2015; Wirtz et al., Reference Wirtz, Bingeman, Bingeman, Fricke, Hook and Young2014). On some occasions, we observed group-feeding behaviour by the blackfish (Figure 4D) without attracting attention from aggressor species such as Lubbock's damselfish or the brown chromis. We suggest that the sheer numeric dominance by the blackfish would result in decreased aggressiveness towards it, which would benefit a potential mimic. The groups of darkened yellow goatfish (Mulloidichthys martinicus) perhaps take advantage of mimicry as well, e.g. social or Batesian mimicry (Randall, Reference Randall2005). We are, however, unable to offer a proper explanation for this colour, as we have not recorded the goatfish joining other similarly coloured fishes, or evading predators while in the dark attire. Some shoaling fish species adopt a dark colour while defending resources, hunting or mating (DeLoach, Reference DeLoach1999; Sazima et al., Reference Sazima, Krajewski, Bonaldo and Sazima2013) and this may be another possibility for darkening in the yellow goatfish.
At Ascension, feeding frenzies by the blackfish are readily observable even from above water (Lubbock, Reference Lubbock1980): when an unpredicted food source is made available, these fish are always the first to reach it, similarly to what was described at Fernando de Noronha by Sazima et al. (Reference Sazima, Sazima and Silva2003). Such frenzies occur in other South Atlantic islands (e.g. Gasparini & Floeter, Reference Gasparini and Floeter2001; Sazima et al., Reference Sazima, Sazima and Silva2003), but their unprecedented group size at Ascension is reflected in the fact that this species was the most important follower of both types of feeding mobs in our study (Figure 6). Kavanagh & Olney (Reference Kavanagh and Olney2006) suggest that, among the factors that contribute to the swarming abundances of the blackfish (Melichthys niger) in isolated islands, its exceptionally long pelagic stage and high plasticity in feeding might be crucial for its success. We add that its adaptability and ability to quickly perceive its surroundings could be just as important. For example, at Fernando de Noronha Archipelago large groups of blackfish associate with groups of spinner dolphins (Stenella longirostris) and follow these cetaceans to feed on their faeces. The fish are even able to recognize a dolphin that is about to void and quickly converge in large groups of up to 300 individuals on a particularly rich discharge (Sazima et al., Reference Sazima, Sazima and Silva2003).
CONCLUSIONS AND NEW PERSPECTIVES
In our study, cleaning activities were performed exclusively by facultative fish species that cleaned disproportionally to their abundance. Either the white-striped cleaner shrimp (Lysmata grabhami) is able to display a specialized cleaning role in Ascension island or this reef community subsists without any obligate cleaner. If this absence of obligate cleaners is confirmed, it could be explained by non-specialized (or less specialized) cleaners being able to control isolated islands’ species poorer fish parasite assemblages. There is compelling evidence that simplified reef food webs have smaller parasite richness than the more complex ones (Lafferty et al., Reference Lafferty, Dobson and Kuris2006, Reference Lafferty, Shaw and Kuris2008; Wood et al., Reference Wood, Sandin, Zgliczynski, Guerra and Micheli2014). Although this has not, to our knowledge, been assessed by experimental design in isolated islands vs continental areas, it seems reasonable to assume that simplified food webs of isolated islands contain fewer parasite species than coastal sites. Further studies on community dependence of cleaners in isolated islands and continental areas might help to clarify these issues. An interesting question to be hereafter addressed would be how non-isolated reefs such as those from mainland Africa are able to sustain themselves in the absence of obligate cleaner fishes (Quéro et al., Reference Quéro, Hureau, Karrer, Post and Saldanha1990).
Mob feeders, especially the African sergeant, showed darkened colours, a possible mimicry strategy to take advantage of the sheer numeric dominance of the blackfish. We propose that mimicry might help the sergeants to approach the brown chromis territories without being perceived as a threat, or being perceived as a threat unworthy of fighting for, until very close. This would allow the sergeants to draw more resources from brown chromis’ reproductive territories. A new avenue would be to understand the reliance of African sergeant on mobs for feeding and if brown chromis’ eggs are in fact an important food resource for this sergeant species.
Cleaning, mobbing, following and other multi-species fish associations are poorly studied in isolated islands such as Ascension. These places are poorer in species than coastal reefs and provide the opportunity for plastic species to take on new ecosystem roles and perform unique behaviours. For instance, the widespread Western and Eastern Atlantic chain moray (Echidna catenata) is reported to strike at sally lightfoot crabs (Grapsus grapsus) out of the water and chase them on the exposed reef at ebb tide only at the oceanic Fernando de Noronha Island (Sazima & Sazima, Reference Sazima and Sazima2004). The same applies to the circumtropical blackfish group-feeding on wastes of spinner dolphins at the same island (Sazima et al., Reference Sazima, Sazima and Silva2003). Recording and reporting such opportunistic or symbiotic relationships might help, for example, to better understand marine species’ degree of adaptability in face of newly set environmental and biological conditions. This is particularly promising in the case of isolated islands, where there is increased probability that the species involved in such associations are endemics. Additional and perhaps surprising examples of associations, interactions and behaviours certainly are waiting to be formally recorded (see Losey, Reference Losey and Mostofsky1978 for an instrumental definition and examples of multi-species symbiotic behaviours).
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at http://dx.doi.org/10.1017/S0025315416001041.
ACKNOWLEDGEMENTS
We are indebted to the Conservation Department of the Ascension Island Government, Ascension Divers and Obsidian Hotel for all of the support they provided. In particular, we wish to thank Caroline Yon for providing us with full membership of the Ascension Dive Club, Dr Nicola Weber for crucial help with permits and logistics, Emma Nolan and Kate Downes for invaluable support during our island time. We appreciate the help of all of the Conservation Department team. Samara Danielski analysed the remote videos in search of cleaning and mobbing events. We also greatly benefitted from the critical comments and suggestions of three anonymous referees.
FINANCIAL SUPPORT
This study was funded by the Brazilian National Council of Research and Development (CNPq) through grant no. 458548/2013-8 to Dr Leticia Veras Costa Lotufo; and by the California Academy of Sciences to LR and SB.









