Introduction
The lithological characteristics of the substrate may influence the richness and dynamics of hard-bottom benthic assemblages (Cerrano et al., Reference Cerrano, Arillo, Bavestrello, Benatti, Calcinai, Cattaneo-Vietti, Puce and Sarà1999; Bavestrello et al., Reference Bavestrello, Bianchi, Calcinai, Cattaneo-Vietti, Cerrano, Morri and Sarà2000, Reference Bavestrello, Bo, Betti, Canessa, Gaggero, Rindi and Cattaneo-Vietti2018; Cattaneo-Vietti et al., Reference Cattaneo-Vietti, Albertelli, Bavestrello, Bianchi, Cerrano, Chiantore, Gaggero, Morri and Schiaparelli2002; Faimali et al., Reference Faimali, Garaventa, Terlizzi, Chiantore and Cattaneo-Vietti2004; Johansen, Reference Johansen2018; Canessa et al., Reference Canessa, Bavestrello, Bo, Betti, Gaggero and Cattaneo-Vietti2019, Reference Canessa, Bavestrello, Bo, Trainito, Panzalis, Navone, Caragnano, Betti and Cattaneo-Vietti2020). In fact, under comparable edaphic conditions, several communities were found to be structurally different on substrates of different lithology, in terms of presence and abundance of some sessile species (Coombes, Reference Coombes2011). However, there is a general scarcity of studies on this topic. During tests performed using experimental blocks of different rocks along the coast of Cornwall, barnacle settling was higher on granites and concrete than on limestones, suggesting that the roughness of the substrate could positively influence settling (Coombes, Reference Coombes2011). The influence of different rock types was particularly evident also for the supralittoral barnacle Chthamalus spp. which, in the Ligurian Sea, appeared more abundant on limestones than on marly substrates (Canessa et al., Reference Canessa, Bavestrello, Bo, Betti, Gaggero and Cattaneo-Vietti2019). Also, comparing barnacle populations living on different ophiolitic rocks, differences in density arose: they were abundant on serpentinite rocks and virtually absent on metagabbros (Bavestrello et al., Reference Bavestrello, Bo, Betti, Canessa, Gaggero, Rindi and Cattaneo-Vietti2018).
In the subtidal zone of the Mediterranean, the species richness of sponges, anthozoans, serpulids and sessile gastropods living on hard bottoms characterized by different lithologies showed differences in density or covering capacity (Cattaneo-Vietti et al., Reference Cattaneo-Vietti, Albertelli, Bavestrello, Bianchi, Cerrano, Chiantore, Gaggero, Morri and Schiaparelli2002; Bavestrello et al., Reference Bavestrello, Benatti, Cattaneo-Vietti, Cerrano and Giovine2003; Schiaparelli et al., Reference Schiaparelli, Guidetti and Cattaneo-Vietti2003; Guidetti et al., Reference Guidetti, Bianchi, Chiantore, Schiaparelli, Morri and Cattaneo-Vietti2004). For example, in coralligenous habitats of north-east Sardinia, some sponges (Axinella sp., Axinella polypoides and Sarcotragus foetidus), although present on both granite and carbonate, showed a clear preference for granite (Canessa et al., Reference Canessa, Bavestrello, Bo, Trainito, Panzalis, Navone, Caragnano, Betti and Cattaneo-Vietti2020).
The bulk of evidence suggests an intricate pattern of interactions exists between sessile organisms, rock morphology and composition, together with several local environmental factors (Aguilera et al., Reference Aguilera, Broitman and Thiel2014; Angiolillo et al., Reference Angiolillo, Gori, Canese, Bo, Priori, Bavestrello, Salvati, Fabrizio, Greenacre and Santangelo2016). In particular, the structure and diversity of algal assemblages seem particularly sensitive to substrate lithology (McGuinness, Reference McGuinness1989; Hadfield & Paul, Reference Hadfield and Paul2001; Bavestrello et al., Reference Bavestrello, Bo, Betti, Canessa, Gaggero, Rindi and Cattaneo-Vietti2018). Crustose epilithic red algae may act as ecosystem engineers, modifying conditions, creating habitat, but also competing for space with sessile animals (Bressan, Reference Bressan1974; Steneck, Reference Steneck1986; Johansen, Reference Johansen2018). Comparing the development of calcareous algae on two contiguous ophiolitic rocks in the upper tidal zone, it was evident that Neogoniolithon brassica-florida was only present on serpentinites, while virtually absent on metagabbros (Bavestrello et al., Reference Bavestrello, Bo, Betti, Canessa, Gaggero, Rindi and Cattaneo-Vietti2018). Moreover, in subtidal habitats within the area of Tavolara Island (north-east Sardinia), the thickness of the crustose calcareous red algae was higher on limestone rocks than on granite ones (Canessa et al., Reference Canessa, Bavestrello, Bo, Trainito, Panzalis, Navone, Caragnano, Betti and Cattaneo-Vietti2020). Here, it is possible that two different genotypes of ‘Lithophyllum stictiforme’ colonized these two different lithotypes, as stated by Pezzolesi et al. (Reference Pezzolesi, Peña, Le Gall, Gabrielson, Kaleb, Hughey, Rodondi, Hernandez-Kantun, Falace, Basso, Cerrano and Rindi2019).
This paper aims to verify the open hypothesis that the settlement of sessile zoobenthic organisms and the consequent development of the assemblage in terms of richness and occurrence could be directly driven by the substrate lithology, without interference due to encrusting calcareous algae. This was achieved by carrying out research in dark habitats, where algae are absent. Although marine caves are the most obvious option, it is virtually impossible to find close caves with similar ecological characteristics but different lithology. A more practical choice has been the study of the sessile fauna settled under boulders, comparable to that of the caves (Harmelin et al., Reference Harmelin, Vacelet and Vasseur1985), even if influenced by the faunistic characteristics of the surrounding communities (Bellan-Santini, Reference Bellan-Santini1962).
