INTRODUCTION
Water quality and increased sediment resuspension or influx into marine habitats are recurring themes in the recent discussions on environmental changes caused by human activities such as deforestation, desertification, farming practices, alterations to river courses, coastal construction, maintenance dredging and material extraction (e.g. Airoldi, Reference Airoldi2003; McKergow et al., Reference McKergow, Prosser, Hughes and Brodie2005). Possible negative effects of sediments on sessile biota include increased energy cost and maintenance needs due to shading, clogging, smothering, reduced reproduction success, settlement and growth, and may lead to mortality of large parts of attached benthic communities, as their members cannot move into more favourable areas (e.g. Koop et al., Reference Koop, Booth, Broadbent, Brodie, Bucher, Capone, Coll, Dennison, Erdmann, Harrison, Hoegh-Guldberg, Hutchings, Jones, Larkum, O'Neil, Steven, Tentori, Ward, Williamson and Yellowlees2001; Fabricius, Reference Fabricius2005; Weber et al., Reference Weber, Lott and Fabricius2006; Alcolado, Reference Alcolado, Custódio, Hajdu, Lôbo-Hajdu and Muricy2007; Fabricius et al., Reference Fabricius, Golbuu and Victor2007; Bell et al., Reference Bell, McGrath, Biggerstaff, Bates, Bennett, Marlow and Shaffer2015; Schönberg, unpublished literature review). Scientists, monitoring and environmental protection agencies increasingly recognize the need to include locally abundant filter feeders and especially sponges into environmental assessment and management (e.g. Wulff, Reference Wulff2001; Butler et al., Reference Butler, Althaus, Furlani and Ridgway2002; Becerro, Reference Becerro2008; Przeslawski et al., Reference Przeslawski, Ahyong, Byrne, Wörheide and Hutchings2008; Kenchington et al., Reference Kenchington, Cogswell, Lirette and Murillo-Perez2009; De Mestre et al., Reference De Mestre, Maher, Roberts, Broad, Krikowa and Davis2012).
Where growing in high density and diversity sponge communities can become habitat-forming and will crucially contribute to the respective ecosystem by providing many significant ecosystem services such as nutrient cycling and water purification (Díaz & Rützler, Reference Díaz and Rützler2001; Wulff, Reference Wulff2001, Reference Wulff2006; Bell, Reference Bell2008; Pawlik, Reference Pawlik2011), partly with cascading or economic consequences (e.g. Hutchings, Reference Hutchings1990; Pronzato & Manconi, Reference Pronzato and Manconi2008; Marliave et al., Reference Marliave, Conway, Gibbs, Lamb and Gibbs2009). This creates the need to understand the responses of sponges to changed environments and whether other consequences are involved (e.g. Herrnkind et al., Reference Herrnkind, Butler IV, Hunt and Childress1997; Wisshak et al., Reference Wisshak, Schönberg, Form and Freiwald2014). Sponges are presently not adequately studied with respect to sediment stress but are thought to be especially vulnerable (Bell et al., Reference Bell, McGrath, Biggerstaff, Bates, Bennett, Marlow and Shaffer2015; Schönberg, unpublished literature review). This is because some of them are slow-growing and long-lived, and as filter feeders they depend on specific concentrations and qualities of particle suspensions in the ambient water and may be at risk of clogging (Bell et al., Reference Bell, McGrath, Biggerstaff, Bates, Bennett, Marlow and Shaffer2015; Schönberg, unpublished literature review). Where sponges occur in shallow waters close to the coast and urbanized or industrialized areas they are more likely to experience altered sediment conditions (intertidal to shelf edge, such sponge habitats occur for example in Australia, e.g. Schönberg & Fromont, Reference Schönberg and Fromont2012; Canada, e.g. Krautter et al., Reference Krautter, Conway, Barrier and Neuweiler2001; the Caribbean, e.g. Díaz & Rützler, Reference Díaz and Rützler2001; and the eastern Atlantic, e.g. Van Soest et al., Reference Van Soest, Boury-Esnault, Vacelet, Dohrmann, Erpenbeck, De Voogd, Santodomingo, Vanhoorne, Kelly and Hooper2012).
However, not all sponge-sediment relationships are negative. Many sponges habitually experience various natural conditions of sediment exposure and have developed strategies not only to deal with these conditions, but to turn these into an advantage (e.g. Tabachnick, Reference Tabachnick, Reitner and Keupp1991; Cerrano et al., Reference Cerrano, Calcinai, Gioia di Camillo, Valisano, Bavestrello, Custódio, Hajdu, Lôbo-Hajdu and Muricy2007a; De Voogd, Reference De Voogd2012; Schönberg, Reference Schönberg2014). In order to correctly understand sponge stress responses to high turbidity, scouring and sediment deposition one needs to be able to recognize whether sponges are used to or profit from a life in natural relationship with sediments, and to distinguish between stress and adaptation. However, such patterns and relationships are not well understood for sponges in general, vary enormously between different taxa, and related information is difficult to glean from published literature (Schönberg, unpublished literature review). Notwithstanding, the occurrence of such relationships has long been recognized by sponge scientists and is often reflected in the sponges’ scientific names that for example contain the Greek word ‘(ps)ammos’ or the Latin word ‘arena’, both meaning ‘sand’ or ‘sand-like’ (Whitaker, Reference Whitaker2007; Kypros-Net, 2014; Van Soest et al., Reference Van Soest, Boury-Esnault, Hooper, Rützler, De Voogd, Alvarez, Hajdu, Pisera, Manconi, Schönberg, Janussen, Tabachnick, Klautau, Picton, Kelly, Vacelet, Dohrmann and Díaz2015).
The present publication reviews information on sponge-sediment relationships, adds related field observations from sites located along coasts of the northern half of Australia and aims to generate a sound overview, pointing out differences between groups, and other patterns where apparent. While it was impossible to exhaustively screen all species descriptions for mentionings of sediment relationships, a large amount of data is presently summarized, showing which sponge groups are more likely to be adapted in which way. The generated material will inform on, for example, which sponges to expect under which sediment conditions, and provide insights into possible trends in sponge communities, for example with regards to shifts towards sediment-tolerant sponges and why they may be more likely to survive than others. The present review will thus assist the reader (1) to distinguish evidence of natural adaptation to sediment stress, and (2) to make prognoses on survival and recovery potential of certain sponge species in environments where sediment conditions are altered.
MATERIALS AND METHODS
This publication is part of a thorough literature review of a wider scope (Schönberg, unpublished literature review) and was prepared during a project on effects of marine dredging on north-western Australian filter feeders (Schönberg et al., unpublished technical report). In order to inform ongoing investigations the present study somewhat favoured Australian literature and background information. In an effort to offset this bias, Van Soest (Reference Van Soest2015) was consulted, which is a guide to NE Atlantic sponges. Present results and observations are on positive relationships of marine sponges with sediments, while other parts of the literature review will be published elsewhere.
Keyword searches were conducted in the Web of Knowledge (Thomson Reuters, 2014) and Google Scholar (2014), and reference lists of obtained literature were again searched for titles that may be useful. The Systema Porifera (Hooper & Van Soest, Reference Hooper and Van Soest2002) was screened for further information, as was a selection of species descriptions. Nevertheless, the literature search was not exhaustive with respect to species descriptions and is thus not listing every species ever described interacting with sediments, but it is still very detailed and will reveal patterns and identify the main taxon groups to be considered. Latest sponge species validities, names and taxon authorities were confirmed on the World Porifera Database (Van Soest et al., Reference Van Soest, Boury-Esnault, Hooper, Rützler, De Voogd, Alvarez, Hajdu, Pisera, Manconi, Schönberg, Janussen, Tabachnick, Klautau, Picton, Kelly, Vacelet, Dohrmann and Díaz2015), and recent changes in taxonomic nomenclature were included (Morrow & Cárdenas, Reference Morrow and Cárdenas2015). To avoid confusion by moving back and forth between all sponge taxa in this publication, species names are not abbreviated anywhere in the main text. Taxon authorities and systematic allocations are given in full in the Appendices to declutter the text, as well as listing name changes there when citing from older literature in which invalid names were used (traced in Van Soest et al., Reference Van Soest, Boury-Esnault, Hooper, Rützler, De Voogd, Alvarez, Hajdu, Pisera, Manconi, Schönberg, Janussen, Tabachnick, Klautau, Picton, Kelly, Vacelet, Dohrmann and Díaz2015 and Van Soest, personal communication). Van Soest et al. (Reference Van Soest, Boury-Esnault, Hooper, Rützler, De Voogd, Alvarez, Hajdu, Pisera, Manconi, Schönberg, Janussen, Tabachnick, Klautau, Picton, Kelly, Vacelet, Dohrmann and Díaz2015) was also the source for searches for sponge species with scientific names reflecting their relationships with sediments. Names were based on Greek, Latin and Italian words that translate into ‘sediment’, ‘sand’, ‘mud’ etc. Kypros-Net (2014), Whitaker (Reference Whitaker2007) and the Italian dictionary of LEO (2014) were consulted for possible translations.
