Introduction
Wood falls serve as a significant organic source to the oligotrophic deep-sea environment (Bienhold et al., Reference Bienhold, Pop Ristova, Wenzhöfer, Dittmar and Boetius2013; McClain & Barry, Reference McClain and Barry2014). Degradation of wood by wood-boring bivalves allows predatory and detritus-feeding organisms to settle and colonize within wood blocks as well as in sediments mixed with wood debris (Bienhold et al., Reference Bienhold, Pop Ristova, Wenzhöfer, Dittmar and Boetius2013). Several members of polychaetes such as polynoids are known as a main indicator species of successional stage during colonization and development of wood-fall benthic communities (McClain & Barry, Reference McClain and Barry2014).
Lacydoniidae Bergström, 1914 currently harbours 14 nominal species from the Pacific, Atlantic, Arctic and Antarctic Oceans (Rizzo et al., Reference Rizzo, Magalhães and Santos2016; Mazurkiewicz et al., Reference Mazurkiewicz, Gromisz, Legeżyńska and Włodarska-Kowalczuk2017). The members have been known to occur among encrusting algae on coral rubbles as well as in sandy and muddy substrata mixed with shell debris, gravel and volcanic rocks (Rouse & Pleijel, Reference Rouse and Pleijel2001; Rizzo et al., Reference Rizzo, Magalhães and Santos2016; Mazurkiewicz et al., Reference Mazurkiewicz, Gromisz, Legeżyńska and Włodarska-Kowalczuk2017). Information on the natural history of Lacydoniidae, including reproductive ecology, feeding behaviour and population biology, is completely lacking due to difficulties in obtaining a large number of specimens from sediment samples (Rouse & Pleijel, Reference Rouse and Pleijel2001; Magalhães et al., Reference Magalhães, Bailey-Brock and Rizzo2012). In this study, we describe a new species of Lacydonia Marion & Bobretsky, 1875 associated with a piece of sunken wood collected at Shoho Seamount of the Nishi-Shichito Ridge in the North-western Pacific during the research project ‘Development of Biodiversity Monitoring Methods for the Management of Deep-sea Marine Protected Areas’. The study area was designated as the first offshore seabed nature conservation area based on the Nature Conservation Act, Japan in 2020. We herein performed morphological examination by use of a scanning electronic microscope (SEM) for the 15th member of the genus, as well as preliminary analyses for phylogenetic relationships within Lacydonia based on partial sequences of the mitochondrial cytochrome c oxidase subunit I (COI), 16S rRNA (16S) genes, and the nuclear 18S rRNA (18S) and 28S rRNA (28S) genes.
Materials and methods
Three specimens were obtained from a piece of sunken wood collected by the remotely operated vehicle (ROV) ‘KM-ROV’ equipped with robotic manipulators during the cruise of RV ‘Kaimei’ (cruise ID: KM20-10C Leg1) under the research project ‘Development of Biodiversity Monitoring Methods for the Management of Deep-sea Marine Protected Areas’ in 2020 (Figure 1). Photographs of living specimens were taken with a digital still camera OM-D E-M1 Mark II (Olympus, Japan). Length and width of the prostomium were measured under a dissecting microscope SMZ-745 (Nikon, Japan) either before or after fixation. Chaetae were examined under a light microscope (LM) CX31 (Olympus, Japan) using oil immersion. Posterior tips of individuals were preserved in 99% ethanol for DNA extraction, while specimens for morphological observation were anaesthetized in a MgCl2 solution isotonic to seawater, then fixed in 10% seawater-buffered formalin or 70% ethanol.