Herein, we present the results obtained by evaluating the species richness and coverage of the sessile zoobenthic assemblage, growing under large boulders of different lithology, without algal presence and assuming the invariance of all other ecological variables.
Materials and methods
Study area and sampling methods
In this study, aiming to describe the diversity and coverage of benthic communities directly settled on two different kinds of substrates, we investigated the lower surface of granite and limestone boulders, characterized by a progressive decrease of algal covering, following the reduction of solar light.
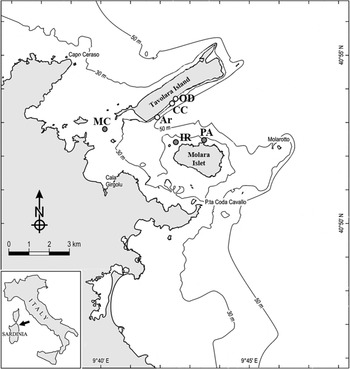
A total of 72 boulders with comparable size at 15–20 m depth were randomly selected and their dark side photographed at six sites, three carbonatic (Archetto (Ar), Occhio di Dio (OD) and Cala Cicale (CC)) and the other three granitic (Isola Rossa (IR), Mezzo Canale (MC) and Punta Arresto (PA)) inside the Marine Protected Area of Tavolara-Punta Coda Cavallo in the north-east coast of Sardinia (Tyrrhenian Sea) (Table 1; Figure 1). The selected sites are characterized by calcareous-dolomitic limestone of Tavolara Island and by granitic granular red pegmatite (Orrù & Pasquini, Reference Orrù and Pasquini1992). Very likely, each boulder has its own specific position with respect to the bottom and therefore specific micro-environmental conditions affecting the development of the benthic communities. Nevertheless, we think that it was reasonable to assume that, due to the number of replicates, the average conditions under boulders of comparable size of the two lithologies can be considered similar.
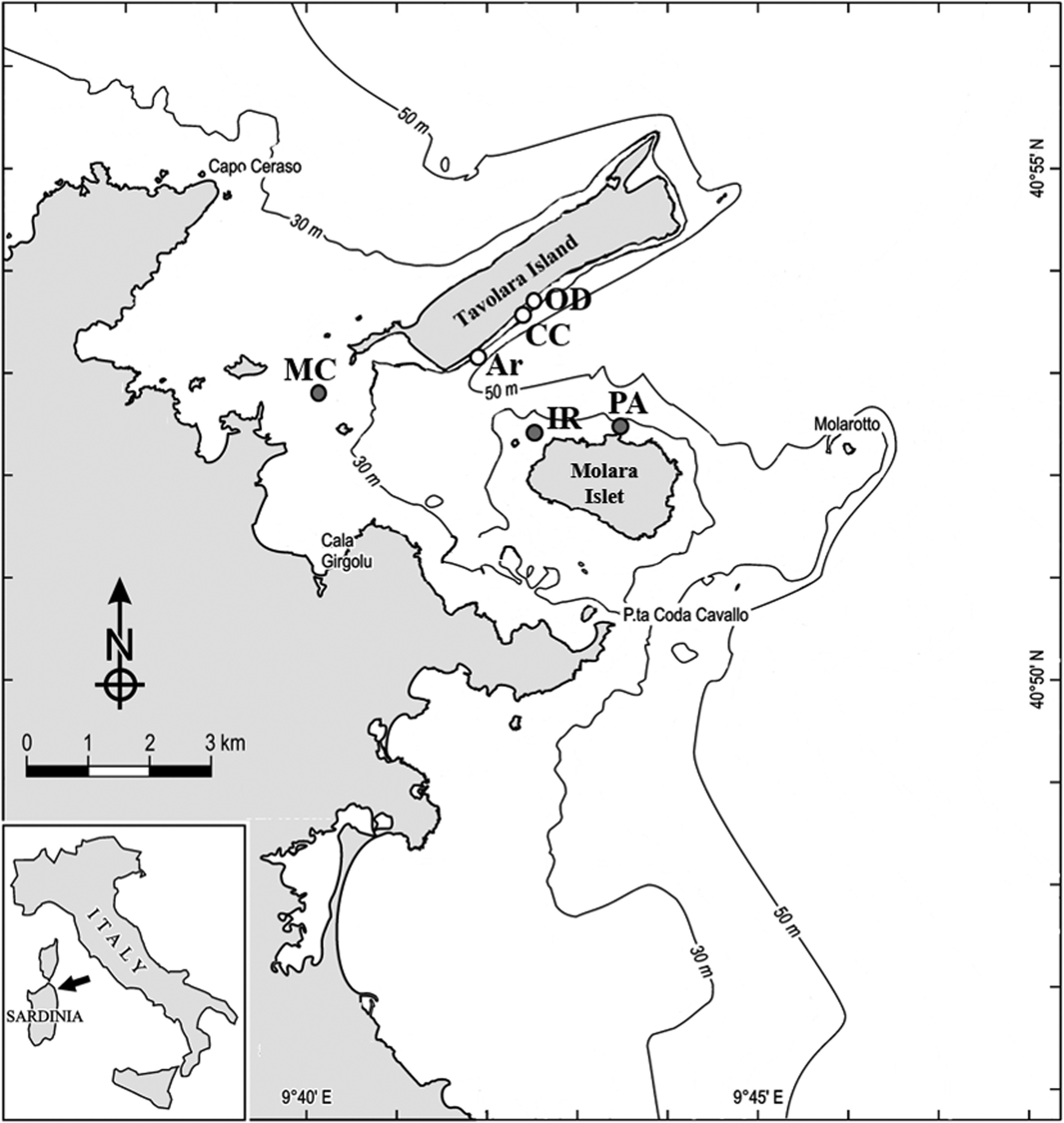
Fig. 1. Study area and sample sites within the Tavolara-Punta Coda Cavallo MPA. White dots, limestone, grey dots, granite. Ar, Archetto; CC, Cala Cicale; OD, Occhio di Dio; MC, Mezzo Canale; IR, Isola Rossa; PA, Punta Arresto.
