INTRODUCTION
Scleractinian corals contribute to the complexity of the marine ecosystems in which they are present, creating shelter and refuge areas for other organisms (e.g. Jaap, Reference Jaap2000; Gratwicke & Speight, Reference Gratwicke and Speight2005). Moreover, the presence of healthy corals has important socio-economic consequences in the regions in which they are present (Jackson et al., Reference Jackson, Kirby, Berger, Bjorndal, Botsford, Bourque, Bradbury, Cooke, Erlandson, Estes, Hughes, Kidwell, Lange, Lenihan, Pandolfi, Peterson, Steneck, Tegner and Warner2001; Edwards & Gomez, Reference Edwards and Gomez2007), supporting fisheries and/or diving tourism (Hughes et al., Reference Hughes, Baird, Bellwood, Card, Connolly, Folke, Grosberg, Hoegh-Guldberg, Jackson, Kleypas, Lough, Marshal, Nyström, Palumbi, Pandolfi, Rosen and Roughgarden2003; Shaish et al., Reference Shaish, Levy, Katzir and Rinkevich2010). These ecosystems are some of the most important and biodiverse around the world (Edwards & Gomez, Reference Edwards and Gomez2007) and must be preserved and/or restored if necessary. However, the dismal fate of coral reefs suggests the need for restoration measures (Epstein et al., Reference Epstein, Bak and Rinkevich2003; Rinkevich, Reference Rinkevich2008), and some authors have proposed that coral transplantation programmes can play a useful role in active rehabilitation strategies (e.g. Raymundo, Reference Raymundo2001; Yap, Reference Yap2003).
Over the past few decades, several coral transplantation techniques have been used to improve degraded or damaged coral habitats, with promising results (e.g. Epstein et al., Reference Epstein, Bak and Rinkevich2003; Nishihira, Reference Nishihira2007). Mainly, these techniques have been carried out in tropical coral reefs, whereas temperate corals have received much less attention. In the Mediterranean Sea, on one hand, the most important anthropogenic threats are habitat loss, degradation and pollution, overexploitation of marine resources, invasion of species and climate change (e.g. Coll et al., Reference Coll, Piroddi, Steenbeek, Kascher, Lasram, Aguzzi, Ballesteros, Bianchi, Corbera, Dailianis, Danovaro, Estrada, Froglia, Galil, Gasol, Gertwagen, Gil, Guilhaumon, Kesner-Reyes, Kitsos, Koukouras, Lampadariou, Laxamana, López-Fé de la Cuadra, Lotze, Martin, Mouillot, Oro, Raicevich, Rius-Barile, Saiz-Salinas, San Vicente, Somot, Templado, Turon, Vafidis, Villanueva and Voultsiadou2010). On the other hand, natural disturbances such as intense catastrophic environmental events are becoming more frequent, with negative effects on key species and habitats (e.g. Teixidó et al., Reference Teixidó, Casas, Cebrián, Linares and Garrabou2013).
The Mediterranean endemic orange coral Astroides calycularis (Pallas, 1766) is an azooxanthellate scleractinian colony coral that inhabits rocky shores from surface to 50-m depth (Zibrowius, Reference Zibrowius1980). Their population densities can be locally high, with colonies covering up to 90% of the sea bottom (Goffredo et al., Reference Goffredo, Caroselli, Gasparini, Marconi, Putignano, Pazzini and Zaccanti2011; Terrón-Sigler et al., Reference Terrón-Sigler, León-Muez, Peñalver, Gálvez-César and Espinosa Torre2016a), but the species has a limited geographic distribution in the Mediterranean Sea (Zibrowius, Reference Zibrowius1995; Bianchi, Reference Bianchi2007). Along the Spanish coasts, the highest orange coral densities can be found on the Andalusian shores (Alborán Sea), where enhanced biodiversity associated with this species was recently reported (Terrón-Sigler et al., Reference Terrón-Sigler, Peñalver, León-Muez and Espinosa2014). The increasing human impacts on the coastal areas, such as marine pollution and/or habitat degradation, negatively affect this species (Moreno et al., Reference Moreno, de la Linde, Arroyo, López-González, Barea-Azcón, Ballesteros-Duperón and Moreno2008; Ocaña et al., Reference Ocaña, Ramos and Templado2009). For example, scuba diving has a direct impact on the coral's populations because colonies can be damaged or dislodged by the impact of fins, hands and diving equipment (Moreno et al., Reference Moreno, de la Linde, Arroyo, López-González, Barea-Azcón, Ballesteros-Duperón and Moreno2008; Terrón-Sigler et al., Reference Terrón-Sigler, León-Muez, Peñalver, Gálvez-César and Espinosa Torre2016b). The susceptibility of Astroides calycularis has been widely perceived and the species is protected by national and international organizations as an endangered species (i.e. the Bern and Barcelona Conventions and the Convention on International Trade in Endangered Species of Wild Fauna and Flora [CITES]).