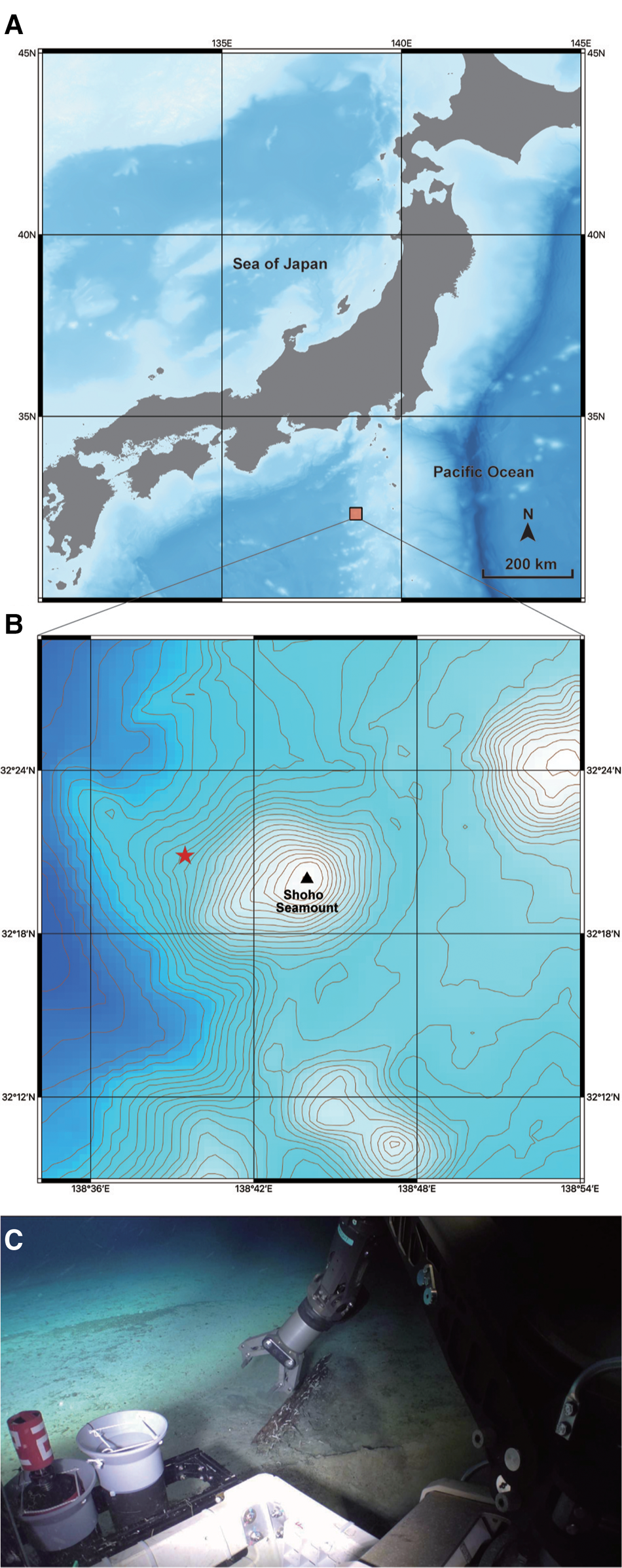
Fig. 1. A collection site for Lacydonia shohoensis sp. nov. (A) Location of Shoho Seamount of the Nishi-Shichito Ridge; (B) magnified map around Shoho Seamount, a red star indicating the sampling site in the present study; (C) the sunken wood discovered at a depth of 2042 m at Shoho Seamount.
For further morphological observation under a SEM, a single specimen was washed with phosphate buffered saline, subsequently dehydrated in an ethanol series (70, 80, 90, 95, 99, 100% ethanol), critical-point dried in a SYGLCP81 (Sanyu, Japan), mounted on an aluminium stub, and coated with osmium in an HPC-20 (Vacuum Device, Japan). The samples were observed with a JSM-7200F scanning electron microscope (JEOL, Japan) at 3.0–15.0 kV accelerating voltage. Type specimens are deposited in the National Museum of Science and Technology, Tsukuba (NSMT), Japan.
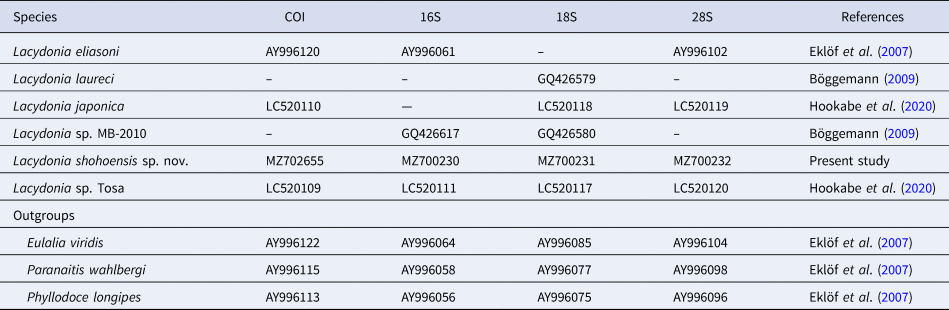
DNA extraction, PCR amplification and nucleotide sequencing were performed for partial sequences of COI, 16S, 18S and 28S genes of the holotype specimen, following the methods in Hookabe et al. (Reference Hookabe, Jimi, Tsuchida, Fujiwara and Kajihara2020). Sequences newly obtained in the present study have been deposited in the DNA Data Bank of Japan (DDBJ) under accession numbers in Table 1.
Table 1. List of phyllodocid species included in the molecular phylogenetic analyses and the GenBank accession numbers
To clarify the phylogenetic position of the new species among Lacydoniidae, maximum likelihood (ML) analyses were performed using concatenated sequences of the four gene markers. Three phyllodocid species were selected as outgroup taxa: Eulalia viridis (Linnaeus, Reference Linnaeus1767), Paranaitis wahlbergi (Malmgren, Reference Malmgren1865) and Phyllodoce longipes Kinberg, 1866. Sequences were aligned with MAFFT ver. 7, employing the L-INS-i strategy (Katoh & Standley, Reference Katoh and Standley2013). Ambiguous sites were trimmed using Gblocks ver. 0.91b (Castresana, Reference Castresana2000) under a less stringent option. As a final dataset a 3599 bp (450 bp for 16S; 602 bp for COI; 1754 bp for 18S; 793 bp for 28S) of the concatenated sequences remained. The best-fit substitution model was selected as GTR + G + I according to PartitionFinder ver. 2.1.1 (Lanfear et al., Reference Lanfear, Frandsen, Wright, Senfeld and Calcott2016) employing the greedy algorithm. The ML analysis was performed with RAxML ver. 8.0.0 (Stamatakis, Reference Stamatakis2014). Nodal values were calculated based on 1000 bootstrap pseudoreplicates (Felsenstein, Reference Felsenstein1985).