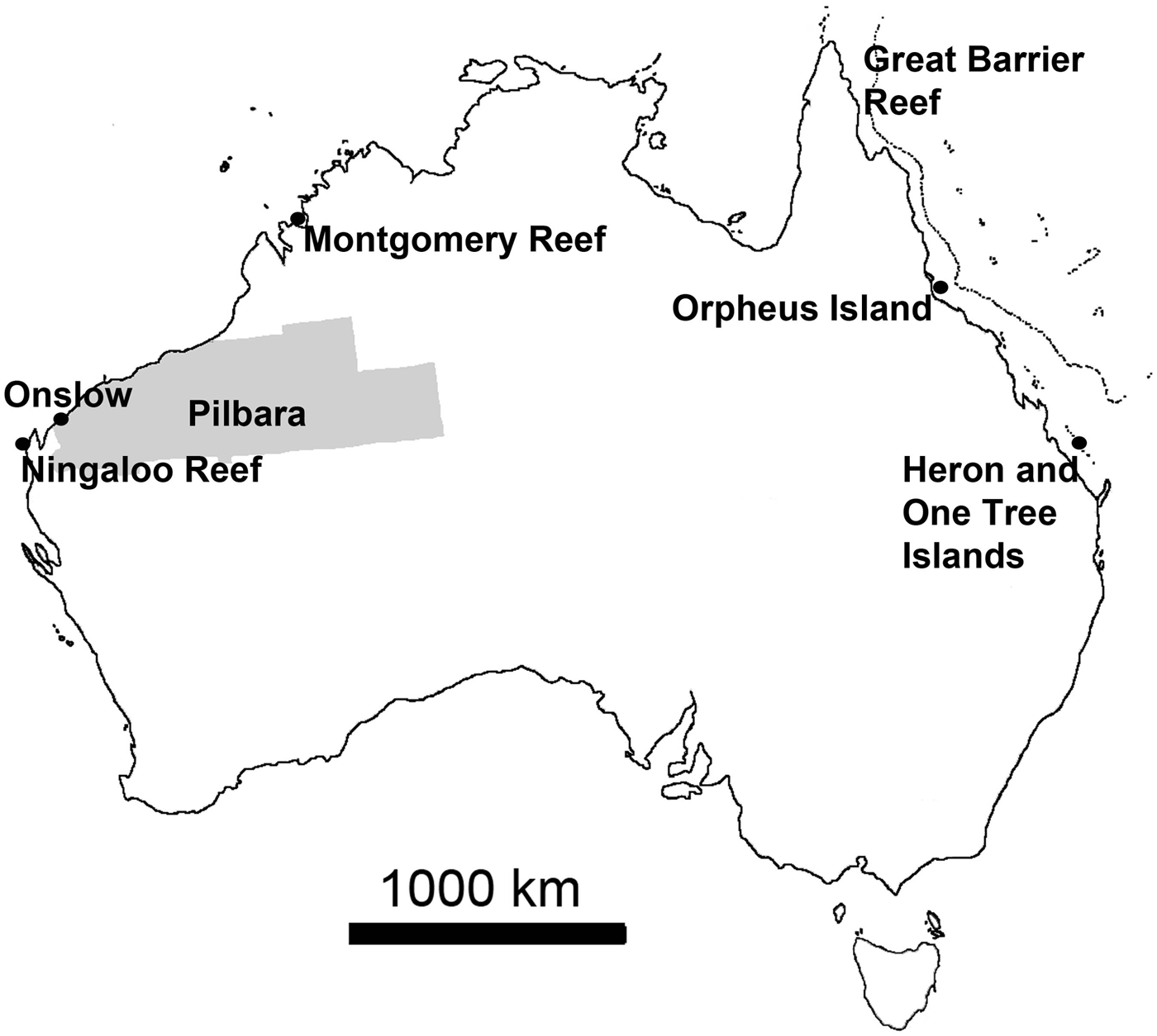
Personal observations obtained from various locations around Australia were used to add information and illustrate some statements (Figure 1). These observations largely relied on field surveys conducted in March 2013 on filter feeder communities near Onslow in the Pilbara, north-western Australia, an area where arid plains of little topography merge into gently sloping coastal flats that are characterized by fluvial and wind-carried influx of fine sediments rich in metals, strong tidal currents and persistently high turbidity, with occasional risk of disturbance events (e.g. Semeniuk, Reference Semeniuk1993; Lafratta et al., unpublished data). Additional information was derived from fieldwork at Ningaloo Reef and the nearby slopes of Carnarvon Shelf Western Australia, where highly diverse and extensive sponge gardens thrive behind the reef edge in ~20 m to the shelf edge in +100 m water depth, the bottoms being largely sandy (e.g. Heyward et al., Reference Heyward, Fromont, Schönberg, Colquhoun, Radford and Gomez2010; Schönberg & Fromont, Reference Schönberg and Fromont2012). Further data were added that originated from fieldwork near Montgomery Reef in the Kimberley (KIM), north-western Australia, a region where the tidal range reaches over 10 m and current speeds two m s−1 (Cresswell & Badcock, Reference Cresswell and Badcock2000; Schönberg, unpublished data). A few examples came from Orpheus and Fantome Islands, central Great Barrier Reef, which have comparatively nutrient-rich and turbid inshore reefs influenced by the Herbert River, with sandy to muddy bottom characteristics (e.g. Anthony, Reference Anthony2000; Schönberg, personal observation), and One Tree Island, southern Great Barrier Reef, which is a platform reef with many microatolls, the historical site of the ENCORE experiments (e.g. Koop et al., Reference Koop, Booth, Broadbent, Brodie, Bucher, Capone, Coll, Dennison, Erdmann, Harrison, Hoegh-Guldberg, Hutchings, Jones, Larkum, O'Neil, Steven, Tentori, Ward, Williamson and Yellowlees2001). Resulting samples were collected under the Commonwealth Environment Research Facilities (CERF) or other projects at AIMS, and are either available from the Western Australian Museum (WAM), through AIMS or the author.

Fig. 1. Map of Australia showing the locations from which new observations were obtained, adding to data from published information. Area highlighted in grey is the Pilbara, along the coasts of which important sponge communities can be found often in very turbid waters and from where most of the recent observations originated.
To tease out patterns of sponge-sediment relationships, retrieved information was synthesized and grouped in tables, partly sorted by taxonomic group and species. This material is provided in the Appendices. Number of species per genus in sediment relationships was then expressed as percentage, and the resulting values were used to calculate means per family, then means per suborder, then per order. In consequence, special sediment relationships per taxon group could be recognized, regardless of the diversity of each taxon. While it needs to be stressed again that not all species in relationship with sediments are listed due to limitations of this review, processing of the data in the described way revealed very clear patterns and preferred strategies by different taxonomic groups.
MARINE SPONGES AND SEDIMENTS – NATURAL AND BENEFICIAL RELATIONSHIPS
Sponges named in reference to their relationships with sediments
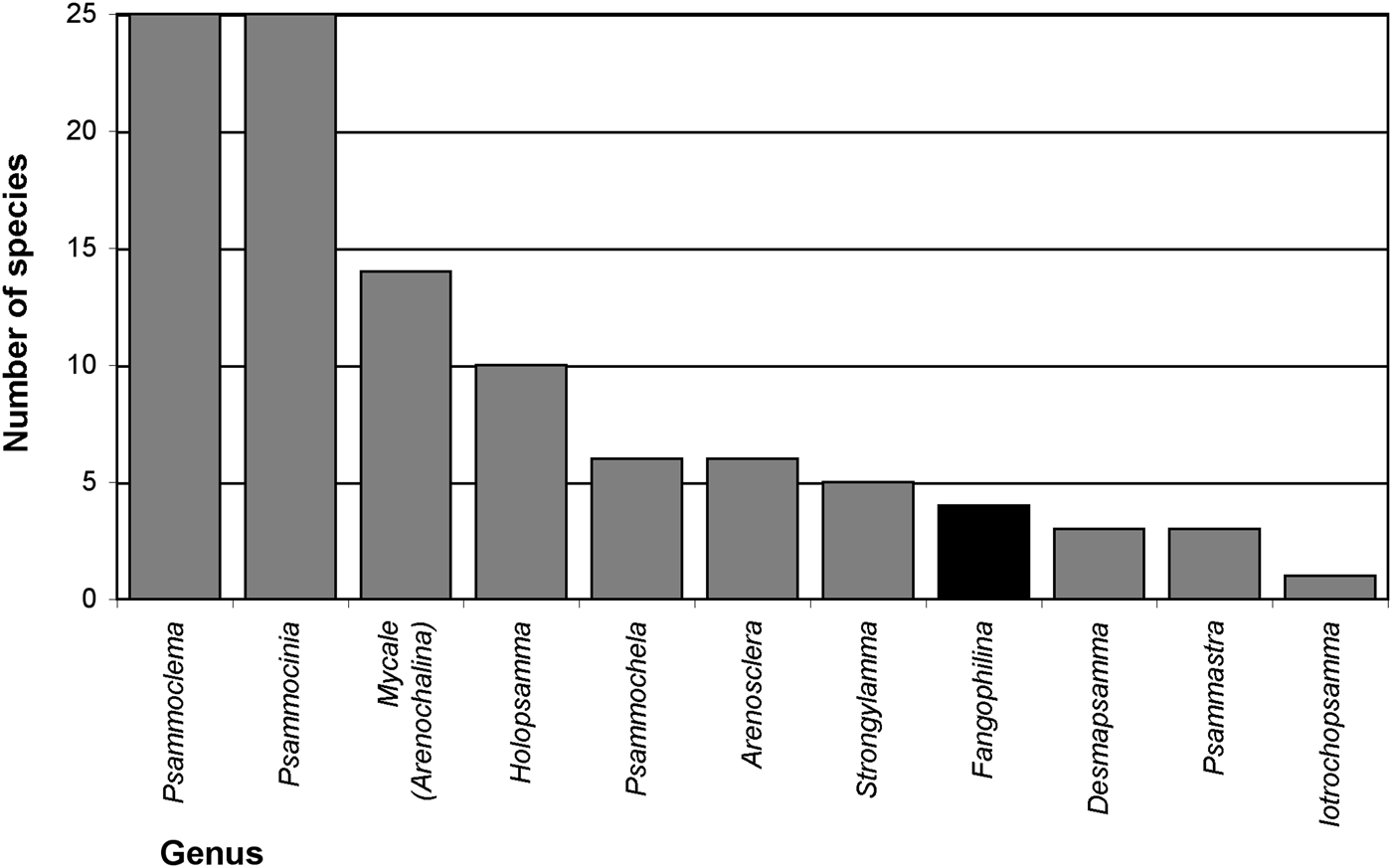
Searching valid scientific names for evidence of relationships of sponges with sediments (Appendix 1), names for 57 distinctive species and three species varieties were found that suggest a natural association with sediments. At higher taxon level 10 genera (Arenosclera, Desmapsamma, Fangophilina, Holopsamma, Iotrochopsamma, Psammastra, Psammochela, Psammocinia, Psammoclema, Strongylamma) and one subgenus (Mycale (Arenochalina)) were named for their association with sediments, with by far most of them referring to sand (e.g. ‘arena’, ‘(ps)ammos’) and only one to mud (‘fango’; Figure 2). All of these are names for marine demosponges, and together they represented 158 valid species, while during the present study 8625 valid sponge species in total, and 8381 marine demosponges were counted (Van Soest et al., Reference Van Soest, Boury-Esnault, Hooper, Rützler, De Voogd, Alvarez, Hajdu, Pisera, Manconi, Schönberg, Janussen, Tabachnick, Klautau, Picton, Kelly, Vacelet, Dohrmann and Díaz2015, as of 30 May 2015 gave 8637 valid sponge species; present count omitted ‘incertae sedis’ etc.). This means 1.8% of marine demosponges or 1.9% of all sponges were named with reference to sediments. Other species were originally described in genera or as species with a reference to sediment in their names, but were later synonymized into other taxa, losing that specification (Appendix 1), e.g. the genera Clathriopsamma and Psammotoxa were included into Clathria (Wilsonella) (Hooper, Reference Hooper, Hooper and Van Soest2002a). As these add up to 25 additional species, this would bring the proportion up to about 2.2% for species of marine demosponges that were at some stage named for their relationship with sediments. In addition, a few genera have no specific name reference but are known to typically contain species in intimate relationship with sediments, e.g. Chondropsis (13 species), Ciocalypta (26 species), Oceanapia (89 species), Tectitethya (five species), Thenea (38 species), and many other tetractinellids, clionaids, many poecilosclerids, dictyoceratids and dendroceratids, as well as many Hexactinellida (Appendix 2). In consequence the proportion of sediment-adapted sponges may likely reach or surpass 10% of all known sponges, and strategies of how to live with sediments appear to play a significant role in sponge biology.

Fig. 2. Marine sponge genera named after their relationships with sand (grey bars) or mud (black bar) and the number of species in these genera. Further invalid names exist, not included in this graph (details listed in Appendix 1).
Utilizing sediment – incorporating it into the body
Cases in which sponges were named for their relationship with sediments are usually those species that are known to actively take up sediments and incorporate them (Figure 2; Appendix 1), and this behaviour was more often recognized compared with other relationships with sediments (Appendix 2). Most studies by far remained descriptive, simply reporting that sediments or coarser foreign materials were found in sponge tissues, where they accumulated in the body of a given species and what kind of materials were present (Appendix 2). Only few investigations aimed at explaining the mechanisms of the incorporation and to what purpose sponges were utilizing sediment. Previous reviews were provided by Rützler (Reference Rützler2004), Cerrano et al. (Reference Cerrano, Calcinai, Gioia di Camillo, Valisano, Bavestrello, Custódio, Hajdu, Lôbo-Hajdu and Muricy2007a) and Giovine et al. (Reference Giovine, Scarfi, Pozzolini, Penna, Cerrano, Müller, Wang and Schröder2013, chapter 6.4), the latter giving a detailed historical overview on relevant research and using Chondrosia reniformis as case example.