The aim of this study is to test an attachment technique and explore the response of attached colonies (both transplantation as translocation) to different levels of hydrodynamism. Coral transplantation techniques have been implemented on zooxanthellate corals, nevertheless this methodology is one of only a few to look at an azooxanthellate brooding coral. This technique could be used as a possible management tool in degraded areas, aiming to improve the conservation status of this endangered species.
MATERIALS AND METHODS
Study site
The experiment was conducted on the Granada coast, southern Iberian Peninsula, between July 2012 and July 2013 (Figure 1). The experiment was carried out on Marina del Este beach, an area frequently visited by recreational scuba divers (Terrón-Sigler et al., Reference Terrón-Sigler, León-Muez, Peñalver, Gálvez-César and Espinosa Torre2016b). Two areas were selected: Punta de la Mona [PM] (36°43′08″N 3°43′38″′W) and Punta del Vapor [PV] (36°43′22″N 3°43′35″W), both at a depth of 8 m. PM is exposed to windstorms from the south, east and west, while PV is primarily exposed to windstorms from the east. In order to quantify the exposure difference, wave exposure analysis was carried out for each site. Following Howes et al. (Reference Howes, Harper and Owens1994), a fetch model index was developed for each area. This model provides good quantitative approximations of wave exposure in order to predict marine community patterns (e.g. Hill et al., Reference Hill, Pepper, Puotinen, Hughes, Edgar, Barrett, Stuart-Smith and Leaper2010). This model relies on two indices of fetch: modified effective fetch and maximum fetch. A combination of the two indices allows for determining the wave exposure class of each area (Table 1) and is calculated using the following equation:
where Fe is the effective fetch in km, θi is the angle between the shore-normal and the direction (0°, 45° to the left and 45° to the right) and Fi is the fetch distance in kilometres (km) along the relevant vector. Maximum fetch is defined as the maximum fetch distance in kilometres measured from the point of interest. A value of 1000 km is conventionally used when open-ocean fetches occur. The mean values of both modified-effective fetch and maximum fetch (hereafter average fetch) for each area were used as continuous variables in subsequent analysis.
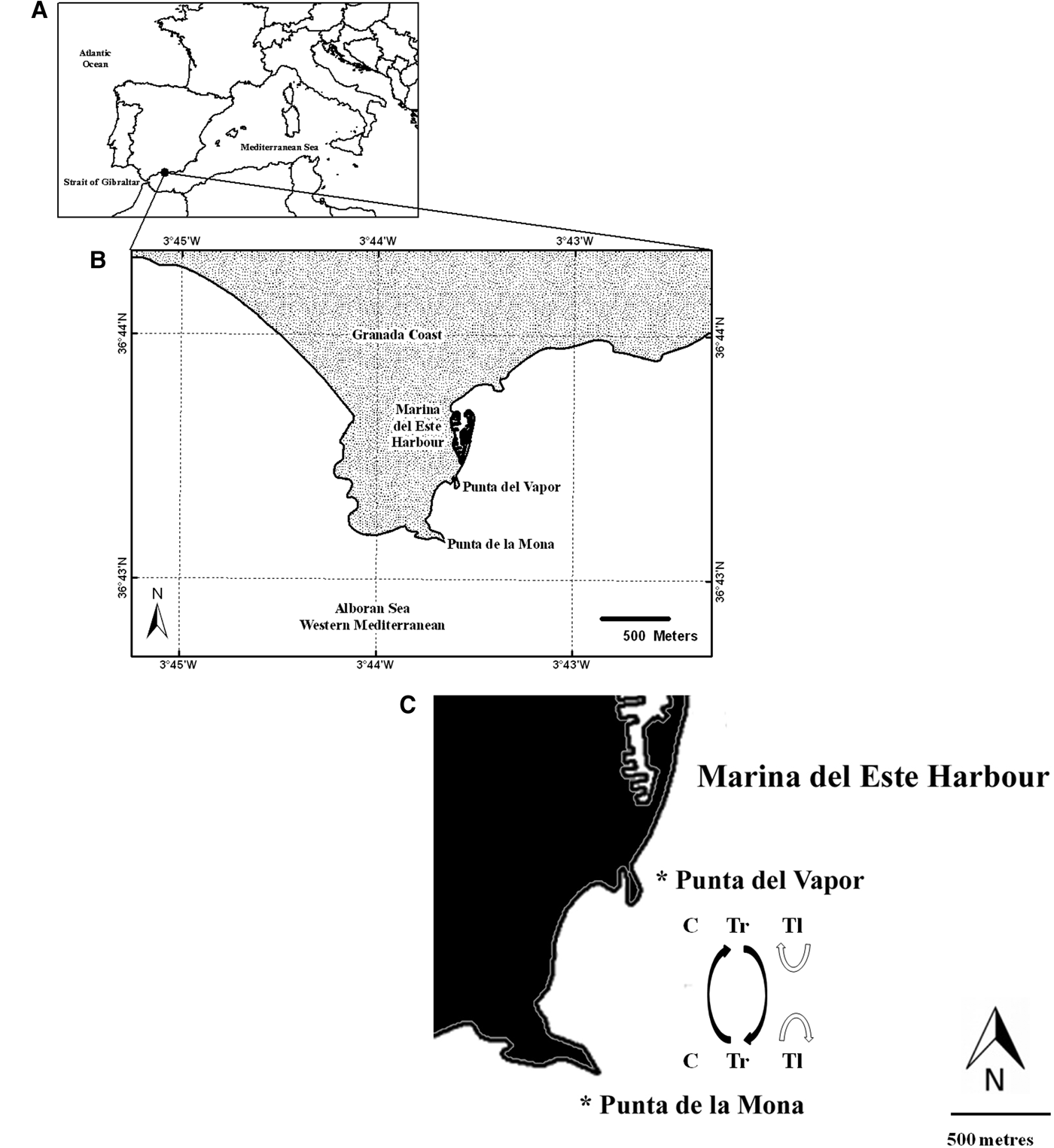
Fig. 1. (A) Map of the study area (Andalusia coastal line; Spain). (B) Areas' position in Granada littoral (Punta de la Mona and Punta del Vapor). (C) Graphic representation of the experimental treatment. (C, Control; Tr, Transplanted colonies; Tl, Translocated colonies).
Table 1. Wave exposure classes based on the modified-effective fetch and maximum fetch matrix (after Howes et al., Reference Howes, Harper and Owens1994).

VP, very protected; P, protected; SP, semi-protected; SE, semi-exposed; E, exposed; n/a, no assessment.
Experiment design
At each area (PM and PV), three sites were selected at the same depth (8 m), in order to distinguish between the effect of a new area (transplantation treatment: PM → PV or PV → PM) on the growth and survival of the coral and the effect of simply being unattached (control treatment), manipulated (transplantation treatment) or reattached (translocation treatment) (following Crowe & Underwood, Reference Crowe and Underwood1999). At each of the three sites per area, we established three treatments: control colonies (undisturbed colonies which were not manipulated); translocation colonies (dislodged and reattached in the same place); and transplantation colonies (dislodged and attached in a different area). Six colonies were used as replicates for each treatment (Figure 2) (N = 108; 54 for each area: PM and PV).

Fig. 2. Summary of the experimental design indicating areas (PM, Punta de la Mona; PV, Punta del Vapor); Sites; treatments (C, Control; TL, Translocated colonies; TR, Transplanted colonies) and number of colonies used.
Attachment methodology
We chose an attachment technique using a marine quick-action epoxy resin (5 min for hardening) that had previously been shown to be effective in transplanting colonies of this species (see Terrón-Sigler et al., Reference Terrón-Sigler, Peñalver, Espinosa and León-Muez2011). As explained above, 18 colonies were transplanted from PV to PM, and vice versa. These colonies were collected via scuba diving and maintained in plastic containers filled with seawater. The colonies remained in the containers for fewer than 15 min before being transplanted to the new area.
In each area (PM and PV), the scuba divers cleaned the substratum with a steel comb, scraped away the surface, and mashed and put the epoxy resin on the clean substrate. Finally, the colonies were attached firmly and observed until the resin had hardened. Each colony was labelled with a plastic tag inserted in the epoxy resin. In the case of control colonies, a piece of resin was placed in the vicinity of each colony in order to attach the plastic tags for labelling purposes.
Monitoring coral survival and growth
Survival and growth are typically used as measures of colony transplantation (Raymundo, Reference Raymundo2001; Dizon & Yap, Reference Dizon and Yap2006; Dizon et al., Reference Dizon, Edwards and Gomez2008). In this study, survival is defined as the presence of the colony after the experiment has been started on each treatment, so the loss of colonies may be the result of the attachment methodology.
Growth was measured as an increase in the colony area and an increase in the number of polyps. We used the length (Lc, major axis of the colony) and width (Wc, minor axis of the colony) as biometric parameters. According to Goffredo et al. (Reference Goffredo, Caroselli, Gasparini, Marconi, Putignano, Pazzini and Zaccanti2011), the Astroides calycularis colony area (Ac) must be calculated using the formula for an ellipse (Ac = π (Lc × Wc)/4). Colony area is a more accurate and representative measure of colony size than colony length (Goffredo et al., Reference Goffredo, Caroselli, Gasparini, Marconi, Putignano, Pazzini and Zaccanti2011), and it is a good parameter for understanding the dynamics of coral populations (Terrón-Sigler et al., Reference Terrón-Sigler, Peñalver, León-Muez and Espinosa2014). Additionally, the number of polyps in each colony was counted in the initial state and at each monitored time. As a result, growth in the colony area and in the number of polyps was estimated by the increase in each parameter between the initial state and the different monitored times.
All the colonies were monitored at 6 and 12 months after the start of the experiment. Each time, we located the labelled colonies and measured the biometric parameters (Lc and Wc) as well as the number of polyps (Figure 3).

Fig. 3. View with the three treatments in one site of PV. Left, control colony; up to the right, translocated colony; and down to the right, transplanted colony. All colonies were labelled.
Data analysis
To test whether the growth was correlated with colony area and/or number of polyps we calculated the Pearson correlation with SPSS 15.0. Additionally, in order to test whether or not the growth was similar between treatments and areas (PM: high hydrodynamism; PV: low hydrodynamism), we used a multifactor ANOVA with the following factors: treatment with three levels (control, translocation and transplantation) and hydrodynamism as an orthogonal with treatment and a fixed factor with two levels (high and low). Prior to ANOVA, the heterogeneity of variance was tested via Cochran's C test. Univariate analyses were conducted with GMAV5 (Underwood et al., Reference Underwood, Chapman and Richards2002). When statistical differences were detected, a post-hoc Student–Newman–Keuls test was applied (Underwood, Reference Underwood1997).
RESULTS
Wave exposure analysis
For the PM area, the maximum fetch was 552.10 km, and the calculated modified effective fetch was 299.82 km. Meanwhile, for the PV area, the maximum fetch obtained was 379.58 km, and the modified effective fetch was 84.27 km. Thus, following the Howes et al. (Reference Howes, Harper and Owens1994) fetch model index (Table 1), PM is considered exposed (E), and PV is semi-exposed (SE). Therefore, according to the fetch model, the PM area has higher hydrodynamic conditions than the PV area.