Table 1. Per cent occurrence of each species/OTU in each site and average occurrence on limestone (Ol) and granite (Og) boulders
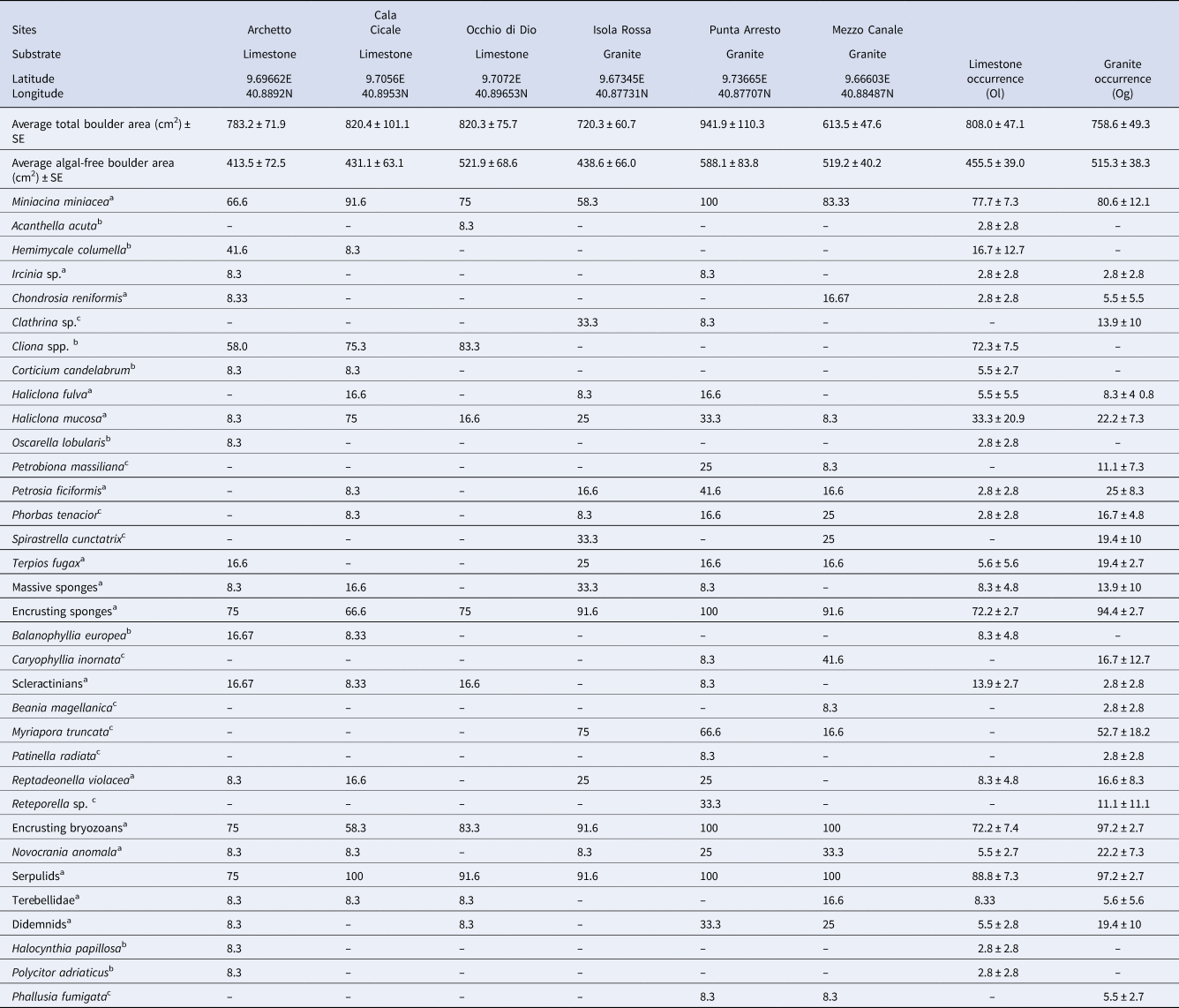
a Species found on both substrates.
b Species/OTU exclusive of limestone.
c Species/OTU exclusive of granites.
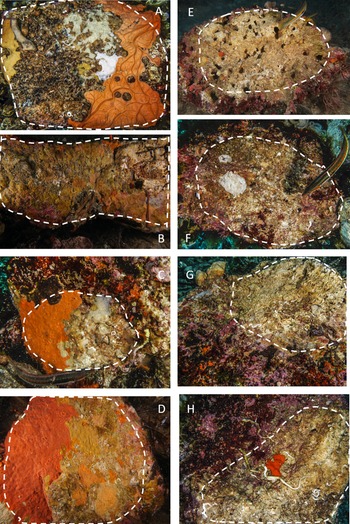
During scuba diving performed in July 2019, we turned the boulders upside down and the lower side was photographed by a Sony A6000 camera, 24 megapixels, 2 Inon S2000 flashes, colour temperature 5000° K; Sony 16-50 at 16 mm focal length; Sea & Sea MDX-a6000 underwater case with a flat porthole. A twin laser pointer, calibrated at 10 cm, was used as a metric reference. The lower surface was evaluated for each image, separating the algal-free portion used for the study from the total area (Figure 2).
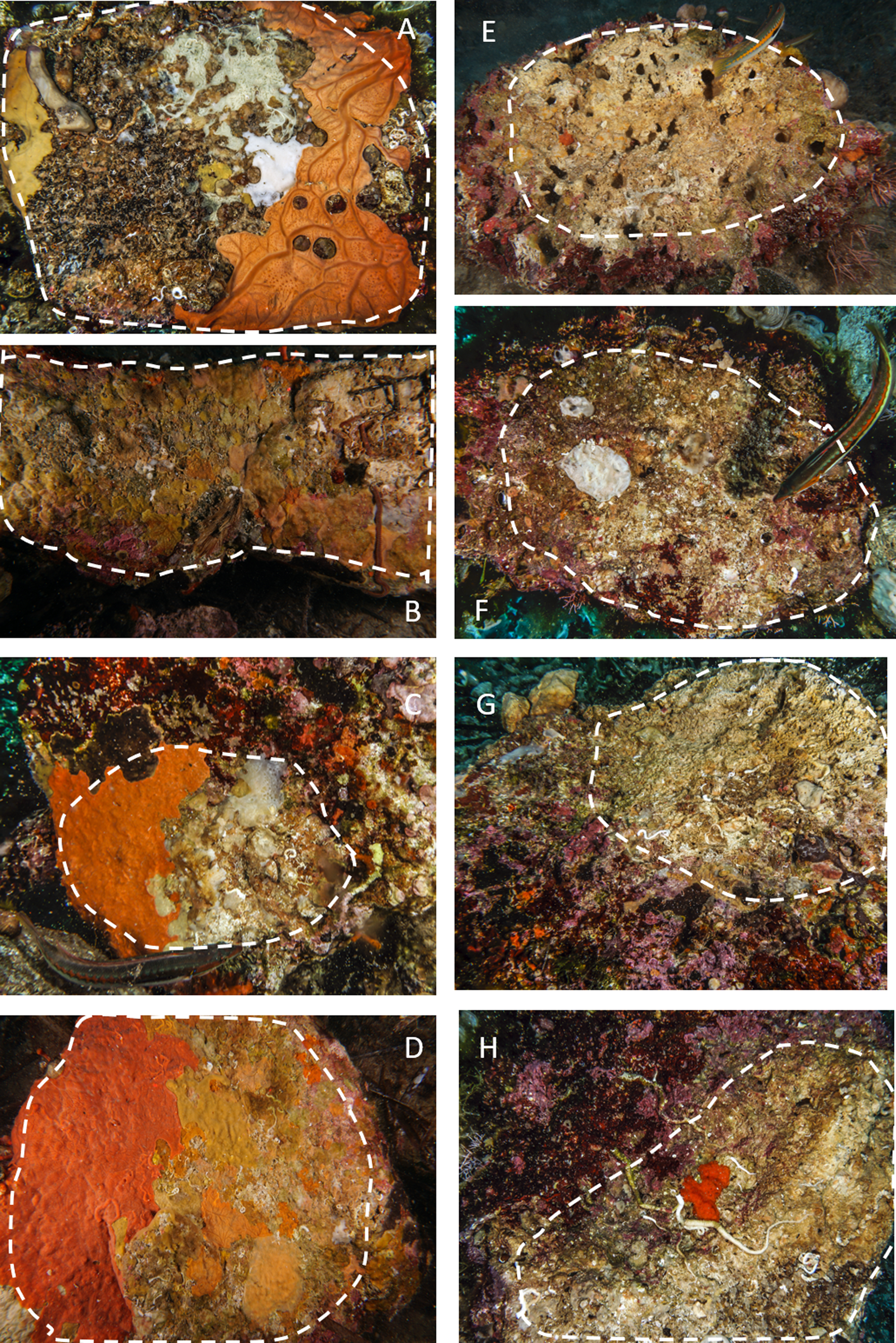
Fig. 2. Examples of limestone and granite boulders photographed during the sampling activity. The stacked lines include the algal-free surfaces on the dark side of the boulders selected for the analysis. A–D, granites; E–H, limestones. Note the extensive coverage of sponges and bryozoans on granites and the sizeable bare area, often bored by date-mussels, recorded on limestones.
Data analysis
The sessile zoobenthic organisms present under the boulders were tentatively classified at a specific level, and their per cent coverage, in terms of main taxonomic groups, calculated by a superimposed grid, using ImageJ Software. When specific or generic identification was not possible, other taxonomic and morphological units (OTUs) were adopted. Also, the bare surface present on the dark side of the boulders was measured.
Values of relative occurrence (Ol and Og, respectively for limestone and granite) were calculated for each species/OTUs, as per cent presence on the 12 photographs shot per site (Table 1). For the statistical analysis, we considered only the species/OTUs present in all the three sites of at least one substrate.
Resemblance similarity matrices, based on the Bray–Curtis index, were constructed for species/OTUs occurrence and per cent coverage of the main taxonomic groups (Anderson, Reference Anderson2001). The multivariate configuration of both the datasets was visualized through non-metric multi-dimensional scaling (nMDS).
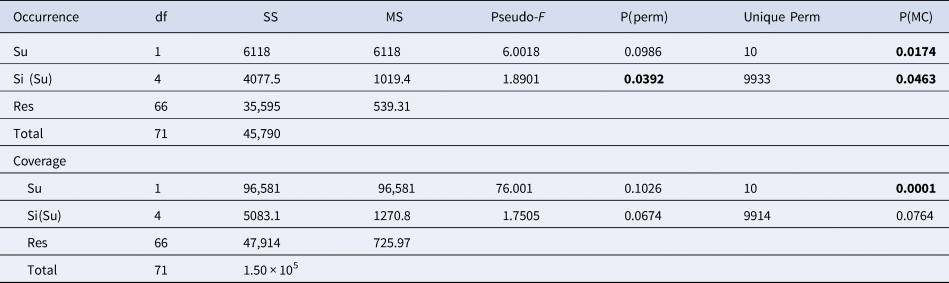
Permutational analysis of variance (PERMANOVA) (Anderson, Reference Anderson2001) was performed using a two-way experimental design, with ‘Substrate’ (Su) as a fixed factor with two levels (Limestone (L) and Granite (G)) and ‘Site’ (Si), a random factor with six levels nested within the substrate. Each analysis used 9999 random permutations; Monte Carlo test was performed for deficient order of permutations; pairwise tests were used to compare condition levels when significant differences were detected by PERMANOVA (Table 2).
Table 2. PERMANOVA performed on the occurrence for the species /OTUs recorded in all the sites of at least one lithology, and coverage of the main taxonomic groups
Bray–Curtis similarity index used for the resemblance matrix construction; permutation N = 9999. Monte Carlo test applied for insufficient unique permutations. Significant values are in bold.
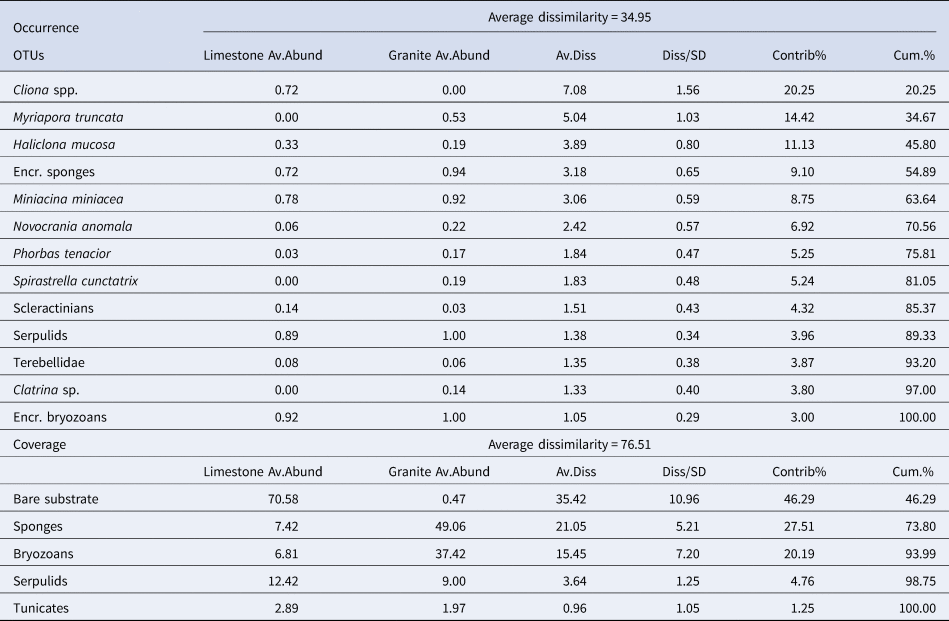
Similarity percentages analysis (SIMPER) (Clarke, Reference Clarke1993) was performed to identify the percentage contribution of each descriptor to the Bray–Curtis similarity among the two substrates (Table 3).
Table 3. SIMPER analysis performed for occurrence of the main species/OTUs recorded in all the sites for almost one substrate, and coverage datasets
Bray–Curtis similarity index used for the resemblance matrix construction.
All the statistics were performed using PRIMER_E (Version 7).
Results
The sessile zoobenthic communities settled on the dark side of large boulders were investigated according to the substrate lithology.
The total area of the lower face of the boulders was, on average, 808 cm2 ± 47.1 SE for limestone and 758.6 cm2 ± 49.3 SE for granite. The average area wholly deprived of algae (the suitable boulder portion for the investigation) was 455.5 cm2 ± 39 SE for limestone and 515.3 cm2 ± 38.3 SE for granite (Table 1). No correlation between the number of species/OTUs and the considered surfaces was found (R = 0.17).
The analysis of the photographic sampling allowed the identification of 34 species/OTUs: 24 of them were observed under granitic boulders (9 exclusives) and 25 under limestone boulders (10 exclusives). The number of species/OTUs recorded on granite ranged from 4 to 10 per image, and that recorded on limestone from 2 to 10; 44.1% of the species were found in common, although with variable occurrences, between the two substrates. Twenty-one species/OTUs were occasional or rare, being recorded in less than 10% of the samples. The most represented taxonomic group was the sponges (with 17 OTUs) (Table 1).
Only considering the species/OTUs recorded in all of the sites of at least one lithology, it is possible to evidence a group of ubiquitarians, OTUs recorded with a high occurrence on both substrata and comprising the encrusting bryozoans, serpulids and encrusting sponges together with the benthic foraminifera Miniacina miniacea. Also, the sponge Haliclona mucosa, although present with lower occurrences, did not show any particular preference for a substratum. In contrast, the boring sponges of the genus Cliona showed, as expected, a complete preference for the limestone, while the brachiopod Novocrania anomala, the sponges Terpios fugax, Phorbas tenacior, Petrosia ficiformis and the branched bryozoan Myriapora truncata were virtually recorded only on granites (Figure 3). Among species recorded with lower frequency, the bryozoan Reptadeonella violacea was similarly recorded on both substrates while the behaviour of the two identified scleractinians was particularly interesting: Balanophyllia italica was recorded only on limestone and Caryophyllia inornata only on granite. Ascidians showed similar trends, with Halocyntia papillosa and Polycitor adriaticus exclusive to limestone and Phallusia fumigata present only on granite (Table 1).