Uncorrected pairwise genetic distances and Kimura (Reference Kimura1980) two-parameter (K2P) genetic distances were calculated based on 658 bp of COI by MEGA ver. 7 (Kumar et al., Reference Kumar, Stecher and Tamura2016).
Results
Systematics
Family LACYDONIIDAE Bergström, Reference Bergström1914 Genus Lacydonia Marion & Bobretsky, Reference Marion and Bobretzky1875 Lacydonia shohoensis sp. nov. (Figures 2–5, Table 3)
http://zoobank.org/750D8377-BBBA-4C14-9ABF-D41342E3CE7A
[New Japanese name: shoho-rakidonia]
Diagnosis
Prostomium anteriorly rounded, twice wider than long, with a groove on median anterior edge; paired lateral antennae and a median antenna same in length; eyes absent; ventral side of prostomial median region slightly depressed; nuchal organs forming an incomplete ring, opening both dorsally and ventrally; peristomium indistinctly separated from prostomium; noto- and neuropodial lobes having ovoid cirrus with reddish pigment spots; capillary notochaetae diagonally striated, sub-distally expanded and marginally serrated; neurochaetae compound spinigerous, diagonally striated; lateral pair of pygidium cirri same length as median one.
Type material
Three specimens: holotype, NSMT-Pol H-844, preserved in 70% ethanol, 33 chaetigers, 4.0 mm in the body length and 1.1 mm in maximum body width, collected from wood falls found at a depth of 2042 m, 32°20.854′N 138°39.472′E, Shoho Seamount, Nishi-Shichito Ridge, Japan, by the ROV ‘KM-ROV’ dive #122 during the KM 20-10C cruise of the RV ‘Kaimei’ on 26 November 2020; posterior tip used for DNA extraction. Two paratypes, both collected from the same wood block as holotype, on 26 November 2020: NSMT-Pol