Introduction
The Magelonidae is a small family of marine annelids, consisting of 81 extant species within the genus Magelona F. Müller, Reference Müller1858 (Mortimer and Mackie, Reference Mortimer and Mackie2006; Mortimer et al., Reference Mortimer, Fitzhugh, dos Brasil and Lana2021a, Reference Mortimer, Kongsrud and Willassen2021b). Magelonid worms are recorded in most of the world's regions and the Temperate Northern Atlantic is one of the most diverse in terms of the number of known magelonid species, which could be partially attributed to geographic bias of taxonomic work in the region. Magelonids are generally described to burrow in muddy and sandy substrates (Uebelacker and Jones, Reference Uebelacker, Jones, Uebelacker and Johnson1984; Parapar et al., Reference Parapar, Mortimer, Capa and Moreira2021) primarily in coastal regions and on continental shelves (Hernández-Alcántara and Solís-Weiss, Reference Hernández-Alcántara, Solís-Weiss, De León-González, Bastida-Zavala, Carrera- Parra, García-Garza, Peña-Rivera, Salazar-Vallejo and Solís-Weiss2009), although several deep-water species are known (Hartman, Reference Hartman1971; Fiege et al., Reference Fiege, Licher and Mackie2000; Aguirrezabalaga et al., Reference Aguirrezabalaga, Ceberio and Fiege2001).
Magelona species are recorded in numerous ecological studies within British and European waters, and many records of occurrence can be found in databases such as the National Biodiversity Network's (NBN Trust 2023) gateway, Global Biodiversity Information Facility (GBIF 2023), Ocean Biodiversity Information System and accessible through platforms such as the World Register of Marine Species (WoRMS) (WoRMS Editorial Board, 2023). However, the application of this information to the assessment of species-specific habitat conditions is not well understood. In the case of European species, Meißner and Darr (Reference Meißner and Darr2009) developed habitat models of four magelonid species in the German Bight based on sediment and depth, finding large variations in the environmental predictors most important for abundance and distribution. However, the study area was relatively shallow (<45 m) and Magelona minuta Eliason, Reference Eliason1962 was not recorded. Fiege et al. (Reference Fiege, Licher and Mackie2000) provided locality data for many European records of Magelona johnstoni Fiege, Licher and Mackie, Reference Fiege, Licher and Mackie2000 and Magelona mirabilis (Johnston, Reference Johnston1865) and subsequent information has been provided for other European magelonids (Mortimer et al., Reference Mortimer, Gil and Fiege2011, Reference Mortimer, Mills, Jordana, Pinedo and Gil2020, Reference Mortimer, Mills and Gil2022; Mills and Mortimer, Reference Mills and Mortimer2018), but descriptions of environmental parameters are still generalised across the family in most cases.
At present, five Magleona species are known to occur in British waters: M. johnstoni, M. mirabilis (Johnston, Reference Johnston1865), Magelona filiformis Wilson, Reference Wilson1959, Magelona alleni Wilson, Reference Wilson1958 and M. minuta. Mackie et al. (Reference Mackie, James, Rees, Darbyshire, Philpott, Mortimer, Jenkins and Morando2006) and Mortimer and Mackie (Reference Mortimer and Mackie2014) reported the former four species to occur both littorally and sublittorally, whilst M. minuta has been considered a distinct offshore species (Mortimer and Mackie, Reference Mortimer and Mackie2014; Mills and Mortimer, Reference Mills and Mortimer2018; Mortimer, Reference Mortimer, Purschke, Böggemann and Westheide2019). Of the British magelonids, M. minuta and M. alleni are the most morphologically (Mortimer et al., Reference Mortimer, Mills, Jordana, Pinedo and Gil2020; Parapar et al., Reference Parapar, Mortimer, Capa and Moreira2021) and behaviourally diverse (Mills and Mortimer, Reference Mills and Mortimer2018). Magelona alleni is a large, stout species known to construct permanent burrows (Mills and Mortimer, Reference Mills and Mortimer2019; Mortimer et al., Reference Mortimer, Mills and Gil2022), while M. minuta is small, fragile (Mills and Mortimer, Reference Mills and Mortimer2018) and regarded as mobile. Mortimer and Mills (Reference Mortimer and Mills2020) highlighted M. minuta to occur in at least 73 works of taxonomic and biotic survey literature by over 60 different authors and numerous ecological reports. They emphasised the species to be dominant in several biological assemblages, the latter being also true for M. alleni (e.g., Mackie et al., Reference Mackie, Oliver and Rees1995). Despite the regularity with which these two species are recorded, information defining the characteristics of their predominant habitat is still lacking, especially regarding sediment characterisation. This information is critical in predicting occurrence and abundance in future monitoring studies.
Both M. alleni and M. minuta are historically associated with muddy sediments (Wilson, Reference Wilson1958, Reference Wilson1982; McIntyre, Reference McIntyre1960; Eliason, Reference Eliason1962; Kirkegaard, Reference Kirkegaard1969; Hartmann-Schröder, Reference Hartmann-Schröder1996; Böggemann, Reference Böggemann1997; Mortimer and Mackie, Reference Mortimer and Mackie2014). Even with the magnitude of records available for these species, habitat descriptions are often based largely on information from the original descriptions, despite several uncertainties. For example, information on the type locality for M. alleni was not clearly defined by Wilson (Reference Wilson1958), the sediment type was based upon visual judgement alone, being noted as abundant in black sandy mud off Rame Head, which Mortimer et al. (Reference Mortimer, Kongsrud and Willassen2021b) concluded to be approximately 60 m deep. The type locality of M. minuta was simply described as shelly mud with a small amount of sand (Eliason, Reference Eliason1962). Comparative material from a re-description of M. minuta by Mills and Mortimer (Reference Mills and Mortimer2018) suggested the species to also occur in sandy mud, muddy sand, sand, and sandy gravel. However, in the absence of detailed assessment of species-habitat records, this remains unconfirmed.
The current study aims to investigate records of M. alleni and M. minuta from waters around the British Isles to increase understanding of abundance and geographical distribution patterns. The previous assumption that both species are predominately from muddy sediments is tested by analysing depth and sediment parameters (mud, sand and gravel content, and mean grain diameter) with abundance information. Furthermore, grains from the tubes of M. alleni are measured to classify the characteristics of the sediments utilised for tube construction. The use of historical visual assessments against quantitative sediment characterisation for the understanding of polychaete species habitat conditions is discussed. It should be noted that M. minuta is an unavailable name because it is an unreplaced junior primary homonym to Magelona filiformis minuta Wilson, Reference Wilson1959 (Read and Fauchald, Reference Read and Fauchald2023). As a replacement name is required, Mortimer and Mills (Reference Mortimer and Mills2020) applied to the ICZN for the suppression of Magelona filiformis minuta in favour of the junior homonym Magelona minuta. As the case (3804) is currently open, and no decision has been made, the name M. minuta is used herein.
Materials and methods
Samples and data
Reanalysed sediment and depth records of M. minuta and M. alleni (Figure 1) held at Amgueddfa Cymru – Museum Wales (NMW) (i.e. verified records where species identification has been checked, available at the Mendeley data repository: doi:10.17632/wzt3bsgmky.1) and additional records from other online sources (unverified records from data held within NBN and GBIF databases, accessed online 18 July 2023) were utilised to better understand distribution and abundance patterns around and beyond the British Coast. Verified records were extracted from the BIOMÔR 1 (Mackie et al., Reference Mackie, Oliver and Rees1995, 73 stations sampled throughout the southern Irish Sea), BIOMÔR 4 (Mackie et al., Reference Mackie, James, Rees, Darbyshire, Philpott, Mortimer, Jenkins and Morando2006, 148 stations located at regular intervals throughout the Outer Bristol Channel, including additional locations of geological interest and a number of coincident stations selected to investigate temporal change) and BIOMÔR 5 (Robinson et al., Reference Robinson, Darbyshire, Van Landegham, Lindenbaum, McBreen, Creaven, Ramsay, Mackie, Michell, Wheeler, Wilson and O'Berin2009, 97 stations in the southern Irish sea, including a few repeat stations from BIOMÔR 1) (Figure 2). Of the stations sampled, magelonids were recorded as present at 44, 30, and 28 stations within the BIOMÔR 1, BIOMÔR 4 and BIOMÔR 5 surveys, respectively. Unverified records extracted from the NBN and GBIF databases covered the entirety of the British coastline (Figure 2).

Figure 1. Anterior regions of the shovel head worms Magelona alleni (NMW.Z. 1991.075.1574, stained with Rose Bengal) and Magelona minuta (NMW.Z.1991.075.1584, stained with Methyl Green); (A) dorsal view of prostomium and chaetigers 1–7 (Left hand palp missing, tube apparent), (B) same, ventral view, (C) same, lateral view, (D) dorsal view of prostomium and chaetigers 1–12 (palps lost), (E) ventral view of prostomium (burrowing organ slightly everted) and chaetiger 1–11 (palp stubs visible).

Figure 2. Map of occurrences of Magelona minuta and Magelona alleni from verified records held at Amgueddfa Cymru – Museum Wales (ACNMW) and unverified records from additional sources (NBN, GBIF).
Data analysis
All surveys with verified records (i.e. BIOMÔR surveys) utilised the same sampling procedure, allowing direct comparison of species abundance at each station. Seabed samples were collected with a 0.1 m2 modified Van Veen grab and sieved with a 0.5 mm mesh sieve (see Mackie et al., Reference Mackie, Oliver and Rees1995; Mackie et al., Reference Mackie, James, Rees, Darbyshire, Philpott, Mortimer, Jenkins and Morando2006; Robinson et al., Reference Robinson, Darbyshire, Van Landegham, Lindenbaum, McBreen, Creaven, Ramsay, Mackie, Michell, Wheeler, Wilson and O'Berin2009 for full methodology). Replicate samples were taken at each site; two for the macrofauna and one for sediment analysis. Quantitative parameters on sediment (mud, sand and gravel contents, and mean grain diameter) and depth were extracted from verified records. Sediment data were originally classified using either Buchanan's trigon (Reference Buchanan, Holme and McIntyre1971, Reference Buchanan, Holme and McIntyre1984) (Mackie et al., Reference Mackie, Oliver and Rees1995) or an extension to the Folk's (Reference Folk1954) classification system within the surveys, allowing the subdivision of gravels (Mackie et al., Reference Mackie, James, Rees, Darbyshire, Philpott, Mortimer, Jenkins and Morando2006; Robinson et al., Reference Robinson, Darbyshire, Van Landegham, Lindenbaum, McBreen, Creaven, Ramsay, Mackie, Michell, Wheeler, Wilson and O'Berin2009). In the current study, sediment size was reclassified following the Udden–Wentworth grain size scale (Wentworth, Reference Wentworth1922) (Table S1). Afterwards, the Krumbein phi (φ) scale, which is defined as a logarithmic phi scale of the Udden–Wentworth scale proposed by Krumbein (Reference Krumbein1934), was calculated to place emphasis on finer grain sizes (Donoghue, Reference Donoghue and Kennish2016) (Table S1). The Krumbein phi (φ) scale is calculated as the negative logarithm to the base 2 of the particle diameter (in millimetres) and is obtained as follows:
where φ is the phi size and D is the grain diameter in millimetres (mm).
All data analysis was carried out in RStudio Version 4.0.3 (R Core Team, 2020). The R code is available from the Mendeley Data Repository at doi:10.17632/wzt3bsgmky.1. Percentage of mud, sand and gravel, together with depth and mean grain diameter for stations where at least one of the two species occurred (Mackie et al., Reference Mackie, Oliver and Rees1995, Reference Mackie, James, Rees, Darbyshire, Philpott, Mortimer, Jenkins and Morando2006; Robinson et al., Reference Robinson, Darbyshire, Van Landegham, Lindenbaum, McBreen, Creaven, Ramsay, Mackie, Michell, Wheeler, Wilson and O'Berin2009 for M. alleni; Mackie et al., Reference Mackie, Oliver and Rees1995; Robinson et al., Reference Robinson, Darbyshire, Van Landegham, Lindenbaum, McBreen, Creaven, Ramsay, Mackie, Michell, Wheeler, Wilson and O'Berin2009 for M. minuta) were investigated using linear regression analyses to understand the relationship between sediment parameters and abundance. This was coupled with principal component analysis (PCA) to allow the number of environmental variables under investigation to be reduced and further explore species–habitat relationships.
Image stacking
To classify the key characteristics of the sediments utilised for tube construction by M. alleni, tube samples were initially mounted on a glass slide. Images were subsequently taken using a Canon EOS 6D 20.2 MP DSLR camera attached to a Nikon Optiphot 2 Trinocular Microscope. Afterwards, the resulting images were stacked using HeliconFocus v6.22 (HeliconSoft Ltd) software. The maximum diameter for grains unobstructed (i.e., grain boundaries that were clearly observed) were measured under a petrographic microscope and reported as the Udden–Wentworth grain size scale. A similar methodology using the same equipment was also employed to take images of the anterior abdominal segments of both M. alleni and M. minuta for comparison of morphology.
Results
Distributions
This study looked at the distribution of two morphologically (Figure 1) and behaviourally diverse species of magelonid – M. alleni and M. minuta. The combined distribution maps of the records (verified and unverified) of both species show a broadly continuous distribution around the British Coast (Figure 2). Yet, there is a distinct lack of records in the English Channel (approximately Weymouth to Brighten for the former species, and a larger gap from Whitby, Yorkshire in the North Sea to Weymouth for the latter).
Quantitative data
Verified survey data from BIOMÔR records for M. alleni showed it to be present in sediments classified as mud to sandy gravel. These sediments ranged from a mud content of between 0.00 and 53.93% (Figure 3a), sand 0.00–98.87% (Figure 3b) and gravel 0.00–96.03% (Figure 3c). Mean grain diameter was between 0.12 and 0.48 mm (Figure 3d), corresponding to fine to coarse sand (Udden–Wentworth scale, 1–3 φ Krumbein phi scale). Occurrences (16) were more frequent in sediments with higher sand contents (80.66–99.56%), less mud (0.00–10.58%) and shallow depths (11–58 m), although abundance at each locality was not strongly influenced by any of the investigated parameters (Figure 3). Total depth range was 11–113 m, but occurrences were higher between 0 and 60 m and the highest abundances (up to 38 individuals per 0.1 m2) occurred between 11 and 32 m.
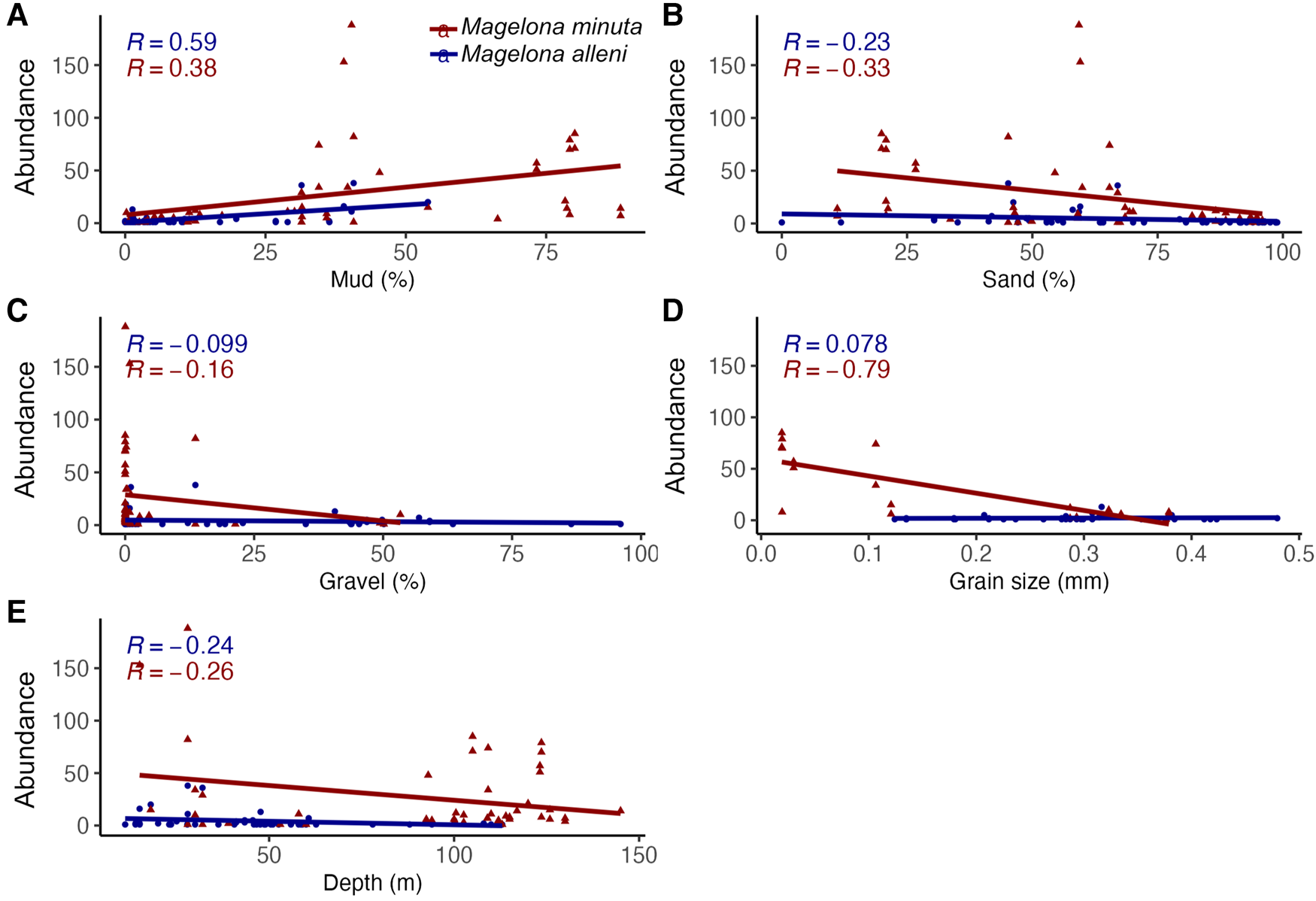
Figure 3. Linear regression analyses and correlation coefficient (R values) abundance per 0.1 m2 of Magelona alleni and Magelona minuta at each station vs (A) mud (%), (B) sand (%), (C) gravel (%), (D) mean grain diameter (grain size) (mm), (E) Depth (m).
Verified survey data for M. minuta revealed the species occurs in mud to sandy gravel. Mud content was between 0.23 and 88.26% (Figure 3a) and sand 11.07–95.90% (Figure 3b). The percentage of gravel was lower than that observed for M. alleni, ranging between 0.00 and 53.34% (Figure 3c). The highest abundances (over 100 individuals per 0.1 m2) for the species occurred in sediments with very little to no gravel (0.00–0.87%) and at least 59.30% sand. Depths ranged between 15 and 145 m, but occurrences were recorded more frequently from deeper waters (>100 m). Mean grain diameter ranged between 0.02 and 0.38 mm (Figure 3d), corresponding to medium silt to coarse sand on the Udden–Wentworth scale (1–6 φ Krumbein phi scale). Grain size strongly influenced abundance (R = −0.79) and more individuals were found in sediments with small mean grain diameters (mud to sandy mud). Overall, there were higher abundances recorded at stations of M. minuta than M. alleni.
PCA was used to explore relationships between investigated environmental variables for both species. PCA defined two principal components covering 83.3% of the cumulative variance (Figure 4). PC1 accounted for 46.3% of the variance, with the highest negative association of gravel and highest positive association of mud. PC2 accounted for 37.0% of the variance, with highest positive association of sand and highest negative association of gravel. Most data points that represented records of M. minuta were situated in the top right quadrant with loadings from mud and depth, while most data points for M. alleni were within the left quadrants, with loadings from sand and gravel.
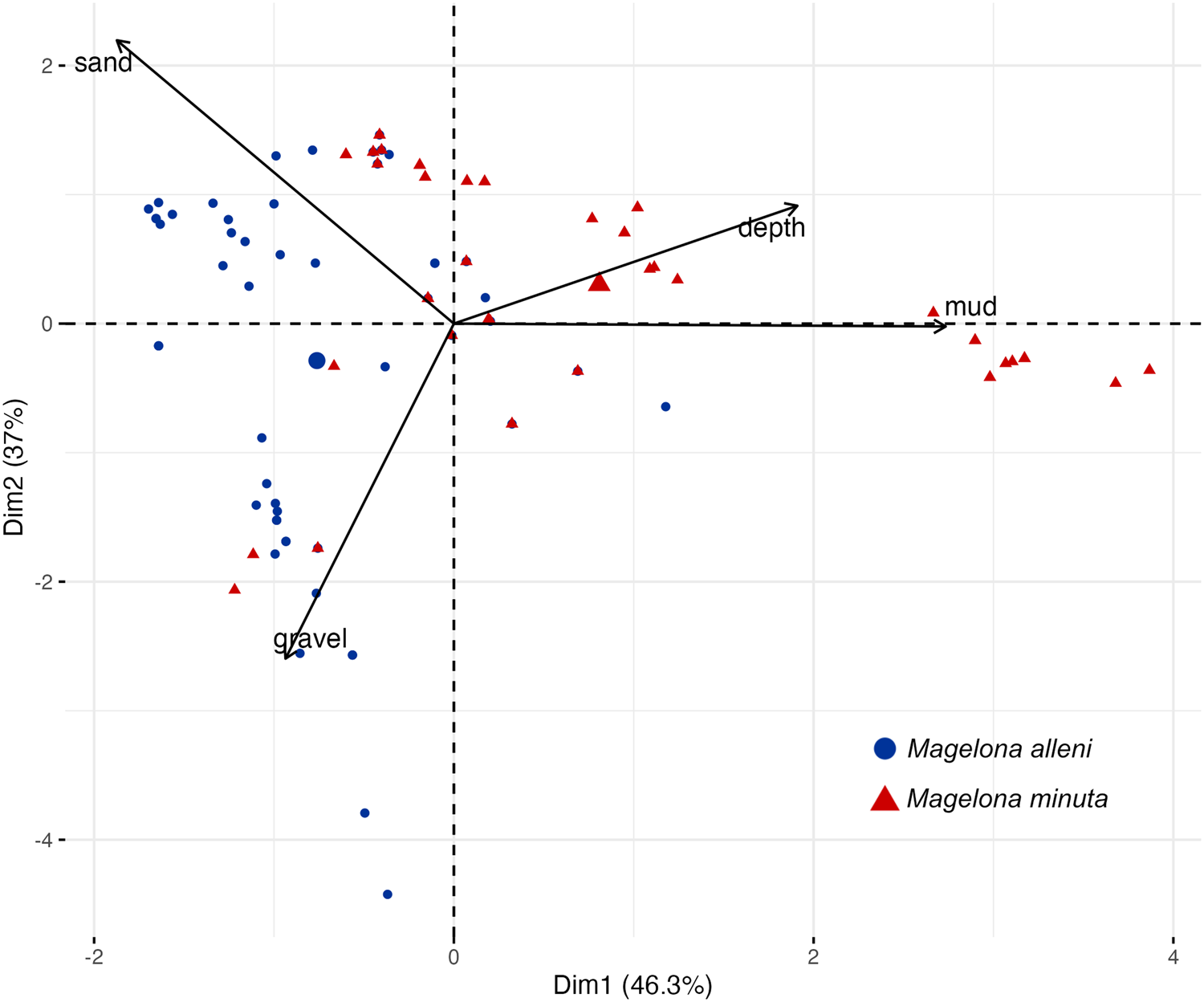
Figure 4. Principal component analysis of environmental variables for occurrences of Magelona alleni and Magelona minuta: mud (%),sand (%)gravel (%),depth (m). Black arrows show the loading of each variable and points show PCA scores. Point sizes represent quality of representation. Superimposed 95% confidence ellipsoids contain group points.
Sediment characteristics of Magelona alleni tubes
The diameter measurements for the majority of sediment grains used to construct the tubes of M. alleni (Figure 5) correspond to fine sand on the Udden–Wentworth scale (3 φ Krumbein phi scale). Measurements ranged between 0.06 and 0.43 mm (2–5 φ Krumbein phi scale) (coarse silt-to-medium sand) (Table S2). Overall, the mean diameter was 0.22 mm and the median diameter 0.20 mm (fine sand).

Figure 5. Magelona alleni tubes with sediment covering. The maximum diameter of unobstructed grains was measured where grain edges can be clearly seen.
Discussion
This study has shown that M. alleni occurs in an extensive range of sediments ranging from mud to sandy gravel. Despite previous reports that it is mostly a muddy sediment species (Wilson, Reference Wilson1958, Reference Wilson1982; McIntyre, Reference McIntyre1960; Hartmann-Schröder, Reference Hartmann-Schröder1996; Mortimer and Mackie, Reference Mortimer and Mackie2014), it is recorded herein more frequently at localities with a high percentage of sand. Although M. minuta was also found to occur in mud to sandy gravel, a strong negative linear relationship between mean grain diameter and abundance corroborates previous descriptions of the species inhabiting finer sediments with small grain sizes (Eliason, Reference Eliason1962; Mortimer and Mackie, Reference Mortimer and Mackie2014). Noteworthy, the observation of M. minuta in coarser sediments occurred at only three stations within the current dataset, suggesting that this is infrequent. It has been previously noted that gravelly sediments in the Southern Irish Sea can comprise of a cobble and shell pavement with mud and sand embedded between the stones which is sufficient for burrowing benthic organisms to live in (Rees, Reference Rees1993; Mackie et al., Reference Mackie, James, Rees, Darbyshire, Philpott, Mortimer, Jenkins and Morando2006). This may explain the infrequent records of M. minuta in coarser sediments as visual assessments from the three stations (Mackie et al., Reference Mackie, James, Rees, Darbyshire, Philpott, Mortimer, Jenkins and Morando2006:12) indicates the presence of some mud within the samples.
An ecological modelling study that investigated the distribution of magelonids in the German Bight (Meißner and Darr, Reference Meißner and Darr2009) has previously shown that the most suitable habitats for M. alleni are sands with elevated mud contents at depths between 30 and 40 m. The records herein show that although M. alleni is found within mud, most occurrences are documented to be under 10% mud and it is not an important predictor of distribution and abundance compared to the records from the German Bight. Despite the differences in the range of depth data between the German Bight and the current study, with depths reaching 45 m in Meißner and Darr (Reference Meißner and Darr2009), compared to over 100 m here, the results are generally similar and show M. alleni is abundant around 30 m, but occurrences are more frequent at approximately 50 m.
In comparison to M. alleni, the current results found that M. minuta is an offshore species, recorded more frequently over 100 m. Although the species has been reported at deeper depths up to 1000 m off the Gulf of Taranto (Fiege et al., Reference Fiege, Licher and Mackie2000) and M. cf. minuta at over 4000 m from Greenland (discussed in Mills and Mortimer, Reference Mills and Mortimer2018), verification of these records is needed, due to their distance from the type locality. It is likely that several undescribed species are present which may account for these records.
Although the records herein indicate both species share some overlap in sediment and depth parameters, the highest species abundances generally occur at localities where they are not in sympatry. Noteworthy, stations where both species co-occur may relate to elevated sand contents for M. alleni, but grain sizes that are sufficiently small for M. minuta. This may explain the observed gaps in distribution around the British Coast. For example, seabed sediments within the English Channel, where an obvious absence of records exists, show gravels with smaller patches of sandy gravel (British Geological Survey, 1987). This may hint that gravel contents within the region are restrictive for both species. Whilst an absence of records could also be related to several other factors such as a lack of sampling, other magelonid species such as M. mirabilis have been recorded within these areas (e.g., NBN atlas). However, the caveat that sediment type and depth alone cannot explain the extent of a macrofaunal community assemblage is acknowledged and additional physical, chemical and biological parameters must be accounted for (e.g., McBreen et al., Reference McBreen, Wilson, Mackie and Nic Aonghusa2008). Characterising complete habitat type is beyond the scope of this study, whose key objective is to highlight if widely used historical occurrence records from original species descriptions of Magelona are reliable predicators of distribution.
It is proposed that the variation in sediment and abundance between species records may be linked to interspecific variation in behaviour and morphology. Magelona alleni is the only known species in British waters that constructs permanent burrows lined with layered tubes (Mills and Mortimer, Reference Mills and Mortimer2019). On analysis of the grain size of sediments attached to tubes of M. alleni, fine sand was identified as the most common. Even with the caveat that some grains may have been dislodged from the tube during collection, handling and preservation, it is likely that sand grains are required for tube construction. Although other tube-dwelling magelonids are known (Mortimer et al., Reference Mortimer, Cassà, Martin and Gil2012; Mortimer, Reference Mortimer, Purschke, Böggemann and Westheide2019, Mortimer et al., Reference Mortimer, Mills and Gil2022), most species are relatively active, likely burrowing continually through sediments (Jones, Reference Jones1968; Fauchald and Jumars, Reference Fauchald and Jumars1979; Mortimer and Mackie, Reference Mortimer and Mackie2014; Jumars et al., Reference Jumars, Dorgan and Lindsay2015). For example, M. minuta is a non-tubicolous, fairly motile species. The elevated mud contents and smaller grains associated with this species may relate to the small size and notable fragility of the species, compared to M. alleni, a robust and stout species (Parapar et al., Reference Parapar, Mortimer, Capa and Moreira2021; Mortimer et al., Reference Mortimer, Mills and Gil2022). McIntosh (Reference McIntosh1911) described a partiality to fine sand for Magelona, noting ‘larger sharp fragments of coarse gravel and sand might injure either snout or proboscis’ (N.B. referring to the prostomium and burrowing organ), which is likely for M. minuta. Investigation of more tube-dwelling magelonid species would be useful to further explore the relationship between life history and sediment requirements.
The current study has demonstrated that sediment type of benthic infaunal polychaetes is easily misinterpreted, especially in concern to historic works that relied upon visual estimation of sediment grain size. These mistakes are often perpetuated in taxonomic and ecological works, compounding the issue, particularly when that data comes from erroneously identified species. This gives a false interpretation for those using that data for species identification. The example herein that M. minuta has been recorded from sediments ranging from mud to sandy gravel doesn't reflect the full picture that coarser sediments are at the edge of the species’ sediment range. Overall, these findings will add further clarity to habitat and distribution patterns for Magelona species, and additionally highlight the implications of interspecific variation for species within the family. The assumption that magelonids are active burrowers is based upon a small number of species. However, it has become increasingly obvious that this is incorrect for some species. Future work should aim to build upon historical data by using quantitative records of sediment and depth for further Magelona species and analysing additional environmental variables that are known to be important predictors of abundance and distribution.
Conclusions
• Magelona alleni occurs in a range of sediments but needs sand for tube building.
• Grain size strongly influenced the abundance of M. minuta and this may be linked to its small body size.
• Sand is the most important variable for M. alleni, whilst mud and depth are the most important for M. minuta.
• Historic visual assessments have led to misinterpretations of habitat preferences for M. alleni. However, an integrated approach is useful as visual assessments can provide additional data on the heterogeneity of sediments.
• These two species overlap in terms of depth records, but M. alleni is more abundant at less than 60 m, compared to M. minuta present more frequently at over 100 m depths.
• Whilst there is much data openly available concerning records of occurrence for magelonid species, it is important to amalgamate findings so that we can understand more about species-specific environmental parameters.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0025315423000814.
Data availability
The data that support the findings of this study are openly available in the Mendeley data repository at https://doi.org/10.17632/wzt3bsgmky.1.
Acknowledgements
The authors would like to acknowledge Andrew S.Y. Mackie and Teresa Darbyshire for provision of information and feedback.
Author's contributions
KM1 and KM2 equally contributed to creation and design of the study. KM1 contributed to data analyses. KM2 contributed to access and analysis of Magelona spp. records and specimens. Both authors contributed to drafting, commenting, and reading the manuscript.
Financial support
Kimberley Mills is a PhD researcher supported by NERC GW4+. This research received no specific grant from any funding agency, commercial or not-for- profit sectors.
Competing interests
None.