Incorporation of very fine sediment can occur but appears to be rare (e.g. Wiedenmayer, Reference Wiedenmayer1989; Van Soest et al., Reference Van Soest, Erpenbeck, Alvarez, Hooper and Van Soest2002; Cerrano et al., Reference Cerrano, Calcinai, Gioia di Camillo, Valisano, Bavestrello, Custódio, Hajdu, Lôbo-Hajdu and Muricy2007a), by far most published accounts are on processes related to coarser material, and it was most commonly recorded as sand grains embedded in spongin fibres (Figure 3A, B, Appendix 2). However, many species descriptions e.g. for dictyoceratids and dendroceratids also speak of incorporation of sponge, ascidian and soft coral spicule debris, of tests or skeletal fragments of diatoms, bryozoans, foraminiferans, calcifying algae, molluscs and corals, all available from local environments in form of sediment (Appendix 2; see also Rützler & Macintyre, Reference Rützler and Macintyre1978; Wiedenmayer, Reference Wiedenmayer1989; Cerrano et al., Reference Cerrano, Pansini, Valisano, Calcinai, Sarà and Bavestrello2004c; Rützler et al., Reference Rützler, Maldonado, Piantoni and Riesgo2007; Cárdenas et al., Reference Cárdenas, Menegola, Rapp and Díaz2009; Łukowiak et al., Reference Łukowiak, Pisera and O'Dea2013). Sponges can contain a mix of these materials and may not prefer one material over another, however, in the majority of known cases they actively choose what they take up and how to use it (e.g. Cerrano et al., Reference Cerrano, Pansini, Valisano, Calcinai, Sarà and Bavestrello2004c, Reference Cerrano, Calcinai, Gioia di Camillo, Valisano, Bavestrello, Custódio, Hajdu, Lôbo-Hajdu and Muricy2007a, Reference Cerrano, Sambolino, Azzini, Calcinai and Bavestrellob). For example, Bavestrello et al. (Reference Bavestrello, Attillo, Benatti, Cerrano, Cattaneo-Vietti, Cortesogno, Gaggero, Giovine, Tonetti and Sarà1995, Reference Bavestrello, Benati, Calcinai, Cattaneo-Vietti, Cerrano, Favre, Giovine, Lanza, Pronzato and Sarà1998a, Reference Bavestrello, Cerrano, Arillo, Calcinai, Lanza, Cattaneo-Vietti, Gaino and Saràb) applied acid purified marine sand to sponges (125–250 µm), laboratory quartz sand (250–500 µm), sand made of organ pipe coral (250–500 µm), biterminate grains (2 mm), sand made of coralline algae (3–5 mm), sponge spicules, chalcedony and opal to Chondrosia reniformis. Grains adhered to the sponge's mucoid surface and were then incorporated (Bavestrello et al., Reference Bavestrello, Benati, Calcinai, Cattaneo-Vietti, Cerrano, Favre, Giovine, Lanza, Pronzato and Sarà1998a, Reference Bavestrello, Cerrano, Arillo, Calcinai, Lanza, Cattaneo-Vietti, Gaino and Saràb, Reference Bavestrello, Benati, Cattaneo-Vietti, Cerrano and Giovine2003; Giovine et al., Reference Giovine, Scarfi, Pozzolini, Penna, Cerrano, Müller, Wang and Schröder2013). Uptake passively depended on supply with regards to grain size and amount available in suspension, but the sponges actively selected for material quality. Lower body parts took up quartz particles, while calcium carbonate grains were incorporated into buds. Detached sponges lost their selectivity for mineralogy.

Fig. 3. Examples of sediment incorporation in demosponges. (A, B) Body reinforcement. (A) Fragments of Chondropsis sp. CERF 1 (CERF-2-46-1-17), showing the grainy, honycomb-like nature of the surface resulting from sediment incorporation. (B) Skeleton preparation of A with almost hexagonal arrangement of sediments held in place by spongin. (C–J) Surface reinforcement of varying thickness – sediment in comparison with spicule use. (C) Psammocinia sp. CERF 1 (CERF-3-99-1-22) with foreign spicules in the uppermost layer and sand grains underneath, overall attaining a very similar structure in crossection as D. (D) Spheciospongia cf. papillosa with proper spicules to structure the skeleton (CERF-3-95-1-21). (E) Psammocinia halmiformis (CERF-2-53-1-3), with one surface having a layer of incorporated spicules, mostly in vertical arrangement, and sediment grains in increasing diameter underneath, and the opposite surface with fine sediments directly at the surface and coarser material deeper in the tissue (see G). (F) Coscinoderma sp. CERF 1 also had a different arrangement of the incorporated material in the opposite surfaces (CERF-2-40-1-34). (G, H) Surface armour of different thickness and structure can provide taxonomic information. (G) Thin surface armour in Psammocinia halmiformis with finer grains on the surface and coarser grains in the fibres (CERF-3-96-1-28). (H) In Dysidea sp. CERF 3 (CERF-2-50-1-7A) sediments in the ectosome and canal walls were finer than in the fibres. Even though this specimen was apparently dead at the time of sampling, the fibre structure was still intact. (I, J) In many geodiid sponges similar surface reinforcement can be created with spicules. (I) Erylus sp. CERF 5 (CERF-3-79-1-1). (J) Erylus sp. WAM SS 2 (CERF-3-82-1-2). All skeletal sections are of sponges sampled from Carnarvon Shelf near Ningaloo Reef, Western Australia and represent Aperio Scanscope images (for further information see Schönberg et al., Reference Schönberg, Fromont, Gomez, Alvarez, Battershill, Goudie, Pisera, Sorokin, Sutcliffe and Case2012).
Some sponges appear to take up sediments in a more passive manner, and form comparatively unconsolidated pockets of sediments within their bodies, or the particles are loosely distributed (e.g. Pulitzer-Finali, Reference Pulitzer-Finali1982; Wiedenmayer, Reference Wiedenmayer1989). Occurrence of sediments in sponge tissues without consolidation by spongin is not well described or understood, and therefore we do not know whether this can occasionally impact negatively on the sponge or whether this is generally a positive process as is incorporating sediments into spongin fibres. In some sponges it may rather be a product of saturation by excessive sediment abundance, when sponges cannot always keep pace with maintenance and cleaning (e.g. Ali, Reference Ali1960). A few cases of pocket-like sediment clusters in Geodia barretti were interpreted as wound reaction, in which ‘unwanted’ sediment settled onto damaged, concave areas that were walled off by forming spicule cortices, and then embedded deeper in the body (Hoffmann et al., Reference Hoffmann, Rapp, Pape, Peters and Reitner2004).
Sediments not bound by spongin or encased by spicule-rich layers may nevertheless serve various specific purposes. A number of species were reported to accumulate, etch and erode such particles: De Laubenfels (Reference De Laubenfels1954) provided an account of dissolving calcareous material in Aplysinella rhax (unconfirmed observation), Calcinai et al. (Reference Calcinai, Cerrano, Bavestrello and Sarà1999) of etched calcareous material in Cliona viridis, Carter (Reference Carter1882) of calcareous material in Suberites spp., Bavestrello et al. (Reference Bavestrello, Attillo, Benatti, Cerrano, Cattaneo-Vietti, Cortesogno, Gaggero, Giovine, Tonetti and Sarà1995), Cerrano et al. (Reference Cerrano, Bavestrello, Cattaneo-Vietti, Giovine, Benatti and Sarà1999) and Giovine et al. (Reference Giovine, Scarfi, Pozzolini, Penna, Cerrano, Müller, Wang and Schröder2013) of quartz in Chondrosia reniformis, and Ise et al. (Reference Ise, Takeda and Watanabe2004) for calcareous material in Spheciospongia inconstans. In the case of Chondrosia reniformis the sponge etched embedded quartz crystals but not hydrated silica such as sponge spicules, chalcedony or opal, clearly showing that some sponges can distinguish between very similar materials and can use them in different, very controlled ways. It is not known whether the sponges derive nutrients or trace elements from etching (Ward & Risk, Reference Ward and Risk1977; Schönberg & Wisshak, Reference Schönberg and Wisshak2012). However, sediment incorporation itself can enhance growth and enable a healthy development (Bakus, Reference Bakus1968; Cerrano et al., Reference Cerrano, Sambolino, Azzini, Calcinai and Bavestrello2007b), and in some sponges fibre growth is hampered without sediment (Teragawa, Reference Teragawa1986a, Reference Teragawab).
Other benefits sponges gain from sediment incorporation are better understood. A sponge body is a composite material made of tissue, organic (spongin) and inorganic skeleton (spicules – or incorporated foreign material). Shifting proportions of those materials will change material properties of a sponge body, with increases in spongin over inorganic skeleton making the sponge more elastic and more resilient, and increases of inorganic skeleton over spongin making the sponge harder and more resistant against physical forces and possibly spongivory (e.g. Palumbi, Reference Palumbi1984, Reference Palumbi1986; Sim & Lee, Reference Sim and Lee1999; McDonald et al., Reference McDonald, Hooper and McGuiness2002; Sim & Lee, Reference Sim and Lee2002; De C. Cook & Bergquist, Reference De, Cook, Bergquist, Hooper and Van Soest2002a; Uriz et al., Reference Uriz, Turon, Becerro and Agell2003). Sediment incorporation will thus reinforce a sponge by shifting the material composition towards inorganic materials. Sediment in Oceanapia spp. can make up over 80% of the dry weight (Bavestrello et al., Reference Bavestrello, Calcinai, Boyer, Cerrano and Pansini2002), a value very similar to proportions of spicules found in sponges with high content of inorganic skeleton (e.g. Rützler & Macintyre, Reference Rützler and Macintyre1978; Desqueyroux-Faúndez, Reference Desqueyroux-Faúndez and Rützler1990; McDonald et al., Reference McDonald, Hooper and McGuiness2002). In some sponges such as Chondropsis spp. the amount of foreign material becomes so large, that they become quite hard and brittle and appear to be built of sand (Figure 3A, B; e.g. Dendy, Reference Dendy1895; Van Soest, Reference Van Soest, Hooper and Van Soest2002a; De Voogd, Reference De Voogd2012).
Based on the above reasoning it is generally accepted that in many sponge species sediment uptake is a strategy to augment or replace spicular skeletons or to obtain surrogate spicules. A number of sponges have their own spicules and incorporate sediments at the same time, with varying ratios of spicules : sediments within the same genus, species or even body region of the same sponge (Appendix 2), further supporting the notion that incorporated sediments and present, innate spicules fulfil similar functions. Sponge taxa such as dictyoceratids and dendroceratids, as well as Holospamma and Psammoclema spp. fully rely on sediment incorporation, and either have reduced or never produced spicules, but often preferentially incorporate foreign spicules and spicule fragments (e.g. various dictyoceratids; Dendy, Reference Dendy and Herdman1905; Wiedenmayer, Reference Wiedenmayer1989; Bergquist & De C. Cook, Reference Bergquist, De, Cook, Hooper and Van Soest2002a, Reference Bergquist, De, Cook, Hooper and Van Soestb; De C. Cook & Bergquist, Reference De, Cook, Bergquist, Hooper and Van Soest2002a, Reference De, Cook, Bergquist, Hooper and Van Soestb, Reference De, Cook, Bergquist, Hooper and Van Soestc, Reference De, Cook, Bergquist, Hooper and Van Soestd; Van Soest, Reference Van Soest, Hooper and Van Soest2002a). Other sponge taxa have low, vestigial amounts of proper spicules that are augmented by significant amounts of foreign material (e.g. Chondropsis, Desmapsamma, Iotrochopsamma, Lissodendoryx, Mycale, Psammochela, Strongylamma; Carter, Reference Carter1882; Dendy, Reference Dendy and Herdman1905; De Laubenfels, Reference De Laubenfels1954; Pulitzer-Finali, Reference Pulitzer-Finali1982; Bergquist & Fromont, Reference Bergquist and Fromont1988; Wiedenmayer, Reference Wiedenmayer1989; Van Soest, Reference Van Soest, Hooper and Van Soest2002a, Reference Van Soest, Hooper and Van Soestb, Reference Van Soest, Hooper and Van Soestc, Reference Van Soest, Hooper and Van Soestd, Reference Van Soest, Hooper and Van Soeste; De Voogd, Reference De Voogd2012), while others yet again may have comparatively low amounts of foreign material added to the existing spicules (e.g. Arenosclera, Clathria, Monanchora, Raspailia, Spheciospongia; Hallmann, 1912; Hechtel, Reference Hechtel1969; Pulitzer-Finali, Reference Pulitzer-Finali1982; Hooper, Reference Hooper, Hooper and Van Soest2002b; Schönberg, personal observation).
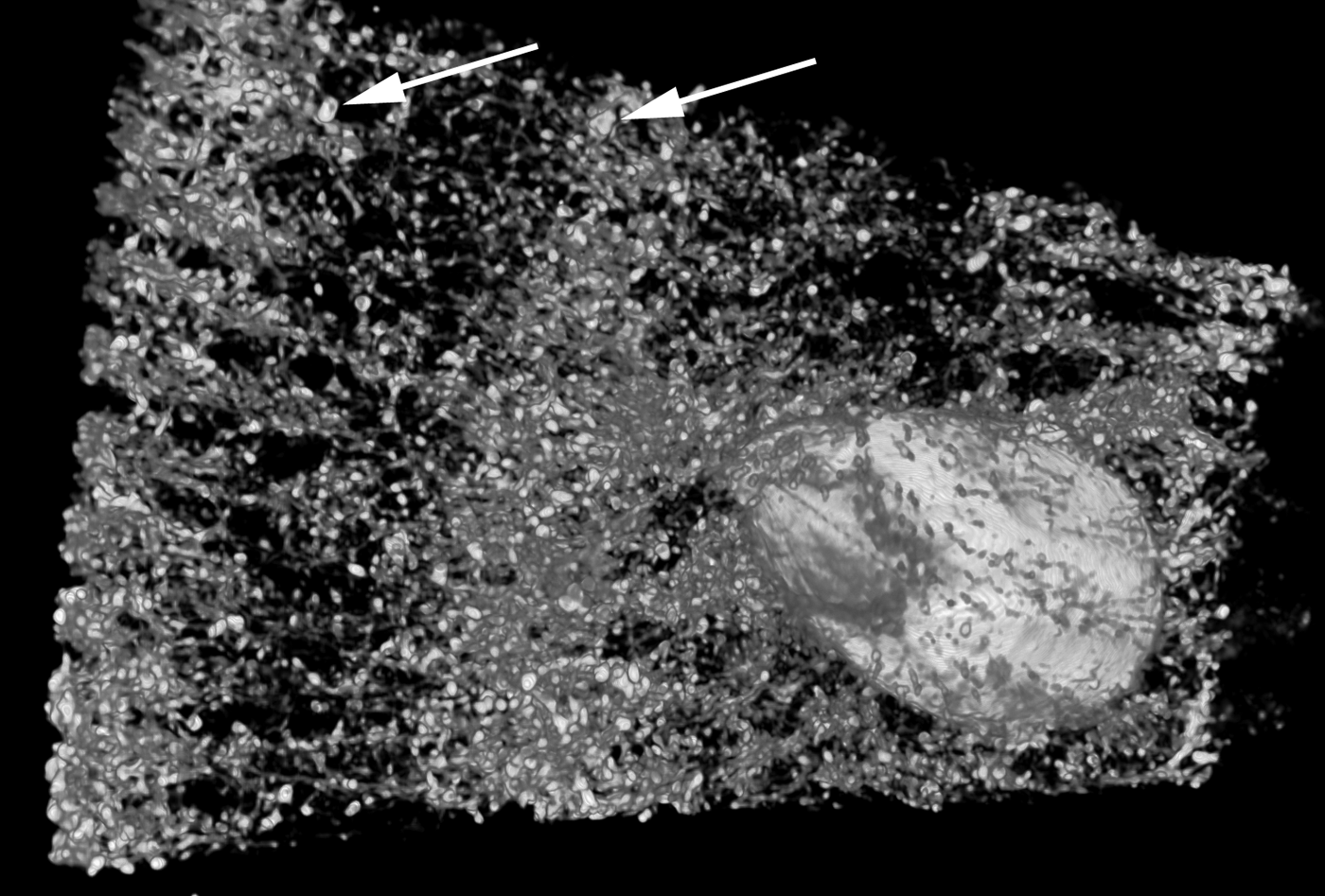
The amount, kind and distribution of embedded materials differ between sponge taxa and can be diagnostic at genus level or for species identification (e.g. Wiedenmayer, Reference Wiedenmayer1989; Bergquist & De C. Cook, Reference Bergquist, De, Cook, Hooper and Van Soest2002a, Reference Bergquist, De, Cook, Hooper and Van Soestb; De C. Cook & Bergquist, Reference De, Cook, Bergquist, Hooper and Van Soest2002a, Reference De, Cook, Bergquist, Hooper and Van Soestb, Reference De, Cook, Bergquist, Hooper and Van Soestc, Reference De, Cook, Bergquist, Hooper and Van Soestd; Van Soest, Reference Van Soest, Hooper and Van Soest2002a; Pronzato et al., Reference Pronzato, Malva and Manconi2004). Most commonly the foreign material is bound into spongin fibres, which are then called ‘cored’, or the particles are held in place by varying amounts of spongin functioning as cement (e.g. Wiedenmayer, Reference Wiedenmayer1989; Bergquist & De C. Cook, Reference Bergquist, De, Cook, Hooper and Van Soest2002a, Reference Bergquist, De, Cook, Hooper and Van Soestb; De C. Cook & Bergquist, Reference De, Cook, Bergquist, Hooper and Van Soest2002a, Reference De, Cook, Bergquist, Hooper and Van Soestb, Reference De, Cook, Bergquist, Hooper and Van Soestc, Reference De, Cook, Bergquist, Hooper and Van Soestd). The details of how foreign material is captured and incorporated by different sponges are largely unknown, but the activity appears to be highly regulated and directed in many species (e.g. Bavestrello et al., Reference Bavestrello, Benati, Calcinai, Cattaneo-Vietti, Cerrano, Favre, Giovine, Lanza, Pronzato and Sarà1998a, Reference Bavestrello, Cerrano, Arillo, Calcinai, Lanza, Cattaneo-Vietti, Gaino and Saràb; Giovine et al., Reference Giovine, Scarfi, Pozzolini, Penna, Cerrano, Müller, Wang and Schröder2013). Amoebocytes are mostly responsible for the transport (e.g. Sarà & Bavestrello, Reference Sarà and Bavestrello1996), and collencytes building the fibre skeleton become involved at the final step of the process, in the same way as if they would cement spicules into place (Uriz et al., Reference Uriz, Turon, Becerro and Agell2003). The end effect is a reinforced, more stable skeleton that in function and appearance resembles a fibre skeleton with coring spicules (Figure 3B–J). Coring and skeleton properties are traditionally studied by light microscopy, and distribution patterns of incorporated materials in three dimensions can nicely be visualized with microcomputed tomography (Figure 4).

Fig. 4. A microcomputed tomography image of Carteriospongia foliascens from the sandy reef flat at Fantome Island, central Great Barrier Reef (fragment ~1.5 × 3 cm2). C. foliascens is a keratose sponge that incorporates sand grains into spongin fibres (arrows pointing towards two large grains). The technique can visualize the embedded sediments and other inclusions such as an associated barnacle without sectioning and clearly shows that sediments are arranged as spicules would have been. Image produced 2013 by E. Büttner and F. Siebler, with friendly permission (Büttner & Siebler, Reference Büttner and Siebler2013).
Sediment-incorporating sponges can therefore reduce efforts for spiculogenesis or have no need to produce their own spicules, which is assumed to save energy. The formation of a single spicule may take 2–7 days (Weissenfels & Landschoff, Reference Weissenfels and Landschoff1977 for the freshwater sponge Ephydatia fluviatilis; Schönberg & Barthel, Reference Schönberg and Barthel1997 for Halichondria panicea), and during or after this process it still needs to be transported to where it will be used (e.g. Custódio et al., Reference Custódio, Hadju and Muricy2002; Uriz et al., Reference Uriz, Turon, Becerro and Agell2003). While sediment incorporation also requires transport, it does not involve formation. Demosponge archaeocytes can move 2–22 µm min−1, and collencytes may move twice as fast, but cells transporting inclusions such as spicules or sediment only develop speeds at the lower end of this range (Kilian & Wintermann-Kilian, Reference Kilian, Wintermann-Kilian, Lévi and Boury-Esnault1979 and Bond, Reference Bond1992 for freshwater sponges; Teragawa, Reference Teragawa1986a, Reference Teragawab for Dysidea etheria; Bavestrello et al., Reference Bavestrello, Benati, Cattaneo-Vietti, Cerrano and Giovine2003 for Chondrosia reniformis; Custódio et al., Reference Custódio, Hadju and Muricy2002 for Mycale spp.). In the calcareous sponge Clathrina clathrus dissociated choanocytes and amoebocytes moved much slower, i.e. at 0.7–2.1 μm min−1 (Gaino & Magnino, Reference Gaino and Magnino1999), and in the hexactinellid Rhabdocalyptus dawsoni organelles were moved at much faster speeds of 0–2.7 µm s−1 (Leys et al., Reference Leys, Mackie and Reiswig2007). It appears to be a logical assumption that building a skeleton consumes significant amounts of energy, and that sediment incorporation in place of spicule production saves on these costs. Provided the existence of an environment that can largely supply materials a given sponge may need, sediment incorporation is assumed to be of significant adaptive advantage.
Other positive effects of sediment incorporation include concurrent cleaning. For example, Columnitis spp. incorporate large amounts of sediments, but have clean surfaces (Sarà & Bavestrello, Reference Sarà and Bavestrello2002). Material incorporation can also have a side-effect of feeding if the material moved from the surface into the sponge can supplement the diet, e.g. with silica. Gaino et al. (Reference Gaino, Bavestrello, Cattaneo-Vietti and Sarà1994) and Cerrano et al. (Reference Cerrano, Calcinai, Cucchiari, Di Camillo, Nigo, Regoli, Sarà, Schiaparelli, Totti and Bavestrello2004a, Reference Cerrano, Calcinai, Cucchiari, Di Camillo, Totti and Bavestrellob) observed the uptake of diatoms into the tissue of Antarctic sponges during times of blooms, presumably to augment the diet during oligotrophic periods and when the surrounding water was silica-depleted, while at other times the diatoms formed an external crust. Similarly, Cárdenas & Rapp (Reference Cárdenas and Rapp2013) found shallow-water specimens of Geodia barretti to incorporate a single species of diatom, but not other diatoms, and this did not occur in sponges in deeper and more silica-rich water.
Another purpose of taking in sediments is anchoring (see section on anchoring). In this case, sand, larger particles, grit, shells or pebbles are predominantly incorporated basally, stabilizing the sponges living on or partly buried in sediments (e.g. Schmidt, Reference Schmidt1870; Rützler, Reference Rützler, Lessios and Macintyre1997; Bavestrello et al., Reference Bavestrello, Calcinai, Boyer, Cerrano and Pansini2002; Cerrano et al., Reference Cerrano, Calcinai, Gioia di Camillo, Valisano, Bavestrello, Custódio, Hajdu, Lôbo-Hajdu and Muricy2007a; Rützler et al., Reference Rützler, Maldonado, Piantoni and Riesgo2007).
Utilizing sediments – incorporating it into superficial parts or accumulating it on the ectosome
Sediment accumulation by sponges is not restricted to incorporation into deeper parts of the sponge body, but some sponges employ sediments to reinforce their surfaces – either by a purely external layer caked over the sponge, by agglutinating coarser particles onto surfaces, or by sediment incorporation into superficial layers. Again, this phenomenon has been described for many species, but functional studies are scarce (Figure 5, Appendix 2).

Fig. 5. Examples of external sediment and particle crusts on sponge surfaces. (A, C) Unidentified spirophorines on Ningaloo Reef with thick external crusts, consolidated by algae, ~5–6 cm in diameter. (B) Cinachyrella cf. CERF 1 from Montgomery Reef, Kimberley, northern Australia (field number KIM-1-1-24), ~7–8 cm in diameter. The pore areas of A–C were kept clean. B–C, Aperio Scanscope images of skeletal sections (for further information see Schönberg et al., Reference Schönberg, Fromont, Gomez, Alvarez, Battershill, Goudie, Pisera, Sorokin, Sutcliffe and Case2012). (D) Cinachyrella sp. CERF 1 from the Carnarvon Shelf showing the thick external sediment crust caught between spicules emerging from the surface (WAM Z45980). (E) In contrast, Tetilla sp. CERF 1 from the Carnarvon Shelf (WAM Z45978) usually had very light surface crusts that were difficult to section. (F) Stelletta sp. CERF 1 from Carnarvon Shelf. (G) Geodia sp. CERF 1 from the Carnarvon Shelf (WAM Z45913). (H) Unidentified tetractinellid sponges from Montgomery Reef with light cover of agglutinated objects (field number KIM-2-3-29), scale coin in background is 2 cm across. F–H with decreasing density of camouflaging attachments.
Sediment incorporation into the ectosome or outer tissue layers of the endosome is well known for various ‘armoured’ fibre sponges (Appendix 2). Depending on species an armour can be thick and coherent or thin, patchy and insubstantial, and this is used as a taxonomic character in the keratose sponges (Figure 3C, E–H; e.g. Pronzato et al., Reference Pronzato, Malva and Manconi2004). Just as for sediments incorporated into the body very different materials are used and act as surrogate spicules, and often in very specific, controlled ways, and they can thus be embedded in a highly ordered or stratified manner (Figure 3E–H). For example in the dictyoceratid Psammocinia halmiformis a surface layer of mostly vertically arranged foreign spicules was found to rest on a layer of sand, and sand of increasingly larger grain size was incorporated into spongin fibres when moving deeper into the sponge (Figure 3E; Schönberg et al., Reference Schönberg, Fromont, Gomez, Alvarez, Battershill, Goudie, Pisera, Sorokin, Sutcliffe and Case2012). A similar arrangement was described from Psammoclema nodosum (Wiedenmayer, Reference Wiedenmayer1989), attesting that this specific, ordered use of foreign material is not restricted to just one taxon.
In contrast to armour, external crusts may passively build up and can be a result of a reduced or selective cleaning effort in which only in- and exhalant areas are kept free of detritus, while the rest of the surface area is allowed to become more or less thickly covered in sediment of variable grain size, debris and sometimes also in algal turf or other epibionts, which can further stabilize the layer (Figure 5A–C; De Laubenfels, Reference De Laubenfels1954; Bavestrello et al., Reference Bavestrello, Benati, Cattaneo-Vietti, Cerrano and Giovine2003; De Voogd & Cleary, Reference De Voogd and Cleary2007; Cárdenas et al., Reference Cárdenas, Menegola, Rapp and Díaz2009; Schönberg et al., Reference Schönberg, Fromont, Gomez, Alvarez, Battershill, Goudie, Pisera, Sorokin, Sutcliffe and Case2012). Trapping sediments on the ectosome is enhanced by strongly hispid surfaces from which spicules extend a significant distance, often involving triaenes that efficiently catch and hold foreign material underneath the forked terminations of their cladomes (Figure 5D–E; e.g. Sarà & Bavestrello, Reference Sarà and Bavestrello1996; De Voogd & Cleary, Reference De Voogd and Cleary2007; Cárdenas et al., Reference Cárdenas, Menegola, Rapp and Díaz2009; Schönberg, Reference Schönberg2014).
Some other sponge species actively form similar, but much coarser external crusts by agglutinating e.g. small pebbles, gravel, coralline algae, diatom and foraminiferan tests onto the ectosome, achieving secure adherence by cell attachment or spongin cementation (Figure 5F–H). This can leave large gaps or largely cover the entire sponge, often attracting epibionts that require hard materials for attachment, thus further obscuring the sponge surface (Figure 5F; Schönberg et al., Reference Schönberg, Fromont, Gomez, Alvarez, Battershill, Goudie, Pisera, Sorokin, Sutcliffe and Case2012 and personal observation for Australian irciniid, spongillid and tretractinellid sponges). Such coarse crusts appear to be relatively common in unattached sponges and may help in avoiding damage when being rolled about or by weighing them down and reducing the risk of being washed around (Figure 5F–H; Rützler et al., Reference Rützler, Maldonado, Piantoni and Riesgo2007 for Iotrochota arenosa).
Any sort of surface armour or crust, in- or externally, provides shading, reinforces the outer sponge surface and reduces the risk of physical damage, including scouring. It may deter predators, especially when the crust is external, thick enough and has an additional camouflaging effect (Figure 5A, C, F–G). External crusts will furthermore reduce effects of desiccation. In fact, intertidal spirophorines are a good example for fine-grained surface crusts that likley reduce evaporation and keep the sponge moist, and many examples were seen in the intertidal near Onslow, north-western Australia (Schönberg, personal observation, in appearance very similar to the sponges shown on Figure 5A, C). A function of forming external crusts or a subdermal armour with coarser sediments to pebble-sized particles is thought to be anchoring (see section on anchoring). None of these functional effects are well studied or understood and are presently based on various incidental observations and assumptions, not on experimental evidence. Nevertheless it stands to reason that such adaptations have a beneficial purpose, and the above suggestions appear to be reasonable.
Colonizing sediments – the need to be anchored
Sponges are usually settling and thriving on hard substrate, and most species cannot colonize sediment, especially not when it is frequently resuspended and moving. However, some specialists have found ways to access these otherwise unsuitable environments. Some may still require first attaching to hard substrates such as mollusc shells, coral or rhodolith rubble or lithic blocks, but eventually they may outgrow that substrate and develop an elongated, vertical body or elevated body parts reaching above the sediments, thus reducing the risk of burial, suffocation and clogging. It is furthermore necessary to be securely anchored and to avoid sinking into the sediments or being uprooted and dislodged e.g. by browsing biota, which is achieved in different ways. Being thus adapted generates a selective advantage, allowing the colonization of habitats that would be inaccessible or hostile to most other sponge species.
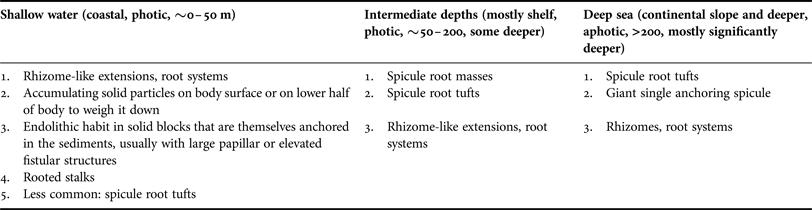
Many deep sea sponges occur and thrive on vast areas of fine sediment. In such environments elevated habit and anchoring is vital and usually facilitated by having an elongated, upright body or a stalk with hexactine or pentactine megascleres extending from the end of the stalk or from the sponges’ sides or bases, a special forte of glass sponges (Figure 6A–D; ‘basalia’; e.g. Gray, Reference Gray1872; Tabachnick, Reference Tabachnick, Reitner and Keupp1991, Reference Tabachnick, Hooper and Van Soest2002a, Reference Tabachnick, Hooper and Van Soestb, Reference Tabachnick, Hooper and Van Soestc; Tabachnick & Menshenina, Reference Tabachnick, Menshenina, Hooper and Van Soest2002a, Reference Tabachnick, Menshenina, Hooper and Van Soestb; Leys et al., Reference Leys, Mackie and Reiswig2007). In these spicules one ray is predominantly developed, while the other ones are reduced (Tabachnick, Reference Tabachnick, Reitner and Keupp1991). This can result in forked, recurving terminations in whirl arrangement, and some spicule shafts are also barbed at the apical ends, increasing the resistance and improving the anchoring properties (Figure 6E; e.g. Gray, Reference Gray1870; Aizenberg et al., Reference Aizenberg, Sundar, Yablon, Weaver and Chen2004; Weaver et al., Reference Weaver, Aizenberg, Fantner, Kisailus, Woesz, Allen, Fields, Porter, Frank, Zok, Hansma, Fratzl and Morse2007). Sponges can be anchored by single spicules (Monorhaphis chuni, Figure 6F–H), a small number of spicules or with one or more spicule tufts that can be made up of thousands of separate spicules (Figure 6A–D; Weaver et al., Reference Weaver, Aizenberg, Fantner, Kisailus, Woesz, Allen, Fields, Porter, Frank, Zok, Hansma, Fratzl and Morse2007). Such spicule bundles can be well preserved in fossils (e.g. Tabachnick, Reference Tabachnick, Reitner and Keupp1991 for Protospongia spp.; Mehl, Reference Mehl1996 described various fossil species; Janussen, Reference Janussen2014 for Hyalonema vetteri). Because basalia are formed differently in different groups they can provide taxonomic information (Tabachnick, Reference Tabachnick, Hooper and Van Soest2002a, Reference Tabachnick, Hooper and Van Soestb, Reference Tabachnick, Hooper and Van Soestc; Tabachnick & Menshenina, Reference Tabachnick, Menshenina, Hooper and Van Soest2002a, Reference Tabachnick, Menshenina, Hooper and Van Soestb).

Fig. 6. Examples of hexactinellid anchoring spicules. (A) Hyalonema owstoni SMF 704 from Sagami Bay, Japan, with long, twisted spicule tuft. (B) Unidentified amphidiscophorid SMF 11254 sampled in 719 m from Tonga Ridge, with short spicule tuft. (C) Euplectella aspergillum. (D) Antarctic Rossella levis from the 2011 ANT XXVII/3 expedition, with numerous smaller root tufts. (E) Apical end of a single anchoring spicule from Euplectella sp. (F) Monorhaphis chuni anchoring spicule SMF 9643. (G) Several Monorhaphis chuni spicules of varying lengths, partly still with tissue. (H) Enlargement of one of the spicules of G, showing that not all layers reach across the entire length of the spicule. Photographs for A, B, D and F were provided by D. Janussen, courtesy of the Senckenberg Museum Frankfurt (SMF). C and G are exhibition specimens of the Western Australian Museum. E courtesy of H. Reiswig.
Asymmetric morphologies and twisted anchoring tufts such as in the Hyalonematidae or for Monorhaphis chuni were explained by environmental effects. The chronic forces of prevailing currents are thought to favour asymmetric growth forms, as they are often exerting shear and drag forces on the sponges, shaping them to turn their inhalants into the prevailing current or to twist their anchoring tuft (Schmidt, Reference Schmidt1870; Levi et al., Reference Levi, Barton, Guillemet, Lebras and Lehuede1989; Tabachnick & Menshenina, Reference Tabachnick, Menshenina, Hooper and Van Soest2002a; Ehrlich et al., Reference Ehrlich, Ereskovskii, Drozdov, Krylova, Hanke, Meissner, Heinemann and Worch2006; Weaver et al., Reference Weaver, Milliron, Allen, Miserez, Rawal, Garay, Thurner, Seto, Mayzel, Friesen, Chmelka, Fratzl, Aizenberg, Dauphin, Kisailus and Morse2010).
Hexactinellid anchoring spicules were found to be especially flexible and fracture-resistant, which in e.g. Euplectella, Hyalonema, Monorhaphis, Rosella and Sericolophus spp. is achieved by an organo-silica layered microarchitecture and by not cementing basalia into a rigid framework as it often occurs in the body (e.g. Sarikaya et al., Reference Sarikaya, Fong, Sunderland, Flinn, Mayer, Mescher and Gaino2001; Aizenberg et al., Reference Aizenberg, Weaver, Thanawala, Sundar, Morse and Fratzl2005; Ehrlich et al., Reference Ehrlich, Hanke, Simon, Goebel, Heinemann, Born and Worch2005, Reference Ehrlich, Ereskovskii, Drozdov, Krylova, Hanke, Meissner, Heinemann and Worch2006; Kul'chin et al., Reference Kul'chin, Bezverbny, Bukin, Voznesensky, Golik, Mayor, Shchipunov and Nagorny2011; Dericioglu et al., Reference Dericioglu, Naumov and Tanaka2012). In Euplectella aspergillum anchoring spicules have a higher organic content and are more hydrated at the core than at the surface (Aizenberg et al., Reference Aizenberg, Sundar, Yablon, Weaver and Chen2004). Monorhaphis chuni is probably the most famous and sought-after sponge for material studies concerning its single, giant anchoring spicule that is the largest biogenic siliceous structure and can become 3 m in length and 8.5 mm in diameter (Figure 6F, G; Tabachnick, Reference Tabachnick, Reitner and Keupp1991, Reference Tabachnick, Hooper and Van Soest2002a; Appendix 2). Several scientists investigated the stability of this layered spicule, finding a ×2.5 fracture resistance of the layered part compared with its unlayered core by crack deflection through the layers, a ×5 breaking resistance compared with pure silica, and a ×10 crack strength compared with synthetic glass (Levi et al., Reference Levi, Barton, Guillemet, Lebras and Lehuede1989; Weaver et al., Reference Weaver, Milliron, Allen, Miserez, Rawal, Garay, Thurner, Seto, Mayzel, Friesen, Chmelka, Fratzl, Aizenberg, Dauphin, Kisailus and Morse2010; Dericioglu et al., Reference Dericioglu, Naumov and Tanaka2012).
Apart from basal spicules many hexactinellids are extremely hispid, with isolated spicules emerging from many places of their bodies and reaching lengths beyond the body diameter (Figure 6B, D). These spicules reduce the risk of sinking into soft substrates if they are angled downwards and away from the sponge in a more or less horizontal alignment (e.g. Tabachnick & Menshenina, Reference Tabachnick, Menshenina, Hooper and Van Soest2002b for Pheronema spp.; Tabachnick, Reference Tabachnick, Hooper and Van Soest2002a, Reference Tabachnick, Hooper and Van Soestb, Reference Tabachnick, Hooper and Van Soestc for lyssacinosid sponges; also known in demosponges: Schmidt, Reference Schmidt1870; Barthel & Tendal, Reference Barthel and Tendal1993; Ilan et al., Reference Ilan, Gugel, Galil and Janussen2003), and act as a filter against clogging around pore areas, where they are usually arranged vertically with respect to the body surface, creating a fence especially around inhalants (Figure 6B; see also Barthel & Tendal, Reference Barthel and Tendal1993 for similar observations in demosponges). Euplectella spp. and other Euplectellidae are tube-shaped and reduce the risk of sediments falling into the atrium by the cemented spicule grid closing off the top (Figure 6C; Tabachnick, Reference Tabachnick, Hooper and Van Soest2002a). Despite all these morphological adaptations only the Hyalonematidae are exclusive soft-bottom inhabitants. The Euplectellinae, Pheronematidae and rossellid genera such as Rossella and Lophocalyx are equipped with spicule tufts and able to live in fine sediments, but overall prefer coarser substrates (Tabachnick, Reference Tabachnick, Reitner and Keupp1991; Tabachnick & Menshenina, Reference Tabachnick, Menshenina, Hooper and Van Soest2002b).
Analogous morphological traits for anchoring can also be found in the demosponges and include spicule tufts and spicule masses. Anchoring spicule masses are predominantly found in the Spirophorina. Cinachyra barbata has a ‘dense spicular basal mass’, becoming larger and more pronounced with age (Van Soest & Rützler, Reference Van Soest, Rützler, Hooper and Van Soest2002). The type specimen was sampled from volcanic mud (Van Soest in Van Soest et al., Reference Van Soest, Boury-Esnault, Hooper, Rützler, De Voogd, Alvarez, Hajdu, Pisera, Manconi, Schönberg, Janussen, Tabachnick, Klautau, Picton, Kelly, Vacelet, Dohrmann and Díaz2015). The genus Craniella has root-like spicule bundles (e.g. Craniella polyura; Van Soest & Rützler, Reference Van Soest, Rützler, Hooper and Van Soest2002). In Fangophilina submersa the tuft is as long as the sponge body (Van Soest & Rützler, Reference Van Soest, Rützler, Hooper and Van Soest2002), and the genus name suggests that it lives in muddy environments. In the intertidal Tetilla euplocamus anchoring is achieved with the help of a long, twisted spicule tuft (Van Soest & Rützler, Reference Van Soest, Rützler, Hooper and Van Soest2002). In shallow water anchoring spicules hold Tetilla mutabilis in place only while they are small, but after reaching a certain size the sponges are dislodged by currents and moved around by tides (McGintie, Reference McGintie1938). Within the astrophorines Thenea spp. have root-like processes made up of flexible spicules holding them in soft substrates in the deep sea (Schmidt, Reference Schmidt1870; Bergquist, Reference Bergquist1968; Steenstrup & Tendal, Reference Steenstrup and Tendal1982; Barthel & Tendal, Reference Barthel and Tendal1993; Maldonado, Reference Maldonado, Hooper and Van Soest2002; Cárdenas & Rapp, Reference Cárdenas and Rapp2012; Figure 7A). Thenea spp. also develop spicule halos or rings radiating out from their horizontal sides that will lie on the sediment surface and prevent the sponge from sinking further into the mud (e.g. Von Lendenfeld, Reference Von Lendenfeld and Chun1907). This strategy was also observed for the apical part of the ocular fistule of the astrophorine, endopsammic Disyringa nodosa (e.g. Von Lendenfeld, Reference Von Lendenfeld and Chun1907) and in Radiella spp. with a ring of spicules framing a disc-like body (Schmidt, Reference Schmidt1870; Barthel & Tendal, Reference Barthel and Tendal1993; Figure 7B). A spicule ring also occurs in some Polymastia spp., but species of this genus typically attach themselves to hard substrate (e.g. Van Soest, Reference Van Soest2015). Another polymastiid, Tentorium semisuberites, lives in the deep sea anchored with root tufts, but also by basal agglutination (Barthel & Tendal, Reference Barthel and Tendal1993; Witte, Reference Witte1996; Pape et al., Reference Pape, Hoffmann, Quéric, Von Juterzenka, Reitner and Michaelis2006). This sponge is so adapted to its life in soft sediments that the buds it forms are usually contained within the mud (also observed in Thenea abyssorum; Barthel & Tendal, Reference Barthel and Tendal1993; Witte, Reference Witte1996).

Fig. 7. Examples of demosponge anchoring. (A) Section through entire specimen of Thenea muricata from Western Norway, embedded in Agar Low Viscosity Resin, sectioned with a diamond wafering blade. The preparation shows the anchoring spicules that emerge from the basal part of the sponge. (B) Radiella hemisphaerica from the Økosystemet 2007 expedition to the Barents Sea (Station 2663). The specimen is pictured from below, showing anchoring rootlets and a ring of spicules protruding from the rim of the disc-like body. Both photographs taken by and courtesy of P. Cárdenas.
While Calcarea are not usually seen as sponges interacting with sediments, some of them also appear to have anchoring structures, including spicule tufts (Schmidt, Reference Schmidt1870 for Amphoriscus synapta and Grantia capillosa; Van Soest et al., Reference Van Soest, Boury-Esnault, Vacelet, Dohrmann, Erpenbeck, De Voogd, Santodomingo, Vanhoorne, Kelly and Hooper2012 for Clathrina lacunosa; Van Soest, Reference Van Soest2015 for Leucilla echina). However, anchoring strategies in the Calcarea are virtually unstudied.
Another means of sponges for anchoring is the development of small ‘roots’, rootlets, rhizomes or rhizoids, i.e. tissue extensions of the body. However, the distinction between small, discrete spicule bundles and small root-like structures is not always clear. Roots are often initially fixed to a solid piece of substrate that can then become covered by sediments (Van Soest et al., Reference Van Soest, Boury-Esnault, Vacelet, Dohrmann, Erpenbeck, De Voogd, Santodomingo, Vanhoorne, Kelly and Hooper2012). In the deep sea this can be seen in demosponges such as Stylocordyla borealis or Cladorhiza spp. that have a body on a stalk with a root system (Barthel & Tendal, Reference Barthel and Tendal1993; Van Soest, Reference Van Soest, Hooper and Van Soest2002f; Van Soest et al., Reference Van Soest, Boury-Esnault, Vacelet, Dohrmann, Erpenbeck, De Voogd, Santodomingo, Vanhoorne, Kelly and Hooper2012) or Chondrocladia (Symmetrocladia) lyra, a stunning, carnivorous sponge from soft abyssal plains (Lee et al., Reference Lee, Reiswig, Austin and Lundsten2012). Many members of the Suberitidae that are able to exist in environments with high sediment pressure have developed rooted stalks. Examples for this group would be Homaxinella balfourensis and Rhizaxinella pyrifera, both living in shallow to intermediate depths and anchored by a system of roots extending from a stalk (Van Soest, Reference Van Soest, Hooper and Van Soest2002g), and Suberites australiensis (Bergquist, Reference Bergquist1968). Even in sponges without stalks roots may most commonly be attached to some fragments of hard substrate that is lodged within the sediments. A good example is the genus Tethya, species of which can occur on soft sediment but mostly appear to prefer attachment to hard substrates or a life on coarse sediments (Carter, Reference Carter1882; Schmidt, Reference Schmidt1870; Bergquist, Reference Bergquist1968; Wiedenmayer, Reference Wiedenmayer1989). De Laubenfels (Reference De Laubenfels1954) counted 5–15 subdividing roots per specimen in the astrophorine Melophlus saranisorum that anchors in rubble and coral sand. Sponges with root systems can thus often attach to hard substrates regardless whether much sediment is present or not, and therefore this adaptation is not exclusively an indicator for existence on soft substrate or an existence in sediment-rich environments.
However, in endopsammic sponges root-like extensions radiating out from the lower body are very common and clearly related to their life buried in sediment, providing additional hold. This type of root can be found e.g. in astrophorine Stelletta and Tribrachium spp., many haplosclerid Oceanapia spp. and some dictyoceratids (Figure 8A–I; Schmidt, Reference Schmidt1870; Werding & Sanchez, Reference Werding and Sanchez1991; Rützler, Reference Rützler, Lessios and Macintyre1997; Cerrano et al., Reference Cerrano, Bavestrello, Boyer, Calcinai, Lalamentik, Pansini, Moosa, Soemodihardjo, Soegiarto, Romimohtarto, Nontji, Soekarno and Suharsono2002, Reference Cerrano, Calcinai, Gioia di Camillo, Valisano, Bavestrello, Custódio, Hajdu, Lôbo-Hajdu and Muricy2007a; Schönberg et al., Reference Schönberg, Fromont, Gomez, Alvarez, Battershill, Goudie, Pisera, Sorokin, Sutcliffe and Case2012 and unpublished technical report).

Fig. 8. Examples for sponge psammobiosis and related anchoring systems. Except for the last, all specimens had obvious fistular structures. (A) Stelletta sp. WAM SS 1 from Carnarvon Shelf had rarely more than one root and only one fistule per specimen. (B, E) Underwater and benchtop views of Onslow Oceanapia cf. sp. PB 7, with roots and some agglutinated material. (C) and (F) Underwater and benchtop views of Onslow Oceanapia sp. WAM SS 13, with roots and agglutinated material. (D, G) Underwater and benchtop views of Onslow Psammocinia cf. bulbosa, with roots and agglutinated material. In B–G the fistules were photosynthetic. (H) This Onslow Oceanapia sp. was deeply buried, with only the far ends of the fistules emerging from the substrate. Only these parts were photosynthetic. (I) Montgomery Reef Tribrachium sp. with anchoring roots and coarse sediment agglutinated to their bodies. (J) Onslow Spheciospongia sp. PB 1. (K) Orpheus Island Siphonodictyon mucosum, living endolithic in buried coral blocks. (L) Onslow Ciocalypta tyleri, attached to a piece of corrugated coral that was buried, with photosynthetic fistules. (M) Montgomery Reef Spheciospongia cf. vagabunda, with much coarse material agglutinated and embedded into the basis. (N) Carnarvon Shelf Polymastia sp., with much coarse material agglutinated and embedded into the basis. Scales on sample labels signify 5 cm.
Anchoring is also achieved without roots when sponges inhabit or attach to rocks buried in the sediment, also often leading to an endopsammic lifestyle. Bioeroding sponges such as Spheciospongia and Siphonodictyon spp. count into this group, as well as suberitid Ciocalypta spp. (Figure 8J–L). Many sponges attach to much smaller particles, however, and accumulate and agglutinate coarse particles to the surfaces buried in sediments and incorporate smaller-grained materials into their lower halves to weigh them down and increase the surface rugosity, thus reducing the risk of being washed out of the stabilizing and protective environment (Figure 8M, N; Cerrano et al., Reference Cerrano, Calcinai, Gioia di Camillo, Valisano, Bavestrello, Custódio, Hajdu, Lôbo-Hajdu and Muricy2007a; see also Schmidt, Reference Schmidt1870 for Chondrosia collectrix; Van Soest & Rützler, Reference Van Soest, Rützler, Hooper and Van Soest2002 for Tethyopsis columnifer).
Living within sediments – psammobiosis
The most extreme strategy of sponges to live with sediments is that of psammobiosis – the ability to live within sediments – previously reviewed by Rützler (Reference Rützler2004) and Cerrano et al. (Reference Cerrano, Calcinai, Gioia di Camillo, Valisano, Bavestrello, Custódio, Hajdu, Lôbo-Hajdu and Muricy2007a). This lifestyle requires a number of morphological adaptations that allow the sponge to retain open water flow and avoid clogging and oxygen depletion, but also to escape dislodgement and being washed out (Ilan & Abelson, Reference Ilan and Abelson1995; Cerrano et al., Reference Cerrano, Calcinai, Gioia di Camillo, Valisano, Bavestrello, Custódio, Hajdu, Lôbo-Hajdu and Muricy2007a). By far most endopsammic sponges have a massive body that is often roughly globular, commonly with elongated, finger-like apical extensions or fistules that reach up into the water column, and usually with rooting structures that reach down and into the sediment (Figure 8). In Oceanapia spp. these root structures are more numerous, longer and slimmer in fine compared with coarse substrate (Cerrano et al., Reference Cerrano, Bavestrello, Boyer, Calcinai, Lalamentik, Pansini, Moosa, Soemodihardjo, Soegiarto, Romimohtarto, Nontji, Soekarno and Suharsono2002, Reference Cerrano, Calcinai, Gioia di Camillo, Valisano, Bavestrello, Custódio, Hajdu, Lôbo-Hajdu and Muricy2007a). Anchoring in endopsammic sponges can be enhanced or replaced by either inhabiting a block of solid material that is buried (Figure 8J, K; Schönberg, Reference Schönberg2000, Reference Schönberg2001 for Siphonodictyon spp.; Rützler, Reference Rützler, Lessios and Macintyre1997; Ise et al., Reference Ise, Takeda and Watanabe2004; Schönberg, personal observation for Spheciospongia spp.), by attaching to rocks (Figure 8L; Ilan & Abelson, Reference Ilan and Abelson1995 for Biemna spp.; Erpenbeck & Van Soest, Reference Erpenbeck, Van Soest, Hooper and Van Soest2002 and Schönberg et al., unpublished technical report, for Ciocalypta spp.) or by incorporating or agglutinating sediments and coarse material to and into the lower half of the body (Figure 8E–I, M, N). The body is entirely or mostly embedded in the sediments (Figure 8B–D, J, K), but the fistular parts always emerge from and are elevated above the sediments (Figure 8A–M). A single case of psammobiosis was found for the Bubarida: Petromica (Chaladesma) ciocalyptoides received its name for its extraordinary resemblance to Ciocalypta, attaching to hard substrate but being covered by a layer of sediment from which it emerges with fistules (Hajdu et al., Reference Hajdu, Peixinho and Fernandez2011; Muricy et al., Reference Muricy, Esteves, Moraes, Santos, da Silva, Klautau and Lanna2014).
The lifestyle has brought about different strategies of water transport. Some species have developed a polar organization, with water taken in at one end expelled at the opposite end. With polarization it appears that most commonly water is inhaled through the fistules and exhaled into the sediments (Rützler, Reference Rützler, Lessios and Macintyre1997 for Cervicornia cuspidifera; Werding & Sanchez, Reference Werding and Sanchez1991 for Oceanapia peltata). However, some species that live in coarse sediments are able to take in the water through the sediments and exhale through the apical fistules (Ilan & Abelson, Reference Ilan and Abelson1995 for Biemna ehrenbergi). Not all Biemna spp. live in coarse sediments, however, some occur in fine sediments (De Laubenfels, Reference De Laubenfels1954 for Biemna fortis, Azzini et al., Reference Azzini, Calcinai, Cerrano, Bavestrello, Pansini, Custódio, Hajdu, Lôbo-Hajdu and Muricy2007 for Biemna megalosigma). While this has not been studied, water flow may be directed from fistules into the sediment in Biemna spp. and other species inhabiting soft sediments. For Tribrachium and Disyringa spp. no field observations are available, and it is not immediately clear in which direction the water is pumped, but the most plausible theory was proposed by Fry & Fry (Reference Fry, Fry, Lévi and Boury-Esnault1979). They suggested that water is drawn in from the sediments and expelled through apical fistules. Many species, however, in- and exhale water only through the apical fistules (e.g. Calcinai et al., Reference Calcinai, Cerrano, Bavestrello and Sarà1999 for Cliona nigricans; Schönberg, Reference Schönberg2000, Reference Schönberg2001 for Siphonodictyon spp.).
The apical, fistular or conical parts of endopsammic sponges can be inhabited by photosynthetic, microbial symbionts such as cyanobacteria or dinoflagellates, while the body, covered in and shaded by sediments is not (Figure 8B–H, L; see also Cerrano et al., Reference Cerrano, Bavestrello, Boyer, Calcinai, Lalamentik, Pansini, Moosa, Soemodihardjo, Soegiarto, Romimohtarto, Nontji, Soekarno and Suharsono2002, Reference Cerrano, Calcinai, Gioia di Camillo, Valisano, Bavestrello, Custódio, Hajdu, Lôbo-Hajdu and Muricy2007a; Schönberg, personal observations from Orpheus and One Tree Islands for Spheciospongia spp.). Bergquist & Fromont (Reference Bergquist and Fromont1988) suggested that this may also be the case for Biemna rufescens. In Oceanapia aff. sagittaria exposed parts are biochemically defended, while body parts embedded in sediments are less well defended, with the defence potential decreasing with distance into the sediment (Schupp et al., Reference Schupp, Eder, Paul and Proksch1999). This confirms how well the sponge body is protected against spongivory when being endopsammic.
Benefits of psammobiosis thus include shading, shelter from spongivores, diseases and desiccation, and a reduced risk to be removed and damaged during storms (Ilan & Abelson, Reference Ilan and Abelson1995; Schupp et al., Reference Schupp, Eder, Paul and Proksch1999; Cerrano et al., Reference Cerrano, Bavestrello, Boyer, Calcinai, Lalamentik, Pansini, Moosa, Soemodihardjo, Soegiarto, Romimohtarto, Nontji, Soekarno and Suharsono2002, Reference Cerrano, Calcinai, Gioia di Camillo, Valisano, Bavestrello, Custódio, Hajdu, Lôbo-Hajdu and Muricy2007a). All these advantages are similar to what endolithic sponges experience (Schönberg, Reference Schönberg2000, Reference Schönberg2001; Schönberg & Wisshak, Reference Schönberg and Wisshak2012; Schönberg & Burgess, Reference Schönberg and Burgess2013), and many psammobiotic sponges belong to taxonomic groups that contain numerous endolithic bioeroders: clionaids and Siphonodictyon spp. (Appendix 2).
Returning favours – binding, ventilating and producing sediments
Sponges that accumulate significant amounts of particles or are capable of colonizing soft and loose sediments and rubble will stabilize these materials through binding them in different ways. Many fast-growing and especially creeping, ramose sponges appear to attach themselves to almost anything in their path of growth, not only including fixed objects, but also loose stones, pebbles, grit and even sediments (e.g. Carter, Reference Carter1882 for Callyspongia tenerrima, Mycale (Mycale) laevis and Spongia (Spongia) officinalis; Bergquist, Reference Bergquist1970 for Ciocalypta polymastia). Sponges are not typically settling directly on soft sediments, but where they do they are often encrusting or have a broad base that binds the sediment (Schmidt, Reference Schmidt1870 for Columnitis squamata; Hechtel, Reference Hechtel1969 for Bubaris spp.; Wiedenmayer, Reference Wiedenmayer1989 for Polymastia crassa; Barthel & Tendal, Reference Barthel and Tendal1993 for Hymedesmia (Hymedesmia) stylata; Schönberg et al., Reference Schönberg, Fromont, Gomez, Alvarez, Battershill, Goudie, Pisera, Sorokin, Sutcliffe and Case2012 for Polymastia spp.). An in situ experiment in the Caribbean involving piles of coral rubble with and without the addition of sponge fragments of Niphates erecta and Aplysina spp. confirmed that sponges quickly attached to loose rubble, binding it and thereby retaining the shape of the piles, while rubble without sponges was moved and redistributed by currents (Biggs, Reference Biggs2013).
Sponges that agglutinate rubble, gravel and coarser sediments for anchoring also consolidate material (Rützler, Reference Rützler, Lessios and Macintyre1997; Cerrano et al., Reference Cerrano, Bavestrello, Boyer, Calcinai, Lalamentik, Pansini, Moosa, Soemodihardjo, Soegiarto, Romimohtarto, Nontji, Soekarno and Suharsono2002, Reference Cerrano, Calcinai, Gioia di Camillo, Valisano, Bavestrello, Custódio, Hajdu, Lôbo-Hajdu and Muricy2007a). This strategy is very common in endopsammic sponges, including the deep sea sponge Forcepia topsenti (Barthel & Tendal, Reference Barthel and Tendal1993), and various shallow-water Spheciospongia and Oceanapia spp. (Figure 8E, F, H, I, M). However, agglutination is not restricted to endopsammic sponges, and some sponges living on top of the substrate were seen with coarse materials adhering to their surface (e.g. Klitgaard & Tendal, Reference Klitgaard and Tendal2004 for Geodia barrettii).
In the context of substrate consolidation hexactinellid spicule mats should also be mentioned. They can build up in areas densely inhabited by glass sponges (e.g. Bett & Rice, Reference Bett and Rice1992; Leys et al., Reference Leys, Mackie and Reiswig2007). When glass sponges die the tissue disintegrates, but their skeletons are often left behind, and over time can create a firm substrate onto which new sponges and other biota recruit and attach (Barthel, Reference Barthel1992).
Sponge-sediment interactions furthermore include the improvement of substrate conditions. Endopsammic sponges such as Oceanapia spp. that inhale water through the fistules and exhale it into the sediments are thought to ventilate the ground around their bodies, as well as contributing nutrients they excrete (Schmidt, Reference Schmidt1870; Werding & Sanchez, Reference Werding and Sanchez1991; Rützler, Reference Rützler, Lessios and Macintyre1997). But even the reverse direction of water flow increases the water transport through the sediments surrounding the body of an endopsammic sponge (Fry & Fry, Reference Fry, Fry, Lévi and Boury-Esnault1979; Ilan & Abelson, Reference Ilan and Abelson1995), making the sediment more amenable to other infauna.
Some demosponge orders of the Heteroscleromorpha also contain sponges that produce sediments by bioerosion, either by expelling silt-sized chips or by weakening the substrate: The well-known groups are the Clionaidae and Spirastrellidae in the Clionaida, the Thoosidae in the Astrophorina, and the genus Siphonodictyon in the Haplosclerida, but odd sponges from other taxa have also been found to contribute, from the haplosclerids, poecilosclerids, suberitids, tethyids and tetractinellids (e.g. Carter, Reference Carter1882; Annandale, Reference Annandale1915; Schönberg, Reference Schönberg2000; Calcinai et al., Reference Calcinai, Bavestrello, Cerrano and Sarà2001; Van Soest & Hooper, Reference Van Soest, Hooper, Hooper and Van Soest2002; Bertolino et al., Reference Bertolino, Pica, Bavestrello, Iwasaki and Calcinai2011; Rützler et al., Reference Rützler, Piantoni, van Soest and Díaz2014). De Laubenfels’ (Reference De Laubenfels1954) account of Aplysinella rhax dissolving shells is unconfirmed, but is not unreasonable when considering how wide the ability to bioerode is spread across different demosponge orders.
Patterns in sponge-sediment relationships
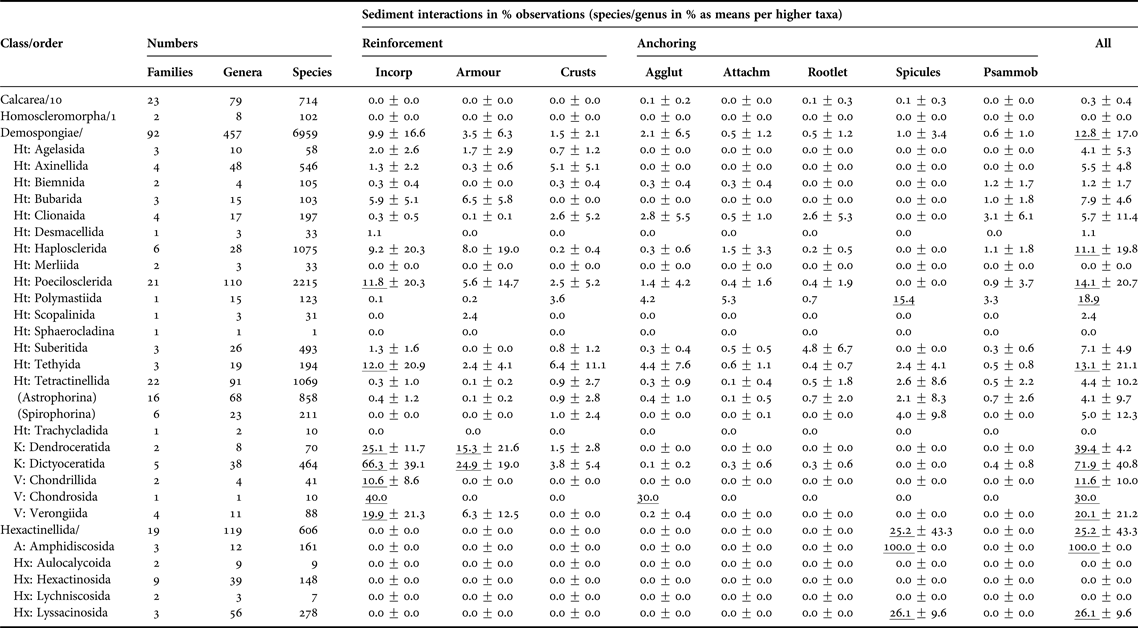
Overall, this non-exhaustive, but nevertheless very substantial literature search found that 10% of all Porifera are well-adapted to a life with sediments, with the Demospongiae and Hexactinellida being best represented (Table 1, Appendix 2). The other two sponge classes, the Calcarea and the Homoscleromorpha, are either not commonly adapted to sediments, avoid them or are inadequately studied.
Table 1. Taxonomic patterns of sponge-sediment relationships in marine environments.

All sediment interactions were quantified as counts of species per genus expressed as per cent to standardise for variable diversities (see first three columns). Percentages were then averaged across families, then across suborders and then orders. Due to the scarcity of accounts for the Calcarea and Homoscleromorpha no information at order level is provided. ‘Incorporation’ includes any sediment uptake into the body, regardless whether cemented into fibres or not. ‘Armour’ is here defined as coherent or interrupted surface crusts contained by the sponges’ ectosome, while ‘crusts’ are any external sediment layers, including pebbly agglutinations to the surface. ‘Anchoring’ was distinguished between ‘by agglutination’ (in contrast to ‘crust’ restricted to lower half of body), ‘by attachment to buried rocks or by endolithic lifestyle’, ‘by tissue rootlets’, and ‘by anchoring spicules’. The last column refers to psammobiosis, i.e. living buried or partially buried in sediments. Counts per taxon for calculations were obtained June 2015 from Van Soest et al. (Reference Van Soest, Boury-Esnault, Hooper, Rützler, De Voogd, Alvarez, Hajdu, Pisera, Manconi, Schönberg, Janussen, Tabachnick, Klautau, Picton, Kelly, Vacelet, Dohrmann and Díaz2015), taking into account Morrow & Cárdenas (Reference Morrow and Cárdenas2015). Only valid, fully accepted taxa were included, not using any observations on OTUs, ‘sp.’, ‘incertae sedis’, ‘nomen quierendum’ or ‘nomen nudum’ species, which is why present taxa counts may vary slightly in comparison to those given by Van Soest et al. (Reference Van Soest, Boury-Esnault, Hooper, Rützler, De Voogd, Alvarez, Hajdu, Pisera, Manconi, Schönberg, Janussen, Tabachnick, Klautau, Picton, Kelly, Vacelet, Dohrmann and Díaz2015) at the same time. Accounts are incomplete and somewhat biased towards groups where relationships with sediments were included into diagnoses. Means reaching >10% are underlined. The error value provided is standard deviation, and where the last step in calculations included only one taxon, no error is provided.
Sediment relationships – Agglut, agglutination; Attachm, attachment; Incorp, incorporation; Psammob, psammobiosis; Subclasses – A, Amphidiscophora; Ht, Heteroscleromorpha; Hx, Hexasterophora; K, Keratosa; V, Verongimorpha.
The Calcarea are not well presented here, because there is almost nothing known about their ways to interact with sediments. It appears that a few species are able to develop anchoring systems, which may usually manifest themselves as small spicule tufts (Schmidt, Reference Schmidt1870 for Clathrina lacunosa and Amphoriscus synapta; Carter (Reference Carter1886) for Clathrina osculum and Ascaltis cavata; Van Soest et al., Reference Van Soest, Boury-Esnault, Vacelet, Dohrmann, Erpenbeck, De Voogd, Santodomingo, Vanhoorne, Kelly and Hooper2012 for Grantia capillosa; Van Soest (Reference Van Soest2015) for Leucilla echina).
I could not find any published accounts on homoscleromorph sediment relationships. A Plakortis sp. in a very turbid environment near Onslow, north-western Australia lived attached to stalks of gorgonians and thus removed itself from risks related to burial and settlement on soft substrate (Schönberg et al., personal observation). Homoscleromorph sponges also commonly encrust vertical surfaces, thereby avoiding effects of settling sediments (e.g. De Moraes, Reference De Moraes2011).
In the demosponges many sediment-related strategies were represented, and this was the most diverse class with respect to such relationships (Appendix 2). Certain taxa had their own special approaches to sediment (Table 1). The orders that had the most species with obvious sediment relationships belonged to the keratose and verongiimorph sponges, strongly relying on incorporation of sediments, in the body and often also in the surface (armour). De Voogd (Reference De Voogd2012) referred to the poecilosclerid ‘sand sponges’ as the most important sediment-incorporating sponges. They certainly utilize sand in concentrations that are not usually found in any other sponges. However, when considering diversity and numbers of species per taxon, the keratose and verongiimorph sponges play a more significant role (Table 1). Apart from a larger number of species per genus this is also evidenced by the comparatively low error values, which indicate that the trait is widely and evenly distributed, unlike for most other taxa that generated standard deviations 2–3× larger than the calculated means. Some tethyids, poecilosclerids and haplosclerids, and a few bubarids also commonly incorporate sediments (Table 1). In addition some families stand out as well: the Chondropsiae (80%, thus reaching levels as known for dictyoceratid families), the Callyspongