Introduction
Jellyfishes are prominent invertebrates, and they serve immense socio-economic and ecological roles in shaping the oceans and human well-being (Pitt and Purcell, Reference Pitt and Purcell2009; Doyle et al., Reference Doyle, Hays, Harrod and Houghton2014). Many jellyfish species form spectacular blooms, which can influence biogeochemical cycling, including the carbon cycle (Doyle et al., Reference Doyle, Hays, Harrod and Houghton2014). Because jellyfish aggregations contain organic matter, they also can regulate climate through carbon sequestration, a factor in controlling atmospheric temperature (Doyle et al., Reference Doyle, Hays, Harrod and Houghton2014; Wright et al., Reference Wright, Le Quéré, Buitenhuis, Pitois and Gibbons2021). Jellyfish are also important to people through a variety of seemingly intricate interactions between jellyfish and humans. Specifically, humans are fascinated with jellyfish because they evoke a positive aesthetic appreciation while at the same time discomfort because they inflict stings (Pitt and Purcell, Reference Pitt and Purcell2009; Doyle et al., Reference Doyle, Hays, Harrod and Houghton2014) with microscopic capsular organelles equipped with harpoons called nematocysts (Arai, Reference Arai1997; Pitt and Purcell, Reference Pitt and Purcell2009; Jarms and Morandini, Reference Jarms and Morandini2019). At the same time, several scyphozoan jellyfish species (phylum Cnidaria; class Scyphozoa) are edible and support significant fisheries, demonstrating their economic importance (Pitt and Purcell, Reference Pitt and Purcell2009; Brotz, Reference Brotz, Pauly and Zeller2016; Brotz et al., Reference Brotz, Schiariti, López-Martínez, Álvarez-Tello, Hsieh, Jones, Quiñones, Dong, Morandini, Preciado, Laaz and Mianzan2017). Despite recognizing their ecological and societal value, our understanding of the taxonomic identity of many jellyfish groups, such as scyphozoans, remains limited (Lawley et al., Reference Lawley, Gamero-Mora, Maronna, Chiaverano, Stampar, Hopcroft and Morandini2021; Gamero-Mora et al., Reference Gamero-Mora, Collins, Boco, Geson, Morandini and Wilson2022; Santander et al., Reference Santander, Maronna, Ryan and Andrade2022). This gap in knowledge is particularly concerning in light of current environmental stressors like climate change, ocean warming, and aquatic pollution (Joppa et al., Reference Joppa, O'Connor, Visconti, Smith, Geldmann, Hoffmann, Watson, Butchart, Virah-Sawmy, Halpern, Ahmed, Balmford, Sutherland, Harfoot, Hilton-Taylor, Foden, Di Minin, Pagad, Genovesi, Hutton and Burgess2016; Girardello et al., Reference Girardello, Martellos, Pardo and Bertolino2018). As these environmental stressors intensify, clarifying the taxonomy of jellyfish has become extremely critical (as in Girardello et al., Reference Girardello, Martellos, Pardo and Bertolino2018; Thomson et al., Reference Thomson, Pyle, Ahyong, Alonso-Zarazaga, Ammirati, Araya and Segers2018; Sandall et al., Reference Sandall, Maureaud, Guralnick, McGeoch, Sica, Rogan, Booher, Edwards, Franz, Ingenloff, Lucas, Marsh, McGowan, Pinkert, Ranipeta, Uetz, Wieczorek and Jetz2023).
One group of particularly prominent scyphozoans is the blubber jellyfishes (genus Catostylus) of the order Rhizostomeae with hemispherical bells shaped by thick mesoglea and oral arms with lace-like surfaces (Mayer, Reference Mayer1910; Arai, Reference Arai1997; Jarms and Morandini, Reference Jarms and Morandini2019). Like other rhizostomes, blubber jellyfishes have a bell with no prominent mouth on the underside, but rather microscopic openings on each gelatinous arm, through which food is passed to the stomach (e.g. Catostylus spp.; see Figure 1A–G; Dawson, Reference Dawson2005a; Boco et al., Reference Boco, Metillo and Papa2014). They also have nematocysts on their oral arms that enable capture of prey such as zooplankton (Peach and Pitt, Reference Peach and Pitt2005; Dawson, Reference Dawson2005a; Boco et al., Reference Boco, Metillo and Papa2014). Catostylus mosaicus (Quoy & Gaimard, 1824), a well-known blubber jellyfish, occurs along the Australian eastern and southern coasts, where it forms large swarms of medusae in estuaries (Dawson, Reference Dawson2005a; Figure 1A). The colour of the medusae may vary depending on the location of this jellyfish. In Sydney's (Australia) waters, they are usually white or brown, while they are mostly blue in northern Australia (Pitt and Kingsford, Reference Pitt and Kingsford2000; Dawson, Reference Dawson2005a). Their medusae also have a mild sting that inflicts pain but does not pose serious risk to humans (Williamson et al., Reference Williamson, Fenner, Burnett and Rifkin1996). Despite their capacity to inflict mild envenomation in humans, blubber jellyfishes remain a crucial component of marine biodiversity (Boco et al., Reference Boco, Metillo and Papa2014; Browne et al., Reference Browne, Pitt and Norman2017; Gueroun et al., Reference Gueroun, Torres, Dos Santos, Vasco-Rodrigues, Canning-Clode and Andrade2021). Thus, further research into their systematics is vital.
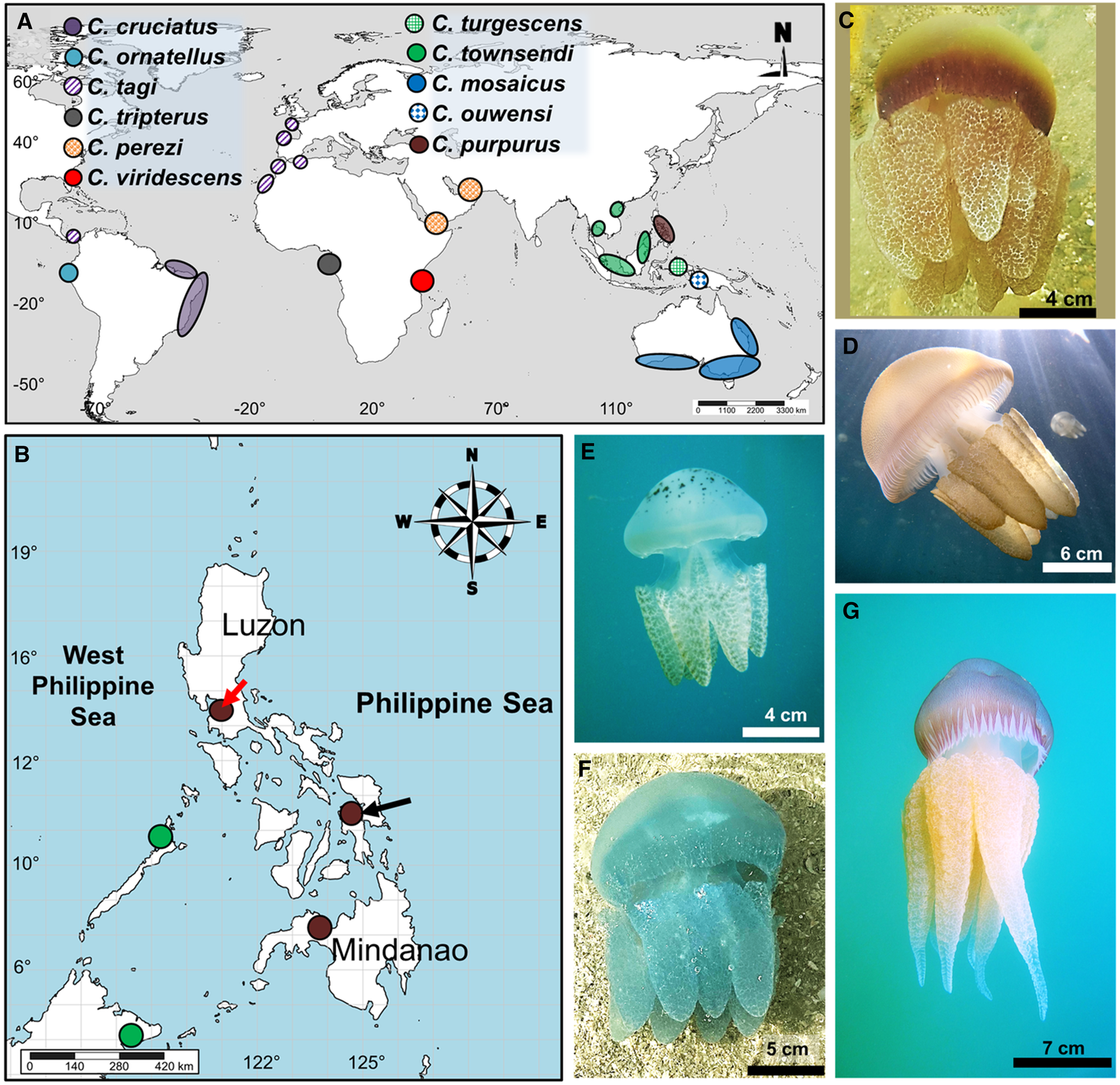
Figure 1. Distribution and images of Catostylus spp.: (A) global distribution of species of Catostylus (see colour and pattern legends), (B) localities of Catostylus spp. in the Philippines (green dot = C. townsendi; brown = C. purpurus; red arrow = type locality; black arrow = new specimen collections), (C) medusa of C. purpurus from central Philippines, (D) C. mosaicus from Manly, northern Sydney, Australia, (E) C. townsendi from Narra town, Palawan, Philippines, (F) C. perezi from Ras Al-Khaimah, United Arab Emirates, and (G) C. tagi from Cascais coast in Lisbon, Portugal. Photos courtesy of J. Turnbull (D), A. Galindez (E), M. Gardner (F), and C. Fletcher (G).
Blubber jellyfishes, such as C. mosaicus (Quoy & Gaimard, 1824) and Catostylus tagi (Haeckel, 1869), play crucial roles in marine ecological processes, including nutrient cycling and transport (West et al., Reference West, Pitt, Welsh, Koop and Rissik2009; Wright et al., Reference Wright, Le Quéré, Buitenhuis, Pitois and Gibbons2021). Although their exact contributions to transfer of nutrients in the marine food web are yet to be quantified, they serve as a food source for various species, notably turtles (Limpus et al., Reference Limpus, Parmenter and Chaloupka2013). For instance, the leatherback turtle, Dermochelys coriacea and the green sea turtle, Chelonia mydas feed on C. mosaicus medusae in Australia (Limpus et al., Reference Limpus, Parmenter and Chaloupka2013; Bjorndal, Reference Bjorndal1997). This highlights the ecological significance of these cnidarians in the context of nutrient cycling and as a critical link in the food chain. Further, the importance of blubber jellyfish extends beyond ecological roles because they are a valuable commodity in coastal societies such as those in Australia and Portugal (Edelist et al., Reference Edelist, Angel, Canning-Clode, Gueroun, Aberle, Javidpour and Andrade2021). For example, in Tagus, Portugal, C. tagi is harvested for food, pharmaceutical purposes, and animal feed during the jellyfish fishing season in October (Edelist et al., Reference Edelist, Angel, Canning-Clode, Gueroun, Aberle, Javidpour and Andrade2021). These multifaceted benefits to ecosystems and societies underscore the importance of understanding the fundamental biology of these jellyfishes, including their taxonomic identity.
Jellyfishes of the family Catostylidae occur in the Philippines, a region of the Coral Triangle with high marine biodiversity richness (Boco and Metillo, Reference Boco and Metillo2018; Pinheiro et al., Reference Pinheiro, Shepherd, Castillo, Abesamis, Copus, Pyle, Greene, Coleman, Whitton, Thillainath, Bucol, Birt, Catania, Bell and Rocha2019; Gamero-Mora et al., Reference Gamero-Mora, Collins, Boco, Geson, Morandini and Wilson2022; Figure 1A, B). Scientific literature indicates occurrence of C. mosaicus in the region (Light, Reference Light1921; Omori and Nakano, Reference Omori and Nakano2001; Jarms and Morandini, Reference Jarms and Morandini2019) going back to the report of one medusa, collected from southwestern Philippines in 1908, i.e. in Malampaya River, Palawan (Mayer, Reference Mayer1915). The jellyfish was speculated to be an immature individual of C. mosaicus. However, our extensive surveys for jellyfish in the Philippines since 2013, combined with social media and verified records of jellyfish from the citizen-science project, The Philippine Jellyfish Stings, have not revealed medusae of C. mosaicus (whether juvenile or mature) in the territory (Boco et al., Reference Boco, Metillo and Papa2014; Boco and Metillo, Reference Boco and Metillo2018; de Vera-Ruiz, Reference de Vera-Ruiz2022; Boco et al., unpublished). The Philippine Jellyfish Stings citizen-science platform (https://rb.gy/77wnb and https://rb.gy/9wey1) successfully aids in recording occurrences of jellyfish, including blubber jellyfishes, in any part of the region, which led to documentation of occurrences of Catostylus townsendi Mayer, Reference Mayer1915 in southwestern Philippines, i.e. Palawan islands (Sabillo, Reference Sabillo2020; Boco et al., unpublished data; Figure 1B; Table S1, Supplementary materials). Further, the Philippine blubber jellyfish (not C. townsendi) with populations of several morphs such as blue and burgundy is currently being identified as Acromitoides purpurus sensu Stiasny (see Boco and Metillo, Reference Boco and Metillo2018). Prior to Stiasny's erection of the genus Acromitoides in 1921, this species was originally described as Catostylus purpurus Mayer, Reference Mayer1910, and indeed, examinations of fresh medusae (without preservation) reveal that the jellyfish fits into Catostylus (Boco and Metillo, Reference Boco and Metillo2018). The medusae have gastrovascular canal systems showing an anastomosing network, which connects to both rhopaliar and inter-rhopaliar canals (Boco et al., Reference Boco, Metillo and Papa2014; see Figure 2E in Boco and Metillo, Reference Boco and Metillo2018), unlike the canal system of the genus Acromitoides (Stiasny, Reference Stiasny1921) but in agreement with that of Catostylus. Examination of additional specimens of this Philippine blubber jellyfish including museum-preserved medusae can help assess the taxonomy of this rhizostome jellyfish.
While morphological features of scyphozoans aid in identifying jellyfish species by eye, characters in their genomes also provide insights on their phylogenetic histories, clarifying species boundaries and taxonomic classifications (Gamero-Mora et al., Reference Gamero-Mora, Collins, Boco, Geson, Morandini and Wilson2022; Santander et al., Reference Santander, Maronna, Ryan and Andrade2022). For example, molecular approaches in the identification of jellyfish, such as by using cytochrome oxidase I marker, effectively aided identification of species in the family Catostylidae, e.g. Acromitus sp. (Gómez Daglio and Dawson, Reference Gómez Daglio and Dawson2017). An even more effective approach in jellyfish taxonomy, uses integrative systematics which combines morphological data and genetic information in identifying scyphozoan jellyfishes, e.g. the upside-down jellyfish Cassiopea spp. (Gamero-Mora et al., Reference Gamero-Mora, Collins, Boco, Geson, Morandini and Wilson2022; Santander et al., Reference Santander, Maronna, Ryan and Andrade2022). Here, we aimed to describe and clarify the taxonomy of a blubber jellyfish in an Indo-Pacific region using a combination of morphological and genetic information. We hypothesized that the blubber jellyfish with populations of different colour morphs in the Philippines does not belong to genus Acromitoides, but instead should be classified among species of the genus Catostylus.
Materials and methods
Type specimens and new collections
Specimens labelled A. purpurus and C. purpurus were examined in the Museum of Comparative Zoology (MCZ) – Harvard University (the USA; n = 1 medusa) and Naturalis Biodiversity Centre (RMNH; n = 2). See also Table 1. Medusae (n = 16) were obtained from various coastal waters in the Philippines during April and July, from 2014 to 2016 and 2022 (see Table 1). They were collected by hand and carefully placed in a plastic bucket filled with filtered seawater to prevent damage to the specimens. In addition, in-situ observations (obs.), photographs, and examination of morphology of live specimens of jellyfish in Manila Bay (Cavite province; June 2016) and Visayan Sea, i.e. Bantayan Island of Cebu province (August 2023) in the Philippines were made (Table 1).
Table 1. Records of preserved specimens and in-situ observations (obs.) of medusae of C. purpurus
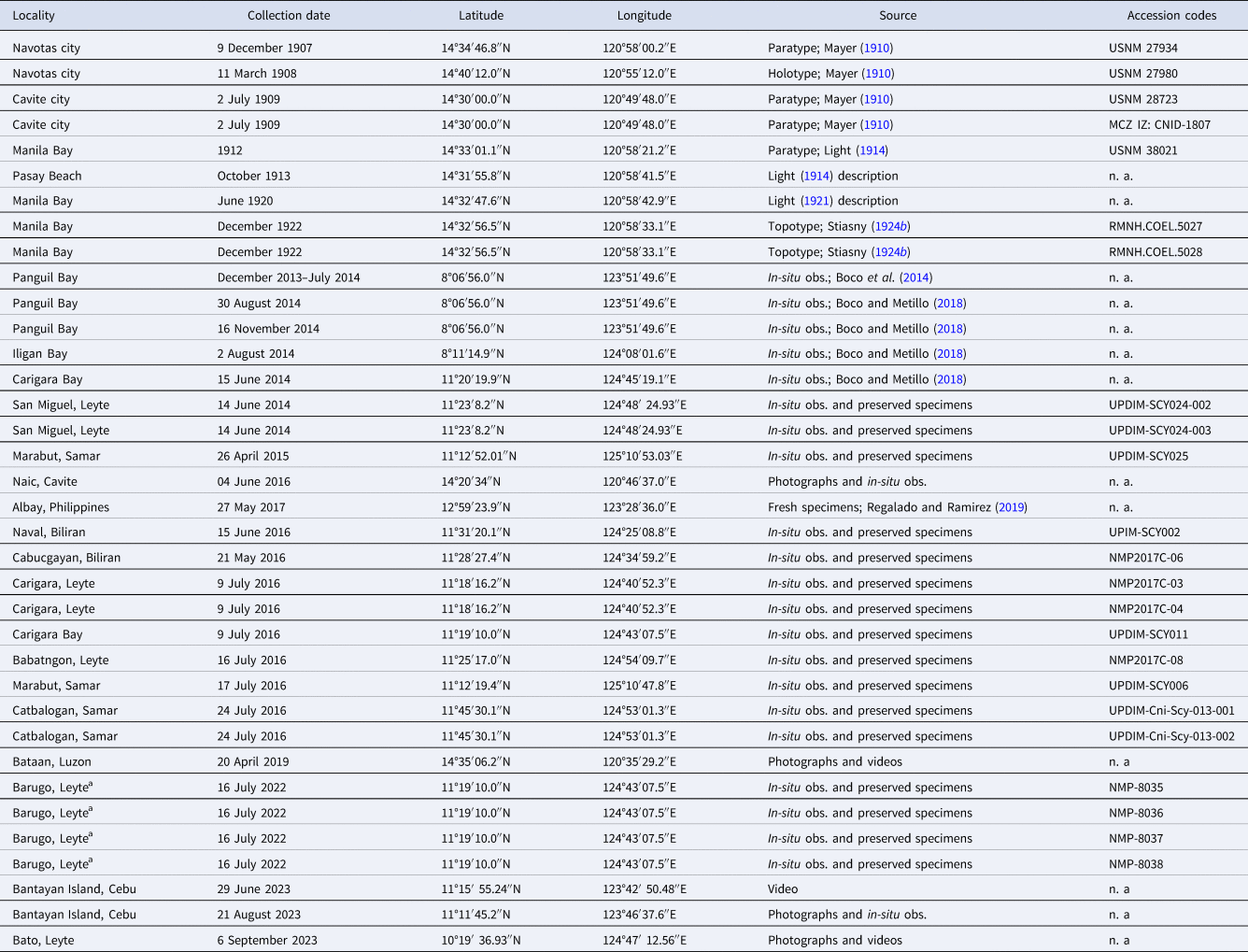
n. a., not applicable; USNM, Smithsonian National Museum of Natural History, USA.
a Tissue was collected for molecular analyses.
Morphological examination
The following morphological features of the medusae (newly collected and type specimens, Table 1) were examined: bell shape, colour, and texture; absence or presence of colour spots or bands on bell; form and texture of marginal lappets including rhopaliar lappets; ostium shape and width; presence or absence and form of subgenital papillae; form, colour and texture of oral arms; absence or presence of appendages connected to the bell or oral arms; and form or shape and position of rhopalia (as in Dawson, Reference Dawson2005a and Boco et al., Reference Boco, Metillo and Papa2014). Sizes of body features like oral arm length and ostium width of the medusae were measured using a metal caliper with 0.1 mm accuracy. Here, the gastrovascular canal system of the medusae, freshly collected, were examined by staining them using a carotene-based pigment, i.e. food dye, and by describing the morphology of the stained canal system, following Boco et al. (Reference Boco, Metillo and Papa2014) and Stiasny (Reference Stiasny1924b). The arrangement of the canal system aids identification of the genus (Stiasny, Reference Stiasny1924b; Thiel, Reference Thiel1978). Voucher specimens were deposited at the National Museum of the Philippines (NMP) and Invertebrate Biology Museum of the University of the Philippines Diliman (UP-DIM).
The jellyfish specimens were identified using the descriptions and images of medusae in Schultze (Reference Schultze1897), Mayer (Reference Mayer1910), Light (Reference Light1914, Reference Light1921), Stiasny (Reference Stiasny1920, Reference Stiasny1921, Reference Stiasny1924b, Reference Stiasny1925, Reference Stiasny1929), Kramp (Reference Kramp1961), Heeger (Reference Heeger1998), Dawson (Reference Dawson2005a, Reference Dawson2005b), Gul and Morandini (Reference Gul and Morandini2013), Boco et al. (Reference Boco, Metillo and Papa2014), Boco and Metillo (Reference Boco and Metillo2018), and Jarms and Morandini (Reference Jarms and Morandini2019). Newly collected medusae (from 2014 to 2022) were identified and compared to other in-situ observations and images of jellyfish for differential diagnosis. The jellyfish were compared to a confirmed record of Crambione mastigophora Maas, 1903 from a bloom in April 2020 in Palawan, southwestern Philippines (Figure S1, Supplementary materials); Crambionella orsini Vanhöffen, 1888 in Wasin, Kenya (https://www.inaturalist.org/observations/39918901; Wasini observations, n = 2); C. mosaicus in Sydney, Australia (https://tinyurl.com/4yhjfnta; J. Turnbull observations, n = 2); C. townsendi observed in June 2018 (A. Galindez observations, n = 4 photo, n = 2 video) and 1 April 2023 (A. Schilde observations, n = 2 photos), both in Palawan, Philippines (Table S1, Supplementary materials); Catostylus perezi Ranson, 1945 of United Arab Emirates (https://www.inaturalist.org/observations/52027645; M. Gardner observations, n = 2), and C. tagi in Portugal (https://www.inaturalist.org/photos/24990680; C. Fletcher observations, n = 1).
In 2015, the citizen-science project with a social media platform, The Philippine Jellyfish Stings (https://rb.gy/77wnb; https://rb.gy/9wey1), was established to precisely locate records of jellyfish in the region. Observations including photos and videos of blubber jellyfish published in the page ‘Philippine Jellyfish Stings’ since 2015–2023 were manually searched, downloaded, and recorded from the social media platform. Contributors of the observations with photographs or videos of jellyfishes in the project were contacted when needed to obtain details related to these jellyfish observations such as GPS coordinates or nearest village and landmarks where the jellyfish was recorded (which provided a maximum of 30 m2 resolution of the location of the jellyfish), date and time of observations, and estimated sizes (e.g. bell diameter) of the jellyfish.
Occurrences of the blubber jellyfish medusae and their blooms among several biogeographic regions (i.e. ecoregions) in the Philippines were recorded using in-situ observations and citizen-science observations, described above. Locations and names of these ecoregions, e.g. Visayan region, were based on Nañola et al. (Reference Nañola, Aliño and Carpenter2011). Further, citizen-science photographs and videos of blooms of Catostylus sp. in Bataan, Luzon (20 April 2019; northern Philippines) and Canigao Channel in town of Bato, Leyte in central Philippines (6 September 2023; Table 1) were obtained and, then, compared to our in-situ records of blooms of this blubber jellyfish to determine whether the blooms recorded using citizen science belong to the species we observed in situ. We compared these citizen-science records of blooms to the appearance of the blooms of Catostylus sp. near Cavite (Manila Bay), off Barugo and Carigara towns (Carigara Bay), Bantayan Island, Cebu (Visayan Sea) (see coordinates in Table 1); blooms of C. townsendi at Palawan, Philippines (A. Galindez observations in June 2018; P. Dimalaluan observations in February 2019; A. Schilde observations in April 2023) and those in Trat province, Thailand (Marine and Coastal Resources Research and Development Centre; Table S1, Supplementary materials); and a bloom of C. mosaicus near Sydney, Australia (J. Turnbull observations).
Interviews (n = 43) with prior informed consent were conducted in Biliran, Leyte, and Cebu provinces to record local names of the jellyfish in several Philippine languages like Waray and Cebuano. Names of the medusae were verified using orthographic sources by Rubino (Reference Rubino2004), Polistico (Reference Polistico2017), and Corpora Project's Waray dictionary (https://corporaproject.org; see Oyzon and Fullmer, Reference Oyzon and Fullmer2014). Furthermore, the etymology of this jellyfish in this study was summarized using the references of Mayer (Reference Mayer1910), Yang et al. (Reference Yang, Zeng and Wang2018), and Bizuneh (Reference Bizuneh2021).
Reported symbionts of the jellyfish A. purpurus were identified using taxonomic references of Hartog (Reference Hartog1888), Wilson (Reference Wilson1911), Boco et al. (Reference Boco, Metillo and Papa2014), and Boco and Metillo (Reference Boco and Metillo2018). We also adapted an identification key for the species of Catostylus by referring to the taxonomic guides of Mayer (Reference Mayer1910), Stiasny (Reference Stiasny1921), Kramp (Reference Kramp1961), Kitamura and Omori (Reference Kitamura and Omori2010), Boco et al. (Reference Boco, Metillo and Papa2014), and Jarms and Morandini (Reference Jarms and Morandini2019, Reference Jarms and Morandini2023).
Molecular analyses
Pieces of tissue, each measuring 1 cm2, were collected from the marginal lappets of each of the four specimens from Carigara Bay (near Barugo town, Leyte Island) and preserved in 99% ethanol in July 2022 (museum accession codes: NMP-8035 to 8038, Table 1). After tissue collection, the medusae were morphologically examined while fresh. They were then preserved in 4% formaldehyde solution in seawater and deposited in Philippine museums (accession codes listed in Table 1). Tissues were collected from several colour morphs (one burgundy, two blue and one white) to account for possible mild genetic variation among morphs. Genomic DNA of the jellyfish was extracted using the protocol which substitutes sodium chloride and absolute ethanol for ammonium acetate and isopropyl alcohol (Miller et al., Reference Miller, Dykes and Polesky1988; Nishiguchi et al., Reference Nishiguchi, Doukakis, Egan, Kizirian, Phillips, Prendini, Rosenbaum, Torres, Wyner, DeSalle, Giribet, DeSalle, Giribet and Wheeler2002). Two genetic markers from the mitochondrial genome, namely cytochrome c oxidase subunit I (cox1) and 16S ribosomal DNA (16S), were amplified using the primers med-cox1-F (5′-ACNAAYCAYAAAGATATHGG-3′) and med-cox1-R (5′-TGGTGNGCYCANACNATRAANCC-3′) for cox1, and med-rnl-F (5′-GACTGTTTACCAAAGACATAGC-3′) and med-rnl-R (5′-AAGATAGAAACCTTCCTGTC-3′) for 16S (Lawley et al., Reference Lawley, Ames, Bentlage, Yanagihara, Goodwill, Kayal, Hurwitz and Collins2016). Each polymerase chain reaction (PCR) sample was carried out with GoTaq® DNA Polymerase (Promega) in a total volume of 25 μl, containing 50 ng of DNA template and following the manufacturer's instructions. Both genes had the same thermocycling conditions, including an initial denaturation step at 95°C for 5 min, followed by 35 cycles of 95°C for 30 s, 51°C for 30 s, and 72°C for 1 min, and a final extension step at 72°C for 7 min, followed by holding at 4°C.
PCR success was confirmed by running the samples on a 2% agarose gel, and the samples were purified using Agencourt AMPure XP (Beckman Coulter). We used the BigDye™ Terminator v3.1 Cycle Sequencing Kit (ThermoFisher Scientific) to prepare the sequencing samples, with 1 μl of BigDye reaction mix for each. The samples were sequenced in a 3730 ThermoFisher sequencing machine at Gate Lab (https://gatelab.ib.usp.br/), and chromatograms were assembled using CodonCode Aligner software (CodonCode Corporation, Dedham, MA). The newly generated sequences were deposited in GenBank and their accession codes are PP786537–PP786540 for cox1 and PP789084–PP789087 for 16S.
Sequenced chromatograms were aligned using CLUSTAL W. Subsequently, phylogenetic trees for cox1 and 16S were constructed using maximum-likelihood under bootstrap method (as in Gómez Daglio and Dawson, Reference Gómez Daglio and Dawson2017) in the Molecular Evolutionary Genetics Analysis 7 (MEGA7) program. The tree was drawn to scale, with branch lengths measured in the number of substitutions per site. These phylogenetic analyses included eight nucleotide sequences for each marker (cox1; 16S) such as those from four Philippine Catostylidae medusae and sequences from GenBank for cox1, i.e. C. mosaicus (AY737247), C. townsendi (MN395694), Acromitus flagellatus (MN395692), Mastigias papua (MN107722), and for 16S, i.e. C. mosaicus (KY610585), C. townsendi (KY610587), A. flagellatus (KY610576), and M. papua (KY610621). Sequences of M. papua and A. flagellatus were used as outgroups. Then, uncorrected pairwise p-distances were obtained using the program Geneious. Sequences from these eight samples were further examined by analysing phylogenetic relationships using the plugin FastTree (Price et al., Reference Price, Dehal and Arkin2010) in Geneious Prime 2024 with all available cox1 and 16S sequences of Catostylus in GenBank (see accession codes in Figures S3 and S4, Supplementary materials). Percent identity of our Catostylus sp. sequences were calculated using NCBI's Basic Local Alignment Search Tool (BLAST) which revealed the jellyfish belongs to family Catostylidae as in Gómez Daglio and Dawson's (Reference Gómez Daglio and Dawson2017) method.
Results
SYSTEMATICS
Class SCYPHOZOA Goette, 1887
Subclass DISCOMEDUSAE Haeckel, Reference Haeckel1880
Order RHIZOSTOMEAE Cuvier, 1800
Family CATOSTYLIDAE Claus, 1883
Genus Catostylus Agassiz, 1862
Catostylus purpurus Mayer, Reference Mayer1910
(Figures 1C; 2A–C; F, G; 3A–C; E–I)
Catostylus purpurus: Mayer (Reference Mayer1910): 671–672, original description; Light (Reference Light1914): 207–209, description; Mayer (Reference Mayer1915): 187–188, description; Mayer (Reference Mayer1917): 213–214, description; Light (Reference Light1921): 41–42, description; Totton (Reference Totton and Sclater1921): 9, mention; Chiu (Reference Chiu1954): 56, mention; Southcott (Reference Southcott, Keegan and MacFarlane1963): 57, sting record; Cleland and Southcott (Reference Cleland and Southcott1965): 107, 158, sting potential; Williamson et al. (Reference Williamson, Fenner, Burnett and Rifkin1996): 213, sting; Heeger (Reference Heeger1998): 314–315, brief description.

Figure 2. Medusae of C. purpurus and morphological features of other species: (A) exumbrella of the topotype (museum accession code, a.c.: RMNH.COEL.5027, Table 1) collected from Manila Bay. Blue arrow = lappet; (B) blue; and (C) burgundy colour morphs of C. purpurus from Babatngon, Leyte (yellow broken line emphasize cruciform-shaped stomach and gonads); (D) Crambione mastigophora from El Nido in Palawan, Philippines; (E) Crambionella orsini from Wasin, Kenya (Wasini Guide observations). Canal systems of a topotype in Naturalis (F; a.c.: RMNH.COEL.5027) and paratype in the MCZ (G; a.c.: MCZ IZ: CNID-1807) with coarse anastomoses = yellow dot; anastomosing canal connections with rhopalar canals = yellow arrows; actual ridges (not canals) of subumbrellar musculature due to slight specimen contraction = red arrows; interrhopalar canal = IRC; rhopalar canal = RC; rhopalium = RHO); gastrovascular canals in the burgundy (H) and blue (I) morphs of C. purpurus (blue and red arrows = subgenital papillae); (J) gastrovascular canals of C. townsendi medusa from Stiasny (Reference Stiasny1921). Photos courtesy of Wasini Guide (E) and Museum of Comparative Zoology (G).

Figure 3. Morphology of C. purpurus: (A) C. purpurus medusa collected from Biliran, central Philippines (museum accession code, a.c.: NMP2017C-06, Table 1) with silver-like spot (statocyst) in the rhopalium (red arrow); (B) section of bell margin of the topotype (RMNH.COEL.5027) with microscopic spots on the rhopalium (yellow arrow) and lappets (red arrow); (C) marginal lappets of burgundy C. purpurus from Babatngon, Leyte (a.c.: NMP2017C-08; yellow arrow = rhopalium; red broken line emphasizes shape of the lappet tip); (D) umbrella margin and lappets (arrows) of C. perezi medusa; (E) rhopalium with lappets (red arrow) of C. purpurus (NMP2017C-08); (F) subgenital ostium (yellow arrows = edges) and oral arms (G) of a C. purpurus topotype specimen (RMNH.COEL.5027; red broken lines = convex-shaped ‘top’ end of oral arms on the subumbrella); (H) oral arm of a living C. purpurus specimen (a.c.: NMP2017C-08); (I) subumbrellar view of a burgundy colour morph, with one small oral arm (blue arrow), from Catbalogan, Samar (a.c.: UPDIM-Cni-Scy-013-001).
Acromitoides purpureus: Stiasny (Reference Stiasny1921): 137–138, brief description, genus change; Stiasny (Reference Stiasny1924b): 39–45, description; Stiasny (Reference Stiasny1926): 246, mention.
Acromitus purpurus: Thiel (Reference Thiel1978): 286, mention [misspelling of genus].
Acromitoides purpurus: Kramp (Reference Kramp1961: 368, diagnosis); Kramp (Reference Kramp1965: 271, brief description); Cleland and Southcott (Reference Cleland and Southcott1965): 261, mention; Raupp et al., (Reference Raupp, Milde, Goerz, Plewig, Burnett and Heeger1996): 49, Table 1, sting potential; Williamson et al. (Reference Williamson, Fenner, Burnett and Rifkin1996): 214, mention; Kitamura and Omori (Reference Kitamura and Omori2010): 114, mention; Boco and Metillo (Reference Boco and Metillo2018): 71, 74, 76, associations; Muffett and Miglietta (Reference Muffett and Miglietta2021): 4, mention; Jarms and Morandini (Reference Jarms and Morandini2019, Reference Jarms and Morandini2023): 554–555, description; Santhanam (Reference Santhanam2020): 81, 294, brief description, venomosity; Hwai et al. (Reference Hwai, Kwang and Miyake2021): 67–68, brief description.
Catostylus sp. – Boco et al. (Reference Boco, Metillo and Papa2014): 63–77, abundance and associations; Regalado and Ramirez (Reference Regalado and Ramirez2019): 531, 534, association with marine fungi; Muffett and Miglietta (Reference Muffett and Miglietta2021): 5, mention.
Materials examined
Type specimens
One medusa (paratype) in the MCZ (accession code, a.c.: MCZ IZ: CNID-1807), coast of Cavite city; all are burgundy morphs; two preserved medusae (topotypes) in RMNH (a.c.: RMNH.COEL.5027), unidentified location in Manila Bay, likely Pasay coast. See Table 1 for the dates of collection, and localities of these type specimens.
Additional materials
Sixteen fresh specimens from several localities in the Philippines, which were subsequently preserved in phosphate-buffered 4% formalin: two juveniles and 14 mature medusae, majority are burgundy morphs (2 blue, 1 orange, and 1 white colour morphs were also examined), collectors: C. Capidos, S. R. Boco, and D. Talacay (see Table 1); photographs and in-situ observations, Manila Bay (Cavite province), Philippines, observer: S. R. Boco, 4 June 2016; photographs (n = 2) and a video, observed near Capinpin Port in Orion, Bataan, Philippines, observer: A. Manlangit; video of the medusa with ectosymbiont fish in Orion, Bataan, observer: J. Gervacio, August 2022; fresh medusae (n = 2, not preserved), photographs (n = 4) and in-situ observations of medusae and a bloom by C. Capidos, Bantayan Island in central Philippines (Table 1); photographs of a blue and burgundy blubber jellyfish (n = 5) currently identified as C. mosaicus obtained from an unidentified location in the Philippines, T. Yoshida and J. Bruce observations (https://tinyurl.com/4r59543c); verified observation (n = 1 video) of burgundy blubber jellyfish medusae called ‘black jellyfish’ and their bloom in 29 June 2023 in Bantayan Island of Cebu, myparadiseonbantayanisland9030 observations (https://tinyurl.com/yeyurtjc) (Table 1); a photo and videos (n = 2) of burgundy jellyfish bloom in 6 September 2023, town of Bato in Leyte, Philippines, anonymous personal communication to S. R. Boco (Table 1).
Diagnosis
Catostylidae possessing oral arms lacking terminal clubs and filaments, and intracircular network of anastomosing canals connected with both rhopaliar and interrhopaliar radial canals, differing from the congeners on the absence of any ornamentation on the exumbrella (ridges, granules, striations).
Description
Holotype: bell with uniform dark brownish to purple colouration in life; bell diameter 115 mm, and height 35 mm; oral arms 58 mm long with smooth proximal section: 7 mm in length and, lower distal portion: 51 mm long (A. G. Mayer in 1910). New specimens typical of Catostylus, with bell diameter ranging from 28.9–42 mm (juvenile) to 52.4–125 mm (mature). Bell hemispherical and slightly flattens after preservation in 4% formalin, exposing the oral arms (Figures 1C and 2A–C). Exumbrella smooth (Figure 2A–C; as in Figure 2D, E) and are unlike the medusae of Catostylus ouwensi, C. mosaicus, and C. tagi with striated to coarsely granulated surface and lappets with ridges (Figure 1D, G) and C. townsendi with dark to greenish constellation-like spots (Figure 1E). Cruciform-shaped gonads and stomach are slightly visible from translucent exumbrella (Figure 2B, C). Gastric canal system has eight rhopaliar and eight interrhopaliar canals. Intracircular anastomosing canal network connects to rhopaliar and inter-rhopaliar canals, typical of Catostylus (Figure 2F–I). Intracircular anastomoses are coarse compared to fine-meshed marginal anastomoses of the canal system (Figure 2F–I), but unlike the canal system of C. townsendi with slightly coarser intracircular anastomoses (Figure 2J). Subgenital papillae, 3–4 per individual, with average width of 4.7 and 6.21 mm length, are positioned parallel to rhopaliar canals and in front of subgenital ostia (Figure 2H, I); 4–5 (among juveniles) and 5–11 (mature) marginal lappets per octant. Thin transparent to translucent membrane connects marginal lappets (Figure 3A–C; E). Marginal lappets have microscopic spots visible at a stereomicroscope's 40× total magnification with leaf-tip- to dome-shaped tips (Figure 3A–C; E). Lappets are smooth macroscopically (Figure 3A–C) unlike those of C. mosaicus with macroscopic striations, Arabian C. perezi with minute warts (Figure 3D), C. townsendi with fine granules, and C. ouwensi with fine striations. Silver-like dots (statocyst) in rhopalia as in original descriptions of paratypes (Figure 3A). Rhopalia are positioned inside a ‘pit’ between paired rhopaliar (ocular) lappets, distinctively smaller than adjacent velar lappets (Figure 3A–C). Smooth subgenital ostia (Figure 3F), 4 per individual, with average width of 13.1 mm. Three-winged oral-arms, eight per individual (Figure 3G–I), with average lengths of 19.5 mm (juveniles) and 34.3 mm (mature). Oral arms without appendages (Figures 1C and 3G–I).
Remarks
Catostylus purpurus medusae in this study resemble C. townsendi. This observation conforms to Light (Reference Light1921) that mentioned this similarity. The smooth bell texture of the jellyfish here looks like the umbrella of C. perezi (Figure 1E), Crambione mastigophora (Figure 2D), and Crambionella orsini (Figure 2E), but C. orsini medusa has prominent ridges on marginal lappets (Figure 2E). Catostylus here has oral arms with no appendages unlike other Catostylidae jellyfish like C. mastigophora with string-like filaments (Figure 2D) and C. orsini with leaf-tip-shaped terminal appendages (Figure 2E). However, the Philippine blubber jellyfish's gastrovascular canal system and morphological features, such as bell shape, texture, and lack of colour spots or bands, affirm its classification as C. purpurus, distinct from Acromitoides species and other Western Pacific species of Catostylus: C. townsendi, C. ouwensi, and C. mosaicus. Moreover, Stiasny (Reference Stiasny1921) deemed Mayer's (Reference Mayer1910, Reference Mayer1915) and Light's (Reference Light1914, Reference Light1921) descriptions insufficient and proposed the new genus to accommodate the taxon under the binomen A. purpureus. This taxon's spelling remained in Stiasny (Reference Stiasny1924b: 39–45, Reference Stiasny1926: 246) until Kramp (Reference Kramp1961) started to use the original epithet purpurus again. Regardless of these historical contexts, our new findings on the morphology including re-examination of type specimens of the Philippine jellyfish validate the species as a member of the genus Catostylus, i.e. as C. purpurus.
We found C. purpurus shares some similarities with Acromitoides stiphropterus (Stiasny, Reference Stiasny1921; see also systematics in Table S2 and Figure S2, Supplementary materials), one of the two species under genus Acromitoides aside from A. purpurus (now a synonym in Catostylus). Notably, A. stiphropterus has a hemispherical bell with smooth exumbrella. However, this jellyfish has brown spots that fade near the bell margin similar to C. townsendi, but unlike C. purpurus. The gastric canal system of A. stiphropterus contains wide-meshed intracircular anastomoses solely connected to inter-rhopaliar (adradial) canals, a feature that deviates from the form in Catostylus spp. (Stiasny, Reference Stiasny1921). Schultze (Reference Schultze1897) originally described this jellyfish (as Crambessa stiphroptera) using one preserved specimen with damage such as the prominent abrasion on coronal (ring) muscles, but he did not provide a preserved specimen for future examination (Table S2 and Figure S2, Supplementary materials). The presence or absence of a subgenital papilla, a diagnostic feature of a Catostylidae genus, in this jellyfish was also not described (Schultze, Reference Schultze1897; Stiasny, Reference Stiasny1921). Without sufficient morphological descriptions maintaining the validity of A. stiphropterus and the morphology of this jellyfish leaning to many features of C. townsendi, A. stiphropterus will remain a species inquirenda until at least morphological features from a series of specimens without damage, will show whether the species is new or belongs to a rhizostome family like Catostylidae. Since no stable sets of morphological traits (e.g. shape of subumbrellar papillae) support the genus Acromitoides, this genus requires further taxonomic investigation. Thus, we establish Acromitoides as genus inquirenda in this study.
Etymology
Mayer (Reference Mayer1910, Reference Mayer1915) did not specify the origin of the name purpurus after establishing this species. The term ‘purpurus’ may be a variant of the Latin word ‘purpureus’, which means ‘purple’ (e.g. Leptolalax purpurus). Purpureus may be used to describe some purple characteristic of a species like Cenchrus purpureus with sometimes purple, foxtail-shaped flower. Purpurus here may refer to the brown, purple, and burgundy colour morphs of the medusae found in Manila Bay.
Local names
Names are salabay and dikya (Tagalog in Cavite and Bataan, northern Philippines); budol, bokya, bukya (Waray in Leyte and Biliran, eastern Philippines); bokya (Cebuano); and bulbog (Cebuano in Ormoc, Leyte and Lanao del Norte, southern Philippines). However, salabay may refer to chirodropid box jellyfishes and other species with prominently trailing tentacles like the compass jellyfish, Chrysaora spp., in Tagalog and Cebuano languages. Here, we also establish English common names such as ‘Philippine blubber jellyfish’ and ‘burgundy blubber jellyfish’ when writing about this species since these terms specifically refer to this jellyfish with brown to burgundy-coloured medusae and other colour morphs such as white and blue morphs.
Molecular analysis
The Philippine C. purpurus medusae exhibit close p-distances of 0.000–0.014 (cox1) and 0.00–0.00 (16S). C. townsendi, found in southeast Asian waters, shows a genetic distance of 0.161–0.166 to C. purpurus in cox1 and 0.0628 in 16S (Table 2). Similarly, C. purpurus differs genetically from C. mosaicus from Australia (0.171 in cox1 and 0.063 in 16S) (Table 2).
Table 2. Uncorrected pairwise distances for cytochrome oxidase I (cox1) and ribosomal RNA gene (16S) between C. purpurus and other scyphozoan jellyfish species, e.g. C. townsendi and M. papua
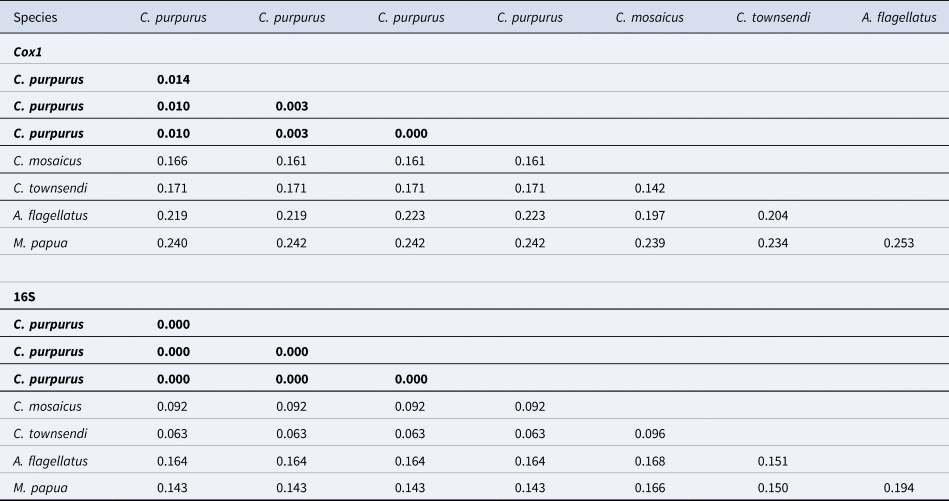
Values in bold are the lowest distances.
Our phylogenetic analyses show C. purpurus specimens form a clade with 100% bootstrap support (cox1; 16S), separated genetically from C. townsendi and the Australian blubber jellyfish, C. mosaicus (Figure 4). Analyses of both markers unite the species of Catostylus into a single clade (bootstrap indices of 81 and 97 for cox1 and 16S), but differ in the specific relationships among the three species. Bootstrap support for the topology based on the cox1 dataset is poor, while the 16S data provide moderate support (bootstrap index = 73) for C. townsendi as the sister species of C. purpurus (Figure 4). Further analyses using phylogenetic trees based on all available sequences of Catostylus for both genes reveal that Western Pacific species (C. purpurus, C. townsendi, C. mosaicus) form a distinct clade with high support (94% for cox1, 98% for 16S), separate from non-Western Pacific species (Catostylus sp. 1 in Central America, C. tagi in Portugal; see Figures S3 and S4, Supplementary materials). In the cox1 tree, C. townsendi forms a clade, and another clade includes Catostylus sp. 2 and C. purpurus, both with 100% bootstrap indices (Figure S3, Supplementary materials). The 16S topology shows a clade of C. townsendi with high support (index = 93%) and another clade uniting C. mosaicus and C. purpurus with 98% support index (Figure S4, Supplementary materials).

Figure 4. Phylogenetic trees based on maximum-likelihood method and Tamura–Nei models for cox1 and 16S genes of C. purpurus and other scyphozoan medusae (e.g. M. papua). Trees are based on the highest log likelihood (cox1: −2210.01; 16S: 1753.65) and eight nucleotides for both genes. Numbers at nodes are bootstrap supports (%).
Ecology
Catostylus purpurus medusae exist in many ecoregions of the Philippines (Figure 1A) including the West Philippine Sea region (i.e. Cavite and Bataan provinces surrounding Manila Bay), Visayan region (i.e. Cebu, Leyte, Biliran, Samar, and Lanao del Norte provinces) and Southern Philippine Sea with Marabut town in Samar (Table 1; Figures 1B and 5A). Recent visits to the type locality (Navotas coast, Metro Manila; coordinates: 14°40′08.3″N, 120°55′57.5″E) of C. purpurus from April to July and November to December 2016 did not reveal the jellyfish in the area due to unknown causes.
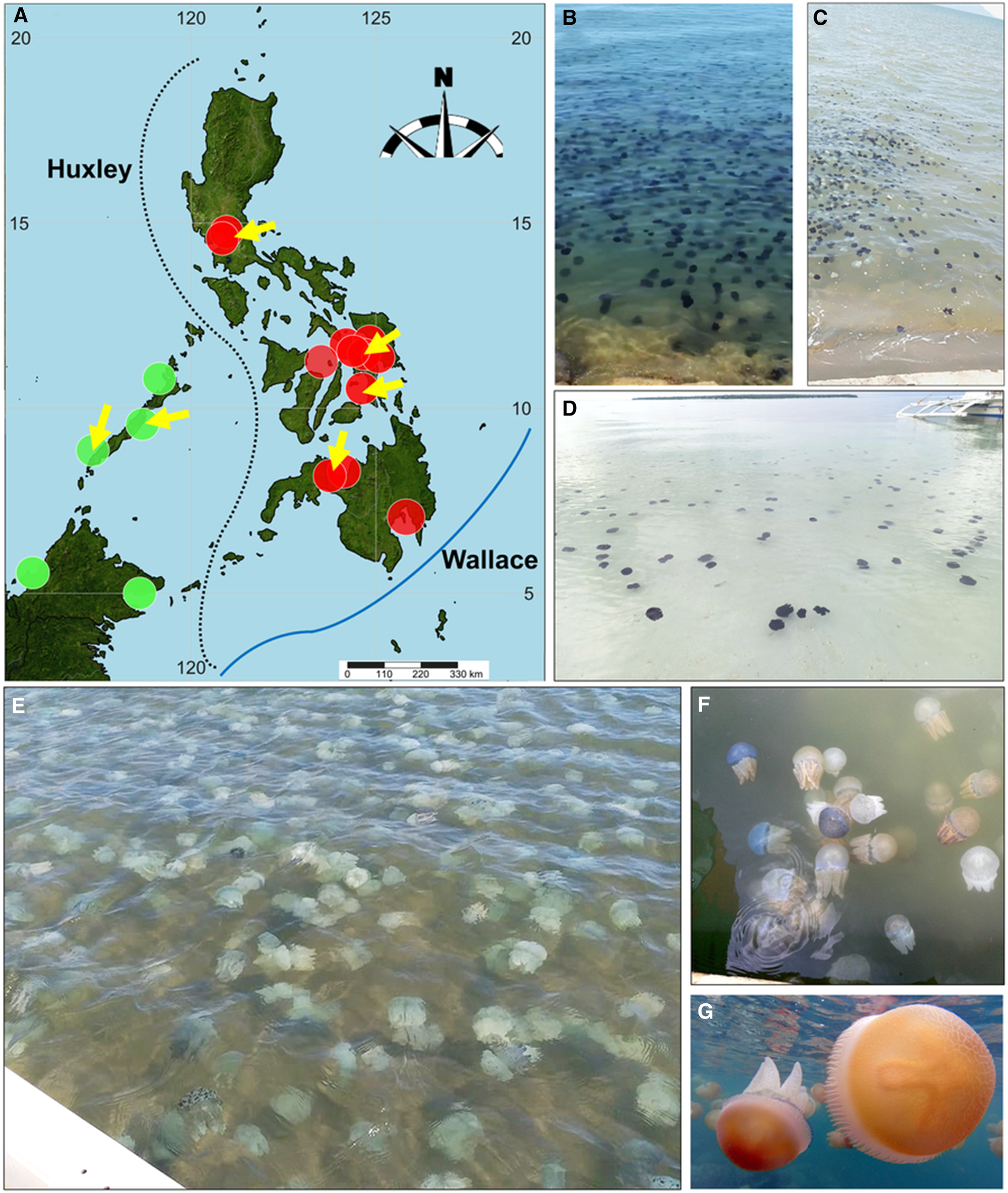
Figure 5. (A) Map of the Philippines with Huxley and Wallace boundaries, indicating locations of confirmed blooms (yellow arrows) and occurrences of C. purpurus (orange dots) and C. townsendi (green dots). Refer also to Table 1 and Table S1 (Supplementary materials) and ‘Ecology’ under the Results section for specific locations of these blooms; (B–D) blooms of C. purpurus in Orion, Bataan (B), at the coast of Carigara town (C) and Bantayan Island (D), Philippines; C. townsendi blooms in Narra, Palawan on February 2019 (E) and in Trat province, Thailand (F); (G) bloom of C. mosaicus in April 2022 in Manly coast, Sydney, Australia. Photos courtesy of A. Manlangit (B), S. Mones (C), P. Dimalaluan (E), Marine and Coastal Resources Research and Development Centre, Thailand, and K. Chanachon (F), and J. Turnbull (G).
Blooms of this jellyfish appear in several coastal waters in the Philippines, particularly in Pasay coast, Metro Manila (14°31′55.8″N, 120°58′41.5″E), Orion town, Bataan (14°35′06.2″N, 120°35′29.2″E), towns of Carigara (11°18′16.2″N, 124°40′52.3″E) and Bato (10°19′36.93″N, 124°47′12.56″E) in Leyte, and Panguil Bay (8°06′56.0″N, 123°51′49.6″E) in northern Mindanao (Figure 5A–D). These blooms are distinguishable from those of C. townsendi with prominent colour spots on the central exumbrella to the lappets, like those that occur in Palawan, Philippines and northern Thailand (Figure 5A; E, F; Table S1, Supplementary materials). Unlike the medusae of Philippine Catostylus, C. mosaicus has a distinct granulated exumbrella, visible among blooms observed in Australia (Figure 5G).
Besides the burgundy morphs of C. purpurus, the species also occurs as other colour morphs, like blue morphs in central Philippines (Cebu, Leyte, and Samar regions), and northern region (Manila Bay) (Figures 2A and 5B; see also records in Table 1). Blue, orange, and white morphs exist in southern Philippines (Panguil and Iligan Bays). The medusae are abundant in warm months (April, May, and June) but also occur year-round in central Philippines (Boco et al., unpublished data; Figures 1A, C; 2B, C; and 5A, D). In addition, thus far, medusae of C. purpurus associate with a number of animal ectosymbionts, including the crucifix crab Charybdis (Charybdis) feriata, the copepod Paramacrochiron sp., and juvenile carangid fish, Alepes djedaba.
Envenomation
Some authors (SRB, CGGC, DT) here, experienced slight burning to itchy stings upon accidental barehand handling of medusae and wading in water where the medusae occur and are abundant (>5 medusae m−3) during extensive field surveys for this jellyfish in the Philippines (2013–2022). Wading in the water when C. purpurus medusae are around may cause stings even without direct contact with the medusa. This observation appears like the stings induced by cassiosomes (i.e. floating tissues with nematocysts) of some jellyfish species like Cassiopea spp. (Lewis-Ames et al., Reference Lewis-Ames, Klompen, Badhiwala, Muffett, Reft, Kumar and Vora2020) and is consistent with Lewis-Ames et al.'s (Reference Lewis-Ames, Klompen, Badhiwala, Muffett, Reft, Kumar and Vora2020) observation that Catostylus mosaicus releases similar stinging structures.
KEYS TO THE IDENTIFICATION OF CATOSTYLUS SPP.
The description of the texture of exumbrella and the type specimen of Catostylus tripterus (Haeckel, Reference Haeckel1880) are unavailable. Further, without description of the number and arrangement of marginal lappets, subgenital papillae, circular musculature, and bell colour, the species of Catostylus turgescens (Schultze, Reference Schultze1898) is extremely doubtful. In 1921, Stiasny recognized this jellyfish as possibly regenerating or had an injury. Due to these morphological descriptions lacking diagnostic characters, we classify C. tripterus and C. turgescens as species inquirendae and they are absent in this key.
1 Catostylidae exumbrella with coarse granulations ……… 2
With completely or macroscopically smooth surface on centre of exumbrella ……… 3
2 Exumbrellar granules fuse into rows on the marginal lappets ……… C. ornatellus (Vanhöffen, 1888)
Without the above arrangement of granules on lappets; smooth proximal part of oral arm short, about 1/6 as long as distal three-winged part ……… C. mosaicus (Quoy & Gaimard, 1824)
3 Radial furrows prominent between centre of exumbrella and bell margin ……… ……… C. cruciatus (Lesson, 1830)
Absence of deep furrows on the bell ……… 4
4 With rows of prominent rugged papillae radiating towards the margin on exumbrella………4. ……… C. perezi Ranson, 1945
Without marginal papillae ……… 5
5 With shallow dendritically branching furrows near umbrella margin, distal end of oral arm tapering to a point ……… ……… C. tagi (Haeckel, 1869)
Smooth exumbrella with fine granulations on exumbrellar margins ……… 6
6 Exumbrella macroscopically smooth but with less prominent minute granulations ………6. ………C. ouwensi Moestafa & McConnaughey, 1966
Entirely smooth exumbrella ……… 7
7 Exumbrella with purple-brown, or light to dark greenish spots that usually fade at the bell margin; length of proximal part of oral arm variable, from 1/2 or 1/4 to the same length of distal three-winged part ……… ……… C. townsendi Mayer, Reference Mayer1915
Exumbrella without constellation-like colour spots ……… 8
8 Bell slightly flattened to hemispherical, slit-shaped subgenital ostia and umbrella colour greyish-yellowish (opaque) and violet oral arms ……… C. viridescens (Chun, 1896)
Flat to hemispherical bell with smooth exumbrella but narrow and long subgenital ostia; with microscopic spots on the lappets; with medusae of burgundy to brown, purple, blue, orange, and white colour morphs ……… ………C. purpurus Mayer, Reference Mayer1910
Discussion
Several jellyfish taxa require reassessment of their validity (e.g. in Jarms and Morandini, Reference Jarms and Morandini2019; Gamero-Mora et al., Reference Gamero-Mora, Collins, Boco, Geson, Morandini and Wilson2022). Modern methods such as integrative systematics with morphological and molecular information effectively clarify taxa of many species of jellyfish like rhizostome medusae (Gamero-Mora et al., Reference Gamero-Mora, Collins, Boco, Geson, Morandini and Wilson2022; Santander et al., Reference Santander, Maronna, Ryan and Andrade2022). Here, the findings support our hypothesis that the Philippine blubber jellyfish, exhibiting different colour morphs, does not belong to the genus Acromitoides, but rather to Catostylus. Thus, in this study, we rearrange the jellyfish species A. purpurus into the genus Catostylus, and confirm the validity of the species, commonly known in English as blubber jellyfish. Our integrative approach shows that C. purpurus can be distinguished from the other Western Pacific species of the genus, C. townsendi and C. mosaicus, with both molecular and morphological features. Hence, this study synonymizes A. purpurus, as a junior synonym of C. purpurus; against Stiasny's (Reference Stiasny1921) and Kramp's (Reference Kramp1961) visions to keep it in a separate genus. This new arrangement with C. purpurus adds one to the eight valid species of the genus Catostylus and confirms occurrences of three Catostylus spp. (C. purpurus, C. townsendi, and C. ouwensi) in the Indo-Pacific (Figure 1A; Jarms and Morandini, Reference Jarms and Morandini2019, Reference Jarms and Morandini2023; Collins and Morandini, Reference Collins and Morandini2023). This revived Philippine taxon enhances our understanding of jellyfish biodiversity in the Indo-Pacific, a region that likely harbours additional undiscovered species of rhizostome jellyfish (Gamero-Mora et al., Reference Gamero-Mora, Collins, Boco, Geson, Morandini and Wilson2022).
This study highlights the importance of examining many morphological features, including the form of the gastrovascular canal system of jellyfish, in order to garner the information necessary to diagnose scyphozoan species. The canal system, combined with other morphological features like bell texture enabled confirmation of many other species of rhizostome jellyfish such as C. mastigophora and Acromitus spp. (Thiel, Reference Thiel1978; Gómez Daglio and Dawson, Reference Gómez Daglio and Dawson2017). However, the same method of diagnosing species using canal system was used to establish A. purpurus (now a synonym), despite preceding evidence indicating it to be a species of Catostylus (in Mayer, Reference Mayer1910, Reference Mayer1915; Light, Reference Light1914, Reference Light1921). Specifically, Stiasny (Reference Stiasny1924b) mentioned that the canal system of topotypes of C. purpurus usually consists of anastomoses that do not connect to all rhopaliar canals. The description of these canals was based on formalin-preserved specimens (Stiasny, Reference Stiasny1924b) and this study opposes this observation. Hence, we speculate that formalin preservation potentially induced slightly rigid musculature and contracted bell of Stiasny's (Reference Stiasny1924b) specimens. This outcome after preservation perhaps influenced the appearance of the canals and led Stiasny's (Reference Stiasny1924b) drawings of canal systems that show rhopaliar canals without connections to coarse anastomoses. The cases in Stiasny (Reference Stiasny1924a, Reference Stiasny1931), similar to observations in Stiasny (Reference Stiasny1924b), support this speculation. Some formalin-preserved specimens of C. mosaicus and Rhizostoma luteum were examined and Stiasny (Reference Stiasny1924a, Reference Stiasny1931) mentioned they contain canal systems that do not conform to established descriptions of the gastrovascular canal network for these two species. Stiasny (Reference Stiasny1924a, Reference Stiasny1931) argued that these are potential ‘population variations’ with canal system ‘anomalies’ of the two species (e.g. C. mosaicus) without considering the possibility of error in staining the canal system due to contraction of specimens after preservation or, simply, a damage to the bell. Stiasny (Reference Stiasny1924a, Reference Stiasny1931) might have examined malformed individuals with deformed gastrovascular canals of these species. This speculation on specimen malformation including the method of staining of canal systems warrants examination in future studies of jellyfish taxonomy. Potential damage of specimens such as predation-based injuries and effect of formalin preservation on the appearance of gastrovascular canal systems need further investigation and must be checked in a series of individuals.
Even without information on the canal system, morphological features of C. purpurus are shared with those of many species of Catostylus. Specifically, C. purpurus shares with C. townsendi and C. mosaicus the shape of the bell and lappets, and texture and shape of three-branched oral arms. Due to similarity of our species here to C. townsendi, we recommend further examination of Catostylus spp. using morphometric statistics. Using data of size-adjusted features of medusae will elucidate potential variation of the morphology of medusae such as the distinction of their bell shapes and texture of oral arms in future studies of Catostylus species (e.g. between moon jellyfish species of Aurelia spp.; Chiaverano et al., Reference Chiaverano, Bayha and Graham2016; Lawley et al., Reference Lawley, Gamero-Mora, Maronna, Chiaverano, Stampar, Hopcroft and Morandini2021). Further, we uncovered the taxonomic status of species similar to C. purpurus. Through careful examination of jellyfish taxonomic literature and advances made in this study, we found that descriptions of C. tripterus and C. turgescens are incomplete, thus they are highly doubtful and not diagnostic for these species. Similarly, the status of the Catostylidae species A. stiphropterus will need taxonomic examination. These doubts on the taxonomy of these species have been echoed before (Mayer, Reference Mayer1910; Stiasny, Reference Stiasny1921; Kitamura and Omori, Reference Kitamura and Omori2010) and our study aligns with doubts presented in these papers. Hence, we classified C. tripterus, C. turgescens, and A. stiphropterus as species inquirendae and genus Acromitoides as genus inquirenda.
Since morphology alone may not always be sufficient to delineate species, clarifying species boundaries sometimes requires genetic analyses (Dawson and Jacobs, Reference Dawson and Jacobs2001; Dawson, Reference Dawson2003). Molecular approaches improved systematics understanding of rhizostomes by confirming the taxonomic lineage of Catostylidae species such as C. mosaicus and Acromitus spp. (e.g. Dawson, Reference Dawson2005a; Gómez Daglio and Dawson, Reference Gómez Daglio and Dawson2017). In this study, analyses of cox1 and 16S sequences clearly separate C. purpurus from other species of Catostylus from the Western Pacific, i.e. C. townsendi, which also occurs in the Philippine region (at the western Philippine area) and C. mosaicus from Australia (see Figure 4). These distinct genetic profiles of C. purpurus, as revealed through two genetic markers, affirm its separation from other Catostylus species in the Western Pacific.
Regarding genetic distances, average intra-species p-distances (%) of 0.7 (±standard deviation) ± 0.5 (cox1) and 0.0 ± 0.0 (16S) of C. purpurus fall within the species-level p-distances measured for other rhizostome jellyfishes (e.g. Cassiopea sp.; Gómez Daglio and Dawson, Reference Gómez Daglio and Dawson2017; Gamero-Mora et al., Reference Gamero-Mora, Collins, Boco, Geson, Morandini and Wilson2022). For example, Cassiopea mayeri, which also occurs in Western Pacific including the Philippines, exhibits 1.4–1.8% (16S) and 3.2–4.2% (cox1) within-species distances (Gamero-Mora et al., Reference Gamero-Mora, Collins, Boco, Geson, Morandini and Wilson2022). Thus, minimal genetic variation exists within the species of C. purpurus despite our samples that originated from different colour morphs. Meanwhile, the average % p-distances between C. purpurus and C. townsendi are higher (17 ± 1.2 for cox1) or around (6.9 ± 1.0 for 16S) inter-clade or genus-level distances of rhizostomes such as between the species of Mastigias with 8.6 ± 3.2 average % p-distance of cox1 in the tropical Western Pacific (de Souza and Dawson, Reference de Souza and Dawson2018). Indeed, the p-distances (%) between Cassiopea mayeri and Cassiopea culionensis are lower (13.6–14.2 for cox1) or around (10.1–12.9 for 16S) the average p-distances between C. purpurus and C. townsendi, although these upside-down jellyfishes occur in sympatry in the Philippines (see Gamero-Mora et al., Reference Gamero-Mora, Collins, Boco, Geson, Morandini and Wilson2022), unlike C. purpurus which exists separately by geography from C. townsendi to date. While the genetic differences between C. purpurus and C. townsendi support their distinction as separate species, the exact threshold for genetic distance that defines separate species remains a subject of research (Smith and Carstens, Reference Smith and Carstens2022). Nevertheless, the genetic evidence presented here is consistent with our morphological results. This underscores the importance of a holistic approach that integrates morphological and molecular data and employs multiple genes to support accurate species identification.
Interestingly, we find some support (through phylogenetic analysis of 16S) that C. purpurus is more closely related to C. townsendi from the Indo-Pacific (sample from Malaysia) than it is to C. mosaicus from Australia. However, topology results using all available sequences of Catostylus showed conflicting phylogenetic relationship of C. townsendi to C. purpurus. C. townsendi formed a 16S clade clearly separating this species to the Philippine C. purpurus (see Figure S4, Supplementary materials). The root of this inconsistency is unclear but could be linked to the unequal number of sequences between the species here. Specifically, there are fewer sequences of C. townsendi compared to C. mosaicus in the cox1 tree, whereas in the 16S topology, there are more samples of C. townsendi than C. mosaicus (Figures S3 and S4, Supplementary materials). While beyond the scope of our study, topologies may shift depending on the number of genetic samples available for a species or between nominated species (refer to Huang et al., Reference Huang, Liu, Zhu and Yang2021). Moreover, without genetic samples from all other congeners, the evolutionary trajectory of the entire genus continues to be a mystery. Nevertheless, our molecular findings mirror significant morphological differences between C. purpurus and other Western Pacific species such as C. townsendi. Notably, C. townsendi displays a constellation-like pattern of colour spots on the exumbrella, and C. mosaicus exhibits ridges or papillae on the exumbrella, both of which are absent in C. purpurus medusae. Despite the lack of genetic data, C. ouwensi from Indonesia presents a unique morphology with macroscopically visible but fine granulations on the exumbrella. These morphological differences seem to be restricted to species with relatively narrow and distinct geographic distributions in the Western Pacific. For example, despite comprehensive surveys, we have not found C. townsendi coexisting with C. purpurus. C. mosaicus appears to be confined to Australia, whereas C. ouwensi has only been observed in Indonesia. Consequently, potential biogeographic boundaries, such as the Sulu Sea and Huxley's line, may govern the distributions of Pacific blubber jellyfish species (discussed below). These boundaries could be the isolating mechanisms that have allowed for the accumulation of distinct morphologies and genetic signatures. Thus, morphological, and molecular findings and available data of biogeographic distributions, support the species distinction of C. purpurus. Given this, we recommend having biogeographic information, morphology, and molecular data (as in this study) when accessible, to explain and/or assess the existence of a separate jellyfish species.
Catostylus purpurus's distribution may represent biogeographic separation of this species to other Catostylus species in the Western Pacific. For example, C. purpurus exists on the east side of Huxley's line, unlike C. townsendi, which occurs on the west side, particularly in western Philippines (Figure 5A). We expect this distribution pattern of this jellyfish, consistent with biogeographic gene separations observed in various marine taxa in Southeast Asia, such as the marine alga, Gracilaria spp. (Chasani, Reference Chasani2017) and squat lobster Uroptychus naso (Poore and Andreakis, Reference Poore and Andreakis2011), which follow Huxley's line (Huxley, Reference Huxley1868). Remarkably, C. purpurus in the north of Wallace's line (Figure 5A) appears geographically separate from C. mosaicus which exists at the line's south (Australia). Although not part of this study, Wallace's line influences geographic distribution of marine species such as the reef ascidian, Polycarpa aurata and fiddler crab Austruca perplexa and we invoke this boundary as a potential factor regulating the distribution of some Catostylus spp. (as in Hardianto, et al., Reference Hardianto, Permata Wijayanti, Shy, Mather, Hughes and Imai2022). If the distribution of C. purpurus here indeed follows these significant biogeographical boundaries (Wallace's and Huxley's), then various factors such as ocean currents, temperature profiles, and distribution of zooplankton prey, perhaps influence separation of this species from jellyfish populations in western Philippines, where C. townsendi occurs. Currents could also regulate transport of planulae or juvenile medusae of C. purpurus and could concentrate them into areas like central and central-eastern Philippines where this jellyfish exists. Therefore, we need to study the life cycle stages including the larvae of this jellyfish similar to studies on the life cycle of many Catostylus species, such as C. mosaicus and C. tagi, to significantly reveal the ecology and distribution of this jellyfish (Pitt, Reference Pitt2000; Phuangsanthia et al., Reference Phuangsanthia, Choosri and Luangoon2018; Gueroun et al., Reference Gueroun, Torres, Dos Santos, Vasco-Rodrigues, Canning-Clode and Andrade2021). However, these are speculations, and further research is needed to understand the exact factors controlling the distribution of Indo-Pacific Catostylus. Studies focusing on life cycle, habitat preferences, and potential dispersal mechanisms of this Philippine jellyfish would provide valuable insights on the occurrence of this species in the Coral Triangle.
Confirming the identity of this jellyfish taxon could benefit local communities, particularly in predicting and understanding blooms and stings by this species (as in Pitt and Purcell, Reference Pitt and Purcell2009). Previously, misidentification has led to this species being wrongly associated with severe envenomation incidents by local communities and news outlets in Cebu, central Philippines (Cordova, Reference Cordova2023; Madarang, Reference Madarang2023). However, unlike other species, jellyfishes of the Catostylidae family, such as C. purpurus, do not appear to cause extreme envenomation such as Irukandji-like stings or fatalities (Williamson et al., Reference Williamson, Fenner, Burnett and Rifkin1996; Peach and Pitt, Reference Peach and Pitt2005; this study). Instead, they can induce ‘stinging water’ like upside-down jellyfishes, Cassiopea spp. (Lewis-Ames et al., Reference Lewis-Ames, Klompen, Badhiwala, Muffett, Reft, Kumar and Vora2020). Further, confirming the presence of this species can potentially aid predictions of the frequency and magnitude of blooms of this jellyfish, since understanding this species means we know they can form large aggregates (e.g. thousands of medusae) of jellyfish during a bloom. This is unlike other blooms such as those of the cubozoan, Carybdea spp., or other scyphomedusae like Chrysaora chinensis whose occurrences are sporadic (Kingsford and Mooney, Reference Kingsford, Mooney, Pitt and Lucas2014; Syazwan et al., Reference Syazwan, Then, Chong and Rizman-Idid2021; Terenzini et al., Reference Terenzini, Boco and Falkenberg2023). The blooms of C. purpurus could influence biogeochemical cycles in an area, affecting the ecosystem's carbon and nutrient flow (sensu Wright et al., Reference Wright, Le Quéré, Buitenhuis, Pitois and Gibbons2021). But, predicting the magnitude of blooms of this jellyfish species and their influence on geochemical cycles will require examination beyond taxonomy such as future ecological studies of C. purpurus. Considering the potential influence of jellyfish blooms such as those of this Philippine jellyfish, on ecosystems and human activities, we need to understand ecological maintenance of populations of this species, a task that can be effectively initiated through accurate taxonomic identification of the species – highlighting the importance of zoological studies and systematics surveys.
Conclusion
Our study provides compelling evidence that the burgundy blubber jellyfish found in the Philippines belongs to the species C. purpurus Mayer, Reference Mayer1910, previously named as A. purpurus. Medusae of C. purpurus are taxonomically separate from related C. townsendi and C. mosaicus, based on combined genetic and morphological findings. Morphological differences between C. ouwensi of Indonesia and this Philippine species were significant to classify C. purpurus as a species separate to the other Western Pacific species, C. ouwensi. Validating this Catostylus species contributes to our understanding of jellyfish biodiversity in the Indo-Pacific region, particularly in the marine species-rich region of the Philippines. Our study also enabled revision of several taxa into species inquirendae (C. tripterus, C. turgescens, and A. stiphropterus) and a genus inquirenda (Acromitoides). This study highlights the need for further research into the occurrences, distribution, and ecology of scyphozoan jellyfishes in the Indo-Pacific.
Supplementary materials
The supplementary material for this article can be found at https://doi.org/10.1017/S0025315424000687.
Data
Data appear in this paper. See also new GenBank sequences.
Acknowledgements
We thank F. C. Baum for help with academic German translation and Manuel Antunes Jr (IB, USP, Brazil) for conducting the molecular protocols. Contributions by citizen-science participants of the Philippine Jellyfish Stings Project are greatly acknowledged.
Author contributions
Study conception and design: S. R. B., C. G. G. C., and A. C. M.. Data collection: S. R. B., C. G. G. C., A. C. M., G. W. M. H., P. A. J. B., D. T., A. G. C., J. C., S. M. H., K. V. D., D. A. R., and P. J. G.. Analysis and interpretation of results: S. R. B., C. G. G. C., A. C. M., G. W. M. H., P. A. J. B., A. G. C., J. C., D. A. R., and P. J. G.. Wrote first draft: S. R. B. and C. G. G. C.. Revision and edits to manuscript: all authors. Final approval of submitted manuscript: all authors.
Financial support
Grants provided for C. G. G. C. and P. J. G. (USC) partially enabled field observations. A. C. M. was supported by CNPq grant (307832/2022-8). This is a contribution of NP-BioMar-USP. This study is part of the Philippine Jellyfish Stings project, partially supported by many citizen-science contributors.
Competing interests
None.
Ethical standards
Permits for genetic sequencing were obtained from the Philippine Department of Environment and Natural Resources to comply for local laws and agreements under the Access and Benefit Sharing of genetic resources of the Pacific (Nagoya Protocol). Citizen-science procedures follow the Philippines's data privacy law of the Republic Act on Data Privacy (10173) and provisions of the United Nations Principles of Personal Data Protection and Privacy.