P-845, preserved in 10% formalin, 2.5 mm in body length and 0.8 mm in maximum body width, posterior segments lost; NSMT-Pol P-846, 2 mm in the body length and 1.2 mm in maximum body width, 33 chaetigers, complete body, Os-coated and mounted on a SEM stub.
Description
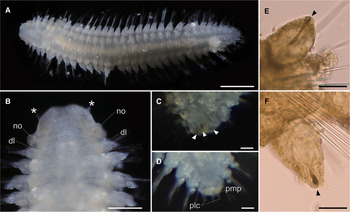
Body flattened, smoothly tapering in posterior region, 4.0–4.2 mm in length, 0.8–1.2 mm in width for 33 chaetigers (Figure 2A, 3A). Body generally transparent; pale-coloured intestine visible through body wall (Figure 2A). Preserved specimens generally pale-yellow. Living specimens with body pigmentation on segments and pygidium; pale-orange pigment spots present in either dorsal or ventral parapodial cirri of all chaetigers except first achaetigerous segment; generally a single pigment spot in each parapodial cirri (Figure 2E, 2F) and rarely in both dorsal and ventral cirri; three pale-orange spots on dorsal side of pygidium (Figure 2C); pigments darker in preserved specimens. Dorsal and ventral lobe of body well developed in all chaetigers (Figure 2B, 4A). Prostomium followed by peristomium, one achaetigerous segment (tentacular segment), three uniramous chaetigers, 30 biramous chaetigers, and pygidium (Figure 3A, 3E).
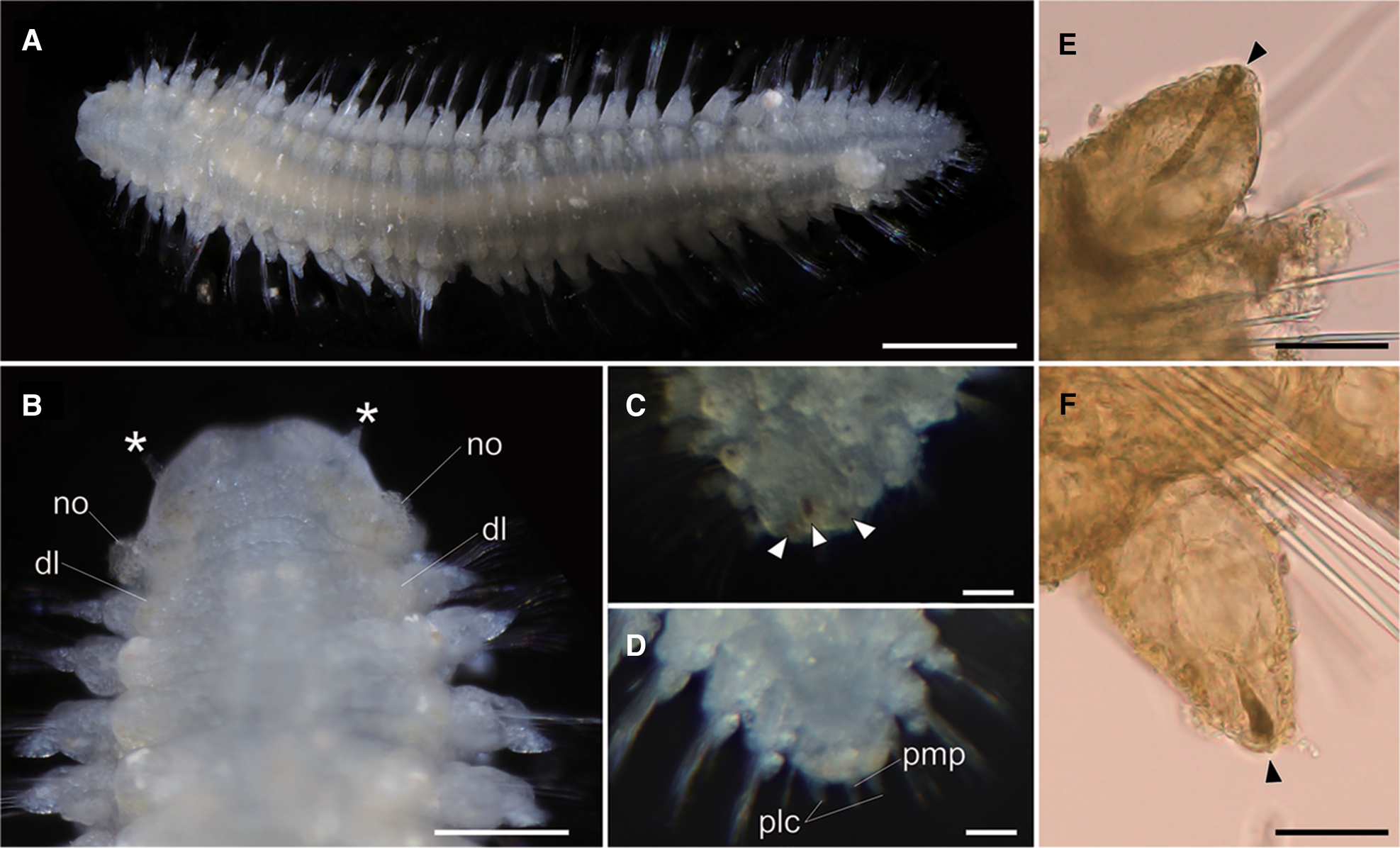
Fig. 2. Lacydonia shohoensis sp. nov., holotype (NSMT-Pol H-844). (A) Whole body of living specimen, dorsal view; (B) head, dorsal view with asterisks pointing to lateral antennae (dl, dorsal lobe of body; no, nuchal organ); (C) posterior end, dorsal view, focusing on three reddish spots on pygidium indicated with arrowheads; (D) posterior end, dorsal view, focusing on pygidium lateral cirrus (plc) and pygidium median papilla (pmp); (E) parapodial dorsal cirrus, an arrowhead pointing to a reddish pigment spot; (F) parapodial ventral cirrus, an arrowhead pointing to a reddish pigment spot. Scale bars: 500 μm (A); 250 μm (B); 30 μm (C–F).
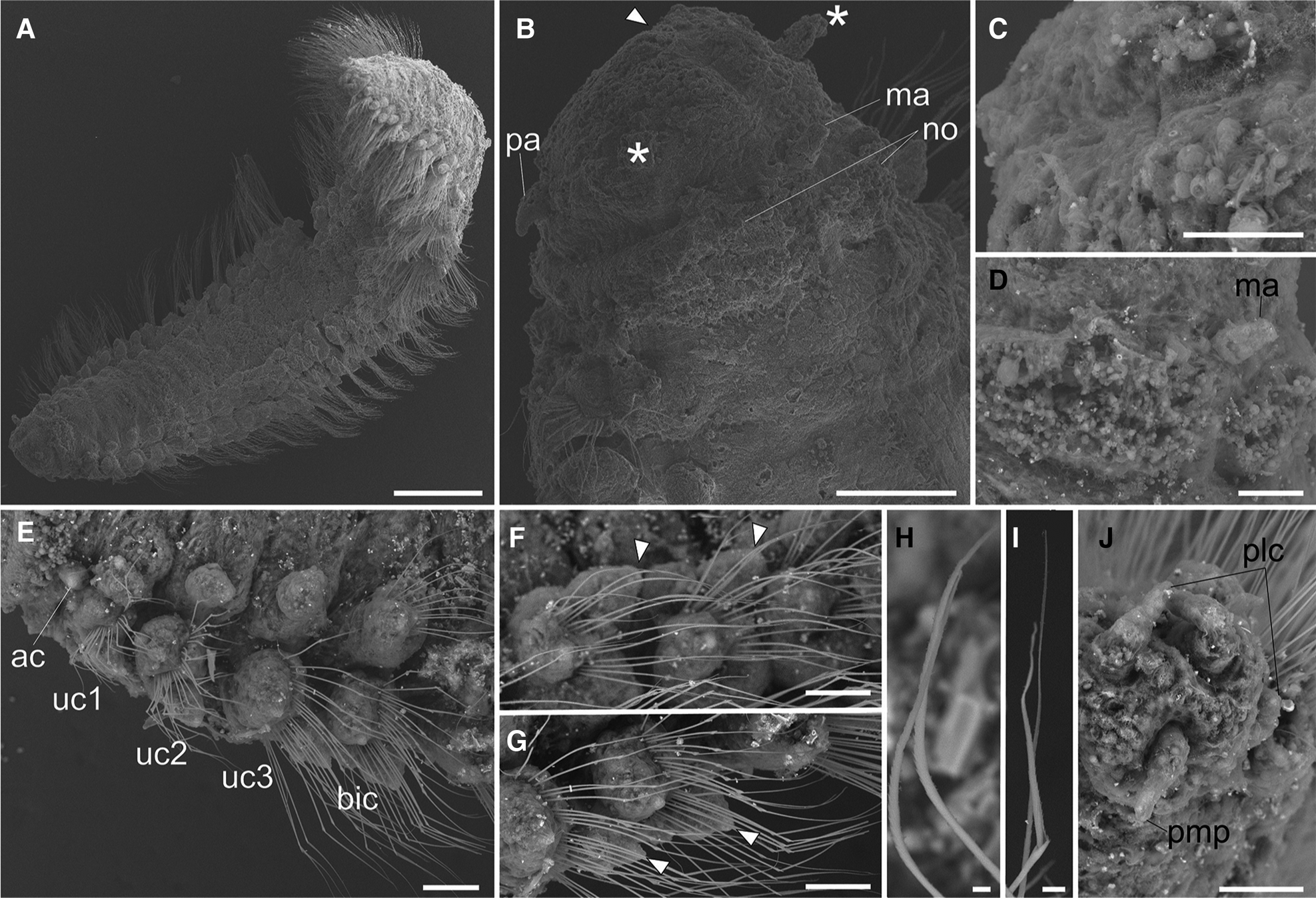
Fig. 3. Lacydonia shohoensis sp. nov., paratype (NSMT-Pol P-846), SEM. (A) Whole body, dorsal view; (B) magnification of prostomium, asterisks pointing to lateral antennae; (C) magnification of prostomium median furrow; (D) magnification of nuchal organ; (E) achaetous segment (segment 1) followed by uniramous chaetigers (segments 2–4) and biramous chaetigers (segment 5–); (F) notopodia of mid-body segments, anterior view, arrowheads pointing to parapodial dorsal cirri; (G) neuropodia of mid-body segment, anterior view, arrowheads pointing to parapodial ventral cirri; (H) notochaeta of mid-body; (I) neurochaeta of mid-body; (J) pygidium. ac, achaetiger; bic, biramous chaetiger; ma, median antenna; no, nuchal organ; pa, palp; plc, pygidial lateral cirrus; pmp, pygidial median papilla; uc1, 1st uniramous chaetiger; uc2, 2nd uniramous chaetiger; uc3, 3rd uniramous chaetiger. Scale bars: 500 μm (A); 100 μm (B); 40 μm (C); 60 μm (D–G); 5 μm (H, I); 50 μm (J).
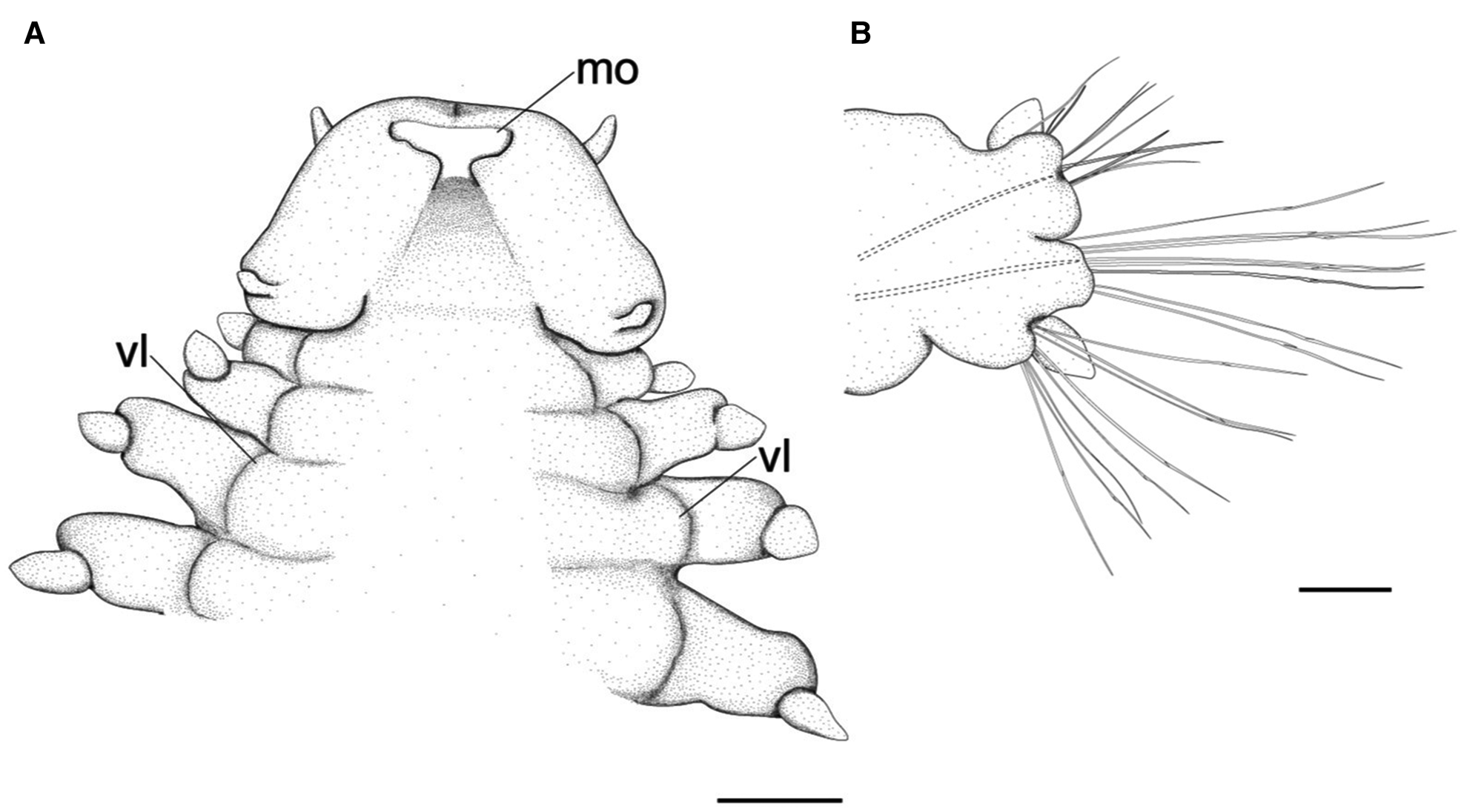
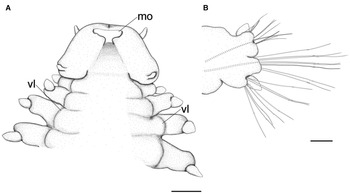
Fig. 4. Lacydonia shohoensis sp. nov., holotype (NSMT-Pol H-844), illustration. (A) Anterior end, ventral view; (B) mid-body parapodium. mo, mouth opening; vl, ventral lobe of body. Scale bars: 150 μm (A); 50 μm (B).
Prostomium anteriorly rounded, twice wider than long, with a furrow on median anterior edge (Figure 3B, 3C). A pair of lateral antennae and a median antenna similar in length, short, conical to digitiform (Figure 2B, 3B). Eyes lacking (Figure 2B). A pair of palps on ventral side of prostomium, short, conical (Figure 3B, 4A). Mouth opening ventral side of prostomium (Figure 4A). Ventral side of prostomial median region slightly depressed (Figure 4A). Palps similar in length with paired lateral antennae (Figure 3B, 4A). Nuchal organ ciliated, well developed in posterior ridge of prostomium (Figure 3B, 3D), forming an incomplete ring, opening both dorsally and ventrally (Figure 3B, 4B). Peristomium indistinctly separated from prostomium (Figure 2B, 3B).
Tentacular segment (segment 1) antero-posteriorly shorter than the other segments, achaetous, with a pair of short ovoid cirri, followed by uniramous chaetigers 1–3 (segments 2–4) bearing neuropodial lobes with dorsal and ventral cirri (Figure 3E). Subsequent parapodia biramous; notopodial lobes slightly shorter than neuropodial lobes (Figure 4B). Notopodial lobes conical, with ovoid dorsal cirrus (Figure 3F). Notopodial cirri 50 μm in length, with reddish pigment spots forming duct-like structure (Figure 2E, 3F). Capillary notochaetae diagonally striated, sub-distally expanded and marginally serrated (Figure 3H, 5A–C). Neuropodial lobe with ovoid ventral cirrus (Figure 2F, 3G, 4B). Neuropodial cirri 60–80 μm in length, with reddish pigment spots forming duct-like structure (Figure 2F, 3G). Neurochaetae compound spinigers, diagonally striated; blades slightly different in length and distally more conspicuous (Figure 3I, 5D–G). Pygidium with ventral cirri between pair of lateral cirri; equal in length (Figure 2D, 3J).
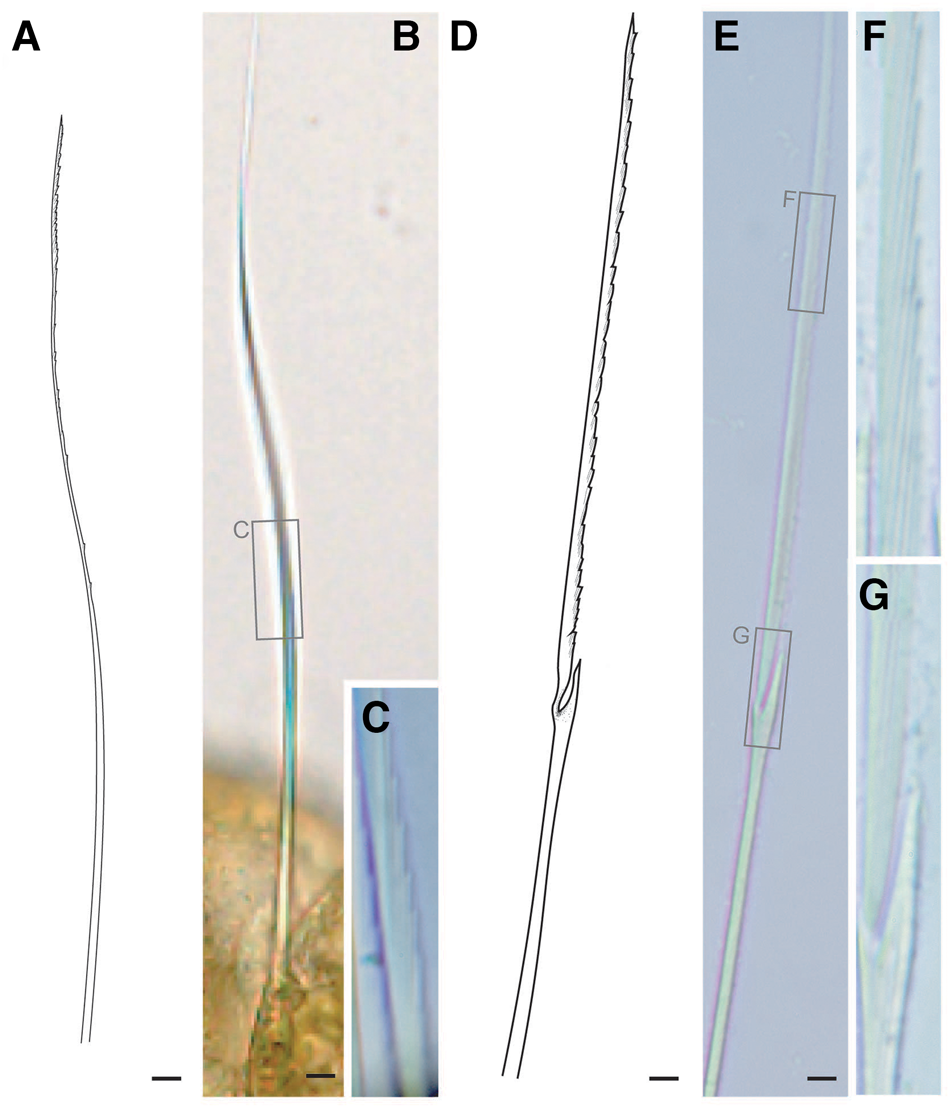
Fig. 5. Lacydonia shohoensis sp. nov., holotype (NSMT-Pol H-844), illustration (A, D) and LM (B, C, E–G). (A–C) Notochaetae; (D–G) neurochaetae. Scale bars: 5 μm.
Distribution and habitat
The species is only known from the type locality, Shoho Seamount of the Nishi-Shichito Ridge, collected from a piece of sunken wood riddled by wood-boring bivalves on sandy mud (Figure 1C).
Ecological notes
The sunken wood was discovered on a gentle slope of Shoho Seamount at a depth of 2042 m (Figure 1B, C). Based on CTD data at the collection site measured with SBE 49 FastCAT CTD Sensor (Sea-Bird Scientific, USA) installed on ‘KM-ROV’, the water temperature and salinity were 2.0°C and 34.6‰, respectively. The wood was degraded by wood-boring bivalves and also colonized by amphipods, munidopsids, asteroids, echinoids and holothroids. Several munidopsids were also associated with a bamboo fragment sunken near the wood block. Members in Ophiuroidea and Holothuroidea also occurred on the bottom sediments around the sunken wood.
Etymology
The new specific name is derived from the type locality, Shoho Seamount of the Nishi-Shichito Ridge in the North-western Pacific Ocean.
Genetic distances and phylogenetic analyses
Lacydonia shohoensis sp. nov. is closest to L. japonica Hookabe, Jimi, Tsuchida, Fujiwara, and Kajihara, Reference Hookabe, Jimi, Tsuchida, Fujiwara and Kajihara2020 in terms of 548 bp of COI sequences; it was 18.4% in p-distance and 21.0% in K2P (Table 2). In the resulting tree, L. shohoensis was a sister taxon to L. laureci Laubier, Reference Laubier1975 sensu Böggemann (Reference Böggemann2009) with 61% of bootstrap value (Figure 6).

Fig. 6. A phylogenetic tree of the genus Lacydonia reconstructed with a maximum likelihood analysis based on concatenated partial sequences of COI, 16S rRNA, 18S rRNA and 28S rRNA genes. The numbers near nodes indicate bootstrap support values generated by maximum likelihood analysis with 1000 replicates. Eulalia viridis, Paranaitis wahlbergi and Phyllodoce longipes were used for outgroup taxa. Solid circles indicate 100% of bootstrap values.
Table 2. Interspecific genetic distance (%) among Lacydonia based on 548 bp of COI sequences. Uncorrected p-distance is shown as values below diagonal, and K2P above diagonal

Discussion
Lacydonia shohoensis sp. nov. is morphologically similar to L. anapaulae Rizzo et al., 2016, L. gordia Hartmann-Schröder, Reference Hartmann-Schröder1993, L. laureci Laubier, Reference Laubier1975, L. laureci sensu Böggemann (Reference Böggemann2009) and L. laureci sensu Rizzo et al. (Reference Rizzo, Magalhães and Santos2016) in the absence of eyes and prostomial width being more than twice its length (Table 3). Among the six species listed in Table 3, L. shohoensis most resembles L. anapaulae in having a depression on the median anterior region and lacking lateral lobes on the posterior margin of prostomium. Morphological differences between the two species can be found in their body colouration – in L. shohoensis the pygidium dorsally pigmented with three reddish spots and the pygidial lateral cirri without pigmentation – although we cannot eliminate the possibility that those features in body pigmentation may have been lost during sample fixation and/or preservation.
Table 3. Comparisons among eyeless species of Lacydonia with prostomium more than twice wider than length

Lacydonia shohoensis sp. nov. was included in the L. laureci sensu Böggemann (Reference Böggemann2009)–L. eliasoni clade in the resulting phylogenetic tree (Figure 6). Lacydonia laureci was originally described from the Eastern Mediterranean Sea by Laubier (Reference Laubier1975), and later reported from the South-eastern (Böggemann, Reference Böggemann2009) and South-western Atlantic Ocean (Rizzo et al., Reference Rizzo, Magalhães and Santos2016), and the Western Mediterranean Sea (Langeneck et al., Reference Langeneck, Busoni, Aliani, Lardicci and Castelli2019) although the species identities remain uncertain because there is a discrepancy between the morphological descriptions. In the original description of L. laureci, prostomium is generally spherical in shape (Laubier, Reference Laubier1975), which could be interpreted as ~1:1 width-to-length ratio of the prostomium, whereas the prostomial width is longer than the length in the illustration of L. laureci sensu Böggemann (Reference Böggemann2009). Furthermore, Rizzo et al. (Reference Rizzo, Magalhães and Santos2016) reported the prostomium as twice as the prostomial length based on the Brazilian specimen. The state of median depression in anterior region of prostomium is also different between the specimens from the three localities; it is present in the Mediterranean (Laubier, Reference Laubier1975) and the South-eastern Atlantic (Böggemann, Reference Böggemann2009) specimens, whereas absent in L. laureci sensu Rizzo et al. (Reference Rizzo, Magalhães and Santos2016). Future taxonomic reappraisals are expected to elucidate species identities of the specimens assigned to L. laureci using DNA barcoding, and then examine if morphological differences in soft parts truly reflect species distinction or an artefact during fixation.
From the North-western Pacific, L. papillata Uschakov, Reference Uschakov1958 (Uschakov, Reference Uschakov1972), L. japonica and Lacydonia sp. Tosa (Hookabe et al., Reference Hookabe, Jimi, Tsuchida, Fujiwara and Kajihara2020) have been reported as eye-less species of Lacydonia. Lacydonia papillata differs from our new species in the lack of depression on anterior tip of prostomium and the presence of four large pigment spots behind chaetiger 1. Lacydonia shohoensis is also distinguished from L. japonica by the depression on the anterior tip of prostomium. As for Lacydonia sp. Tosa, we could not compare the morphology since the specimen was heavily damaged during sledging. The genetic distance based on COI between L. shoehoensis and Lacydonia sp. Tosa was 21.3% in p-distance and 25.0% in K2P; these values are comparable with interspecific genetic distances observed among Phyllodocidae (Nygren & Pleijel, Reference Nygren and Pleijel2011), inferring that the two would not be the same species. Apart from the eye-less lacydoniids, a single unidentified species has been reported by Rouse & Pleijel (Reference Rouse and Pleijel2001). The species apparently differs from our new species as it possesses a pair of large eye spots and rather resembles the type species of the genus, L. miranda Marion, 1874.
In general, members in Lacydonia have been found from bottom sediments collected with van Veen grabs and box corer grabs (e.g. Böggemann, Reference Böggemann2009; Magalhães et al., Reference Magalhães, Bailey-Brock and Rizzo2012; Rizzo et al., Reference Rizzo, Magalhães and Santos2016; Mazurkiewicz et al., Reference Mazurkiewicz, Gromisz, Legeżyńska and Włodarska-Kowalczuk2017), with the one exception of L. hampsoni Blake, Reference Blake, Blake and Hilbig1994 reported from experimentally deployed wood parcels (Judge & Barry, Reference Judge and Barry2016). During our faunal survey, sediments around the sunken wood were collected by the use of a suction sampler, agitated in seawater as well as fresh water, and sieved with a 64-μm mesh net to extract meio- and macrofaunal species live on or a few centimetres below the sediment surface; nevertheless, we could not find any lacydonid species from the nearby sediments. This may suggest colonization preference of L. shohoensis for wood-fall habitats rather than bottom sediments, which might result from the surface complexities and/or availability of food sources on sunken wood degraded by wood-boring bivalves.
Acknowledgements
We thank the captain and crew of the RV ‘Kaimei’, the ‘KM-ROV’ operation teams and all the research members participated in KM20-10C cruise of the R/V ‘Kaimei’ organized under the research project ‘Development of Biodiversity Monitoring Methods for the Management of Deep-sea Marine Protected Areas’. We also thank Dr Toru Miura and members in Misaki Marine Biological Station (the University of Tokyo) for providing facilities for morphological observation and molecular works. NH is grateful to Mr Tomohiro Hatano (JEOL, Ltd) and the other staff in JEOL for kindly supporting our morphological observation with a SEM.
Financial support
This study was supported by the Environment Research and Technology Development Fund (JPMEERF20S20700) of the Environmental Restoration and Conservation Agency of Japan.