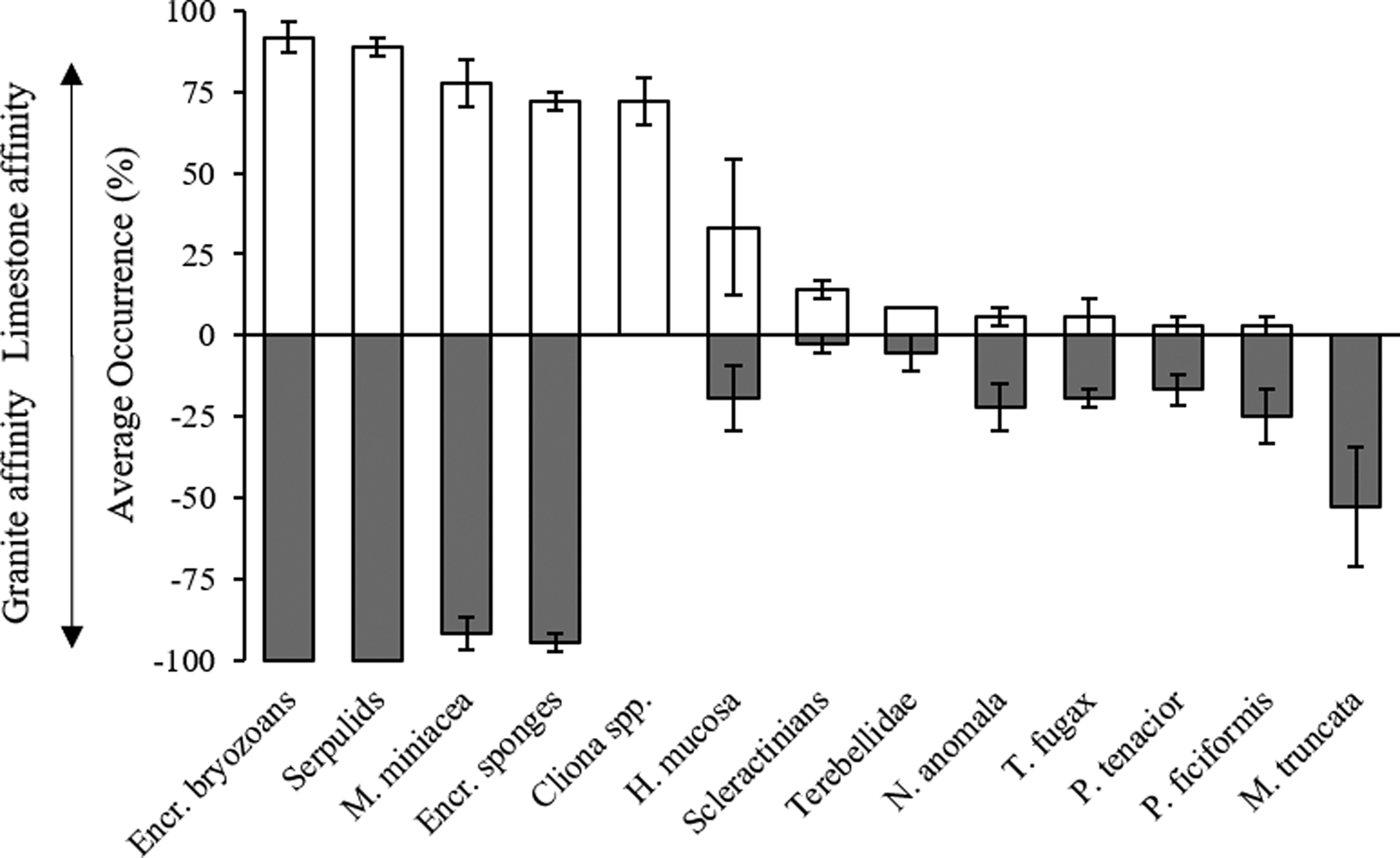
Fig. 3. Lithological affinity of species/OTUs ± SE present in all the sites of at least one substrate. White bars, limestone; grey bars, granite.
Only considering the species/OTUs recorded in all the sites of at least one lithology, nMDS and PERMANOVA showed significant differences for both the factors (‘Substrate’ and ‘Site’), with a marked effect of ‘Substrate’ in clustering sites (Figure 4A, Table 2). According to the SIMPER analysis, the percentage occurrence of the boring sponge Cliona spp., of the bryozoan M. truncata and the sponge H. mucosa contributed 20.25%, 14.42% and 11.13% respectively to the dissimilarity between the two substrates. At the same time, the contribution of the other OTUs was lower (Table 3).
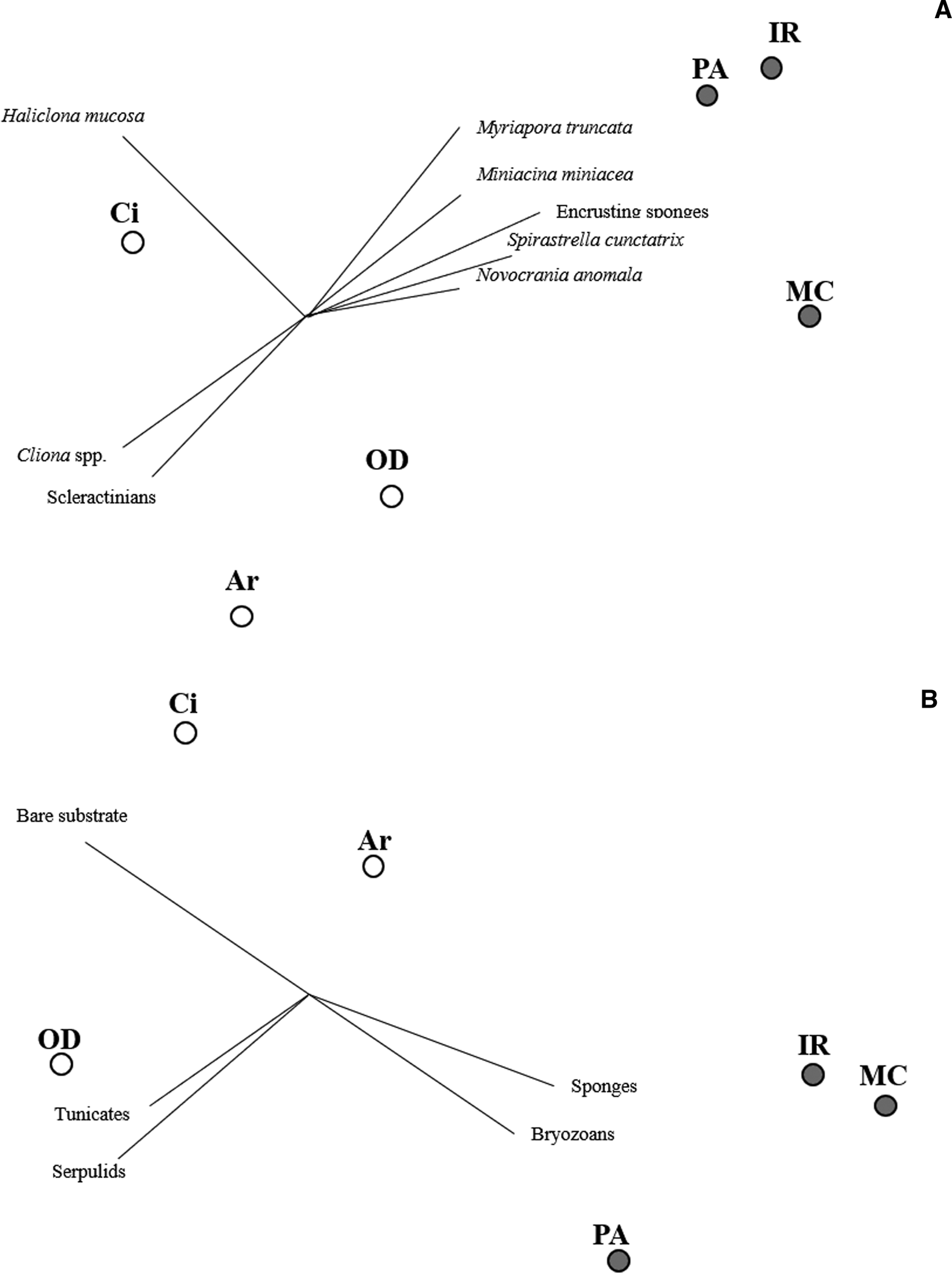
Fig. 4. Non-metric multi-dimensional scaling (nMDS) performed considering the occurrence of species/OTUs present in all the sites of at leat one substrate (A) and per cent coverage (B) of main taxonomic groups and bare substrate. White dots: limestone; grey dots: granite.
Also, the comparison of the cover per cent on the two different substrata confirmed this trend. On limestone, a large portion of the substrate was bare (on average 70%), while granite was always completely bio-covered (Figures 2 & 5; Table 1).
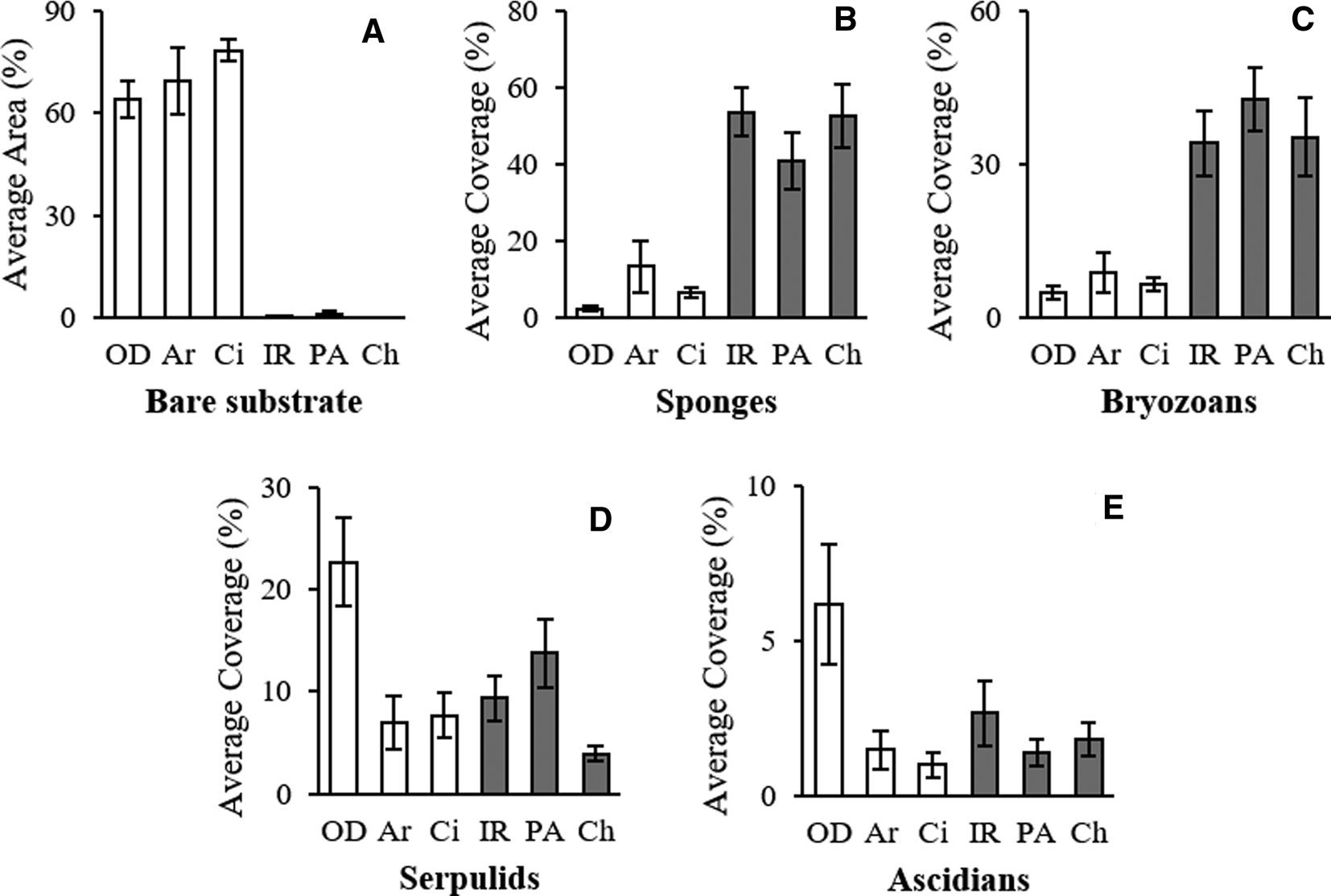
Fig. 5. Average per cent area of bare substrate ± SE (A) and average per cent coverage ± SE (B–E) of the main taxonomic groups in each site.
Considering the per cent coverage of the main taxonomic groups, the nMDS (Figure 4B) and PERMANOVA (Table 2) highlighted two well-defined clusters, according to ‘Substrate’ factor, but not for ‘Site’.
SIMPER analysis reported the contribution of sponges (27.51%) and bryozoans (20.19%) to describe the dissimilarity between the two assemblages and marked the critical contribution of bare substrate (46.29%) (Table 3).
Discussion
In several hard-bottom communities of the photic zone, the presence of secondary substrates of biological origin frequently prevents the direct contact of larvae with the rock. In particular, the development of the epilithic corallines is often so broad that it produces a virtually continuous secondary substrate on which other organisms grow (Johansen, Reference Johansen2018). Consequently, the presence of crustose red algae can mask the influence of the primary substrate on zoobenthos settling. Several invertebrate larvae, in fact, recruit in response to chemical traits of specific crustose coralline algae (Hadfield & Paul, Reference Hadfield and Paul2001). In this situation, some conclusions previously obtained about the influence of rock composition on the development of benthic communities could be biased by the interference due to the coralline coverage. It was, in fact, generally stated that, when comparing benthic communities settled on limestone and granite, the latter is less abundant in species diversity and the bio-coverage is lower (Bavestrello et al., Reference Bavestrello, Bianchi, Calcinai, Cattaneo-Vietti, Cerrano, Morri and Sarà2000; Canessa et al., Reference Canessa, Bavestrello, Bo, Trainito, Panzalis, Navone, Caragnano, Betti and Cattaneo-Vietti2020). The present study was planned to evaluate, in the field, the direct effect of limestone and granite on the settling and development of zoobenthic species, without the influence of the epilithic crustose corallines. The dark sides of large boulders turned out to be particularly fit for this purpose, providing light conditions comparable to those found in caves, as the presence of the sponge Petrobiona massiliana, considered exclusive to the biocenosis of dark caves, confirms (Harmelin et al., Reference Harmelin, Vacelet and Vasseur1985; Manconi et al., Reference Manconi, Ledda, Serusi, Corso and Stocchino2009).
The results obtained were mostly unexpected. In fact, we have shown that the dark sides, coralline-free, of limestone boulders, were widely bare, while the cover per cent was almost total on granite. The only exclusive species with a high occurrence recorded on limestones were clionids, able to bore carbonates due to acid secretions of their etching cells (Ruetzler, Reference Ruetzler1975). In contrast, several species, mainly sponges and bryozoans, were almost exclusive to granite. A pool of other species or morphological OTUs indifferently occupied both substrates, although with different occurrences.
The leading cause that might explain these findings could be the different level of chemical stability of the two types of rocks, due to their different composition and textural characteristics. In the sea, carbonates precipitate in the light zone and dissolve in dark ones where the CO2 increases, favouring a progressive bacterial-induced carbonate dissolution (Golubić & Schneider, Reference Golubić, Schneider, Trudinger and Swaine1979). Moreover, directed carbonate removal is carried out by specialized macroborers as bivalves (mainly Lithophaga lithophaga) and clionid sponges which produce a further, more in-depth erosion with a consequent increase of substrate instability (Schneider & Torunski, Reference Schneider and Torunski1983). In contrast, granites are much more stable, as indicated by Coombes (Reference Coombes2011). A high level of substrate superficial instability has already been demonstrated to strongly affect larval settling and assemblage development. Some friable rocks, such as chalk and sandstones, appeared much more unsuitable compared with harder granites for settling of the barnacle Chthamalus montagui in the English Channel, where the survival rate of its cyprids was relatively low even in the presence of high recruitment (Herbert & Hawkins, Reference Herbert and Hawkins2006).
Substrate roughness has always been claimed as one of the main features of the rock to determine the success of larval settlement (Barnes, Reference Barnes and Manly1970; Raimondi, Reference Raimondi1988; Holmes et al., Reference Holmes, Sturgess and Davies1997; Berntsson et al., Reference Berntsson, Jonsson, Lejhall and Gatenholm2000; Coombes, Reference Coombes2011). Our data do not support this statement. In our opinion, it is possible to hypothesize that when two substrata have comparable chemical stability, the roughness can enhance the settlement and the development of epilithic communities, but when a substrate is subject to a continuous superficial dissolution this characteristic overcomes any other surface feature. Although it is evident that some species were positively attracted by one or the other substrate, in general, the lithology did not affect the richness of the communities. Our data support, in contrast, a profound influence of the lithology on the cover capacity of modular organisms: on limestone, a pool of 29 species produced coverage of about 30% of the available substrate. In contrast, 26 species/OTUs resulted in virtually complete coverage on granites.
In our study, the first conclusion is that the lithology and in particular the substrate stability is one of the primary drivers of colonization, also taking into account that the rock roughness is directly proportional not only to the type of lithology but also to its greater or lesser resistance to erosive processes. A second conclusion picks up on the pivotal role of epilithic corallines in the colonization of rocky substrates. Canessa et al. (Reference Canessa, Bavestrello, Bo, Trainito, Panzalis, Navone, Caragnano, Betti and Cattaneo-Vietti2020) have demonstrated a strong preference of these macroalgae for calcareous substrates, and it is possible to hypothesize that their settling affects the following phases of colonization producing a stabilization of the limestone, preserving it from surface dissolution. This scenario could explain the differences observed in the richness of coralligenous communities dwelling on granites and limestones within the Tavolara MPA.
Martin & Gattuso (Reference Martin and Gattuso2009) have demonstrated changes in the balance between algal carbonate production and dissolution induced by elevated pCO2 and temperature in coralligenous communities. In light of these considerations and of our results, marine acidification, causing a loss of coralline layer, could also have an impact on the structure of zoobenthic assemblages living on limestone and, consequently, a significant adverse effect on the biodiversity of whole Mediterranean coastal ecosystems.
Acknowledgements
We thank the Tavolara Marine Protected Area staff for their permission to undertake sampling and the section of Lega Navale Italiana of Loiri Porto San Paolo for the logistical support during sampling activity.