Survival and growth
Translocated colonies presented higher survival than transplanted ones (Figure 4). For translocated colonies on PM, survival was slightly higher than it was on PV, with the two areas obtaining 87.5 and 85.7%, respectively (Figure 4). These differences between areas were also recorded for transplanted colonies, which showed a higher survival for PM (81.2%) than for PV (64.3%). However, survival in the control colonies was lower on PV than it was on PM (77.8% vs 89.9%, respectively). It is important to note that all colonies that were lost occurred in the first 6 months of the experiment. In general, transplanted and translocated colonies appeared healthy with a similar bright orange colour to the control colonies.

Fig. 4. Percentage colonies survival per zone (PM, Punta de la Mona; PV, Punta del Vapor) and treatments (C, Control; TL, Translocation; TR, Transplantation).
Regarding the growth of the colony (Figure 5), we detected high growth in all treatments within PM 6 months after the start of the experiment, ranging from 4 cm2 for translocated and transplanted colonies to a slightly higher figure for control colonies (5 cm2). However, this pattern was not observed for the PV area, where growth was much higher in translocated and transplanted colonies than in control colonies. Generally, growth was lower for all treatments and areas in the period of 6–12 months, without a clear pattern. Translocated and control colonies obtained higher values on PM, whereas for PV, the values were higher for control and transplanted colonies (Figure 5).

Fig. 5. Astroides calycularis colonies growth per zone (PM, Punta de la Mona; PV, Punta del Vapor) and treatments (C, Control; TL, Translocation; TR, Transplantation) 6 and 12 months after initial experiment. Error bars are confidence interval at 95%.
The number of polyps increased notably during the first 6 months (Figure 5). In the environment with high hydrodynamic conditions (PM), this increment was similar for both control and transplantation treatments, but it was less for the translocated treatment. Nevertheless, in low hydrodynamic conditions (PV), it was lower overall, and in these conditions, the control treatment yielded fewer polyps than did the other treatments. However, after 12 months, the increased number of polyps did not increase overall. Still, again, it was lower on PV than it was on PM for all treatments, and for inside treatments, control colonies presented a smaller increase in number of polyps.
Regarding growth, Pearson correlation did not show correlation either with colony area (R = 0.165, P = 0.157) or number of polyps (R = 0.017, P = 0.887). Moreover, ANOVA analyses did not show significant differences among treatments (Table 2), but differences were significant between areas with different hydrodynamic conditions. For PM (high hydrodynamic conditions), the mean and confidence interval at 95% values of the colony area were 5.19 cm2 ± 1.04, but for PV (low hydrodynamic conditions), these values were 3.30 cm2 ± 0.91. Regarding number of polyps, ANOVA analyses did not show significant differences for any treatment or for hydrodynamic conditions (Table 2).
Table 2. Two-way ANOVA results for the influence of treatment and area on the growth (measured as change in number of polyps and increment of area) of Astroides calycularis colonies after 12 months.
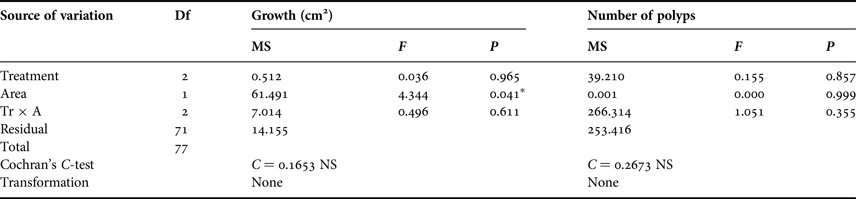
NS, not significant.
*P < 0.05.
DISCUSSION
Little information is available about transplantation experiences associated with scleractinian corals in the Mediterranean Sea (Zibrowius, Reference Zibrowius1995; Ocaña et al., Reference Ocaña, Ramos and Templado2009; Terrón-Sigler et al., Reference Terrón-Sigler, Peñalver, Espinosa and León-Muez2011). The high survival and the observed growth for the colonies under study (both for transplant and translocate treatments) prove the success of the restoration techniques used in this study on the orange coral Astroides calycularis. The selection of an appropriate attaching material is crucial depending on the different substrates and/or coral species (Dizon et al., Reference Dizon, Edwards and Gomez2008). Usually, massive corals survive transplantation better than do branching corals (Raymundo et al., Reference Raymundo2001; Omori, Reference Omori2011). Ocaña et al. (Reference Ocaña, Ramos and Templado2009) used cement-like adhesive to transplant Astroides calycularis colonies in the Strait of Gibraltar, and less than 50% of colonies survived. Previously, Zibrowius (Reference Zibrowius1995) tested transplanting the orange coral from the south of Spain to French Mediterranean waters, but he did not achieve success because recreational scuba divers harvested the unusual orange coral. Recently, Terrón-Sigler et al. (Reference Terrón-Sigler, Peñalver, Espinosa and León-Muez2011) tested different epoxy resins as an adhesive material and concluded that quick epoxy resin (coraFix ®) was better than other epoxy types.
Some of the control colonies of Astroides calycularis died by detachment during the study period at both sites. Some studies involving tropical seas explain this mortality as a possible species strategy focused in a turnover, with dead and recruitment colonies (e.g. Yap et al., Reference Yap, Porfirio and Gomez1992; Raymundo et al., Reference Raymundo2001). On the other hand, A. calycularis is a suspender feeder species and their abundance is related to currents and available particulate organic matter (Cebrián & Ballesteros, Reference Cebrián and Ballesteros2004). Survival and growth of A. calycularis colonies were observed to be higher in PM where the hydrodynamic conditions are stronger. This could be explained by the high support of particulate organic matter present in PM. Additionally, both growth and the number of polyps of the colonies were higher in the first 6 months because it was possible to obtain more nutrients, as particulate organic matter, in the autumn and winter conditions. The A. calycularis strategy needs further study.
Growth and survival should not be considered the sole criteria in the evaluation of transplantation efforts (Edinger et al., Reference Edinger, Limmon, Jompa, Widjatmoko, Heikoop and Risk2000; Raymundo et al., Reference Raymundo2001), as environmental conditions and life strategy must also be taken into account (Yap & Gomez, Reference Yap and Gomez1984; Dizon & Yap, Reference Dizon and Yap2006). The interaction of physical and biological factors is also related to the intrinsic physiological and behavioural characteristics of the species concerned (Gates & Edmunds, Reference Gates and Edmunds1999). Thus Dizon & Yap (Reference Dizon and Yap2006) demonstrated that there are distinct differences among species with different growth forms and life history strategies. Therefore, species with submassive and massive forms have slower growth but better survival than branching species, which present higher growth rates but lower survivorship. Additionally, Fava et al. (Reference Fava, Bavestrello, Valisano and Cerrano2010) observed that sea fans transplanted in the Mediterranean Sea showed a mean negative growth rate and a reduction of survival during a high temperature stress season. On the other hand, Linares et al. (Reference Linares, Coma and Zabala2008) observed that environmental conditions did not affect the mortality of the red gorgonian (Paramuricea clavata) in transplantation experiments, though methodological failure rates have to be taken into consideration. The three treatments displayed significant differences between hydrodynamic conditions, showing higher survival and growth in the more exposed area of PM. High hydrodynamism promotes the suspension-feeder strategy (Zabala & Ballesteros, Reference Zabala and Ballesteros1989), explaining the higher growth observed in PM in comparison to PV. Thereby, A. calycularis shows different responses to different hydrodynamism factors. However, this response does not have to be assigned to a single factor (Dizon & Yap, Reference Dizon and Yap2006) such as hydrodynamism, since many factors could interact, producing complex patterns in the responses (Todd et al., Reference Todd, Ladle, Lewin-Koh and Chou2004).
On the other hand, the attachment method can be a main factor for coral survival and/or growth. Some authors have found differences in the survivorship of colonies between epoxy resin and/or cyanoacrylate adhesive (Borneman & Lowrie, Reference Borneman and Lowrie2001; Dizon et al., Reference Dizon, Edwards and Gomez2008). Nevertheless, Forrester et al. (Reference Forrester, O`Connell-Rodwell, Baily, Forrester, Giovannini, Harmon, Karis, Krumholz, Rodwell and Jarecki2011) did not find significant differences in the growth and survival of fragments using different attachment methods and, therefore, hypothesized that any method that keeps the coral firmly attached should be successful.
Cleaning organisms from the substrate where the colony will be placed by scraping it away before transplantation has proved to improve the success of the transplants (Dizon et al., Reference Dizon, Edwards and Gomez2008). This approach is useful for the transplanted fragments; they can grow substantially larger when the substrate is cleared of surrounding algae (Forrester et al., Reference Forrester, O`Connell-Rodwell, Baily, Forrester, Giovannini, Harmon, Karis, Krumholz, Rodwell and Jarecki2011). In this sense, the orange coral also seems to benefit from this methodology, considering the growth and survival observed in this study. Skipping this step in the attachment procedure may lead to poor success as the colonies of the orange coral can be colonized by benthic organisms as observed in the nearby area of the Strait of Gibraltar (Ocaña et al., Reference Ocaña, Ramos and Templado2009; colonies colonized by surrounding macroalgae). Thus, we recommend cleaning the surrounding algae and biological material in order to facilitate the transplant's success.
Finally, it is important to know the cost-efficiency of transplantation programmes, including the cost of the attachment methodology in terms of the person-hours needed for coral transplantation (Dizon et al., Reference Dizon, Edwards and Gomez2008). In the coral restoration literature, few studies go into adequate detail on the costs involved, and those that do generally ignore some costs and resource implications altogether (Spurgeon, Reference Spurgeon2001). In some cases, restoration costs can vary enormously; for example, some methods require a significant amount of labour and a complex approach to construction and substrate preparation, while others do not (Spurgeon, Reference Spurgeon2001). Creating and maintaining a farming or nursery structure in a seabed is a laborious and expensive method in comparison with other techniques, such as transplanting corals using an adhesive (Forrester et al., Reference Forrester, O`Connell-Rodwell, Baily, Forrester, Giovannini, Harmon, Karis, Krumholz, Rodwell and Jarecki2011). In the present study, an epoxy resin was selected as the adhesive because it is easy to use, is inexpensive, requires little labour, does not require the use of an artificial structure on the seabed, and is easy to monitor. For example, the reattachment of one colony of A. calycularis by the present method had a cost of € 1.83, including the cost of scuba divers, epoxy resin and monitoring campaigns. At a cost per square metre, a high density orange coral populations rehabilitation would have a cost of € 60 m−2, and low densities € 15 m−2. Therefore, marine managers can easily implement this methodology scheme.
In conclusion, we have experienced a high level of success in the transplantation and translocation of A. calycularis in both areas (high and low hydrodynamism), but it is crucial to take into account environmental conditions, methods (such as scraping) and attachment material type. Coral colonies are broken and fragmented either naturally by storms or by human activities, such as diving, anchoring and boat grounding, as has already been demonstrated in the study area (Terrón-Sigler & León-Muez, Reference Terrón-Sigler and León-Muez2009); using these fragments for restoration could be beneficial (Raymundo, Reference Raymundo2001). Therefore, this attachment methodology for transplanting A. calycularis colonies or fragments should be considered in conservation strategies for this species as a potential management tool in areas affected by local disturbances.
ACKNOWLEDGEMENTS
Our research was supported by the Regional Government of Andalusia (General Office of Environment Management, Regional Ministry of Environment), and we thank Eduardo Fernández Tabales for technical assistance. Special thanks to our colleague Lucas Moreno for his help during the fieldwork and Manuel González O'Sullivan for his assistance during the English review process. The experiments complied with current Spanish laws. The authors are grateful to two anonymous reviewers for their useful comments and suggestions, which improved the manuscript.
FINANCIAL SUPPORT
Financial support for this work has been provided by the Asociación Hombre y Territorio (http://www.hombreyterritorio.org) and by the Laboratorio de Biología Marina (Departamento de Zoología, Facultad de Biología, Universidad de Sevilla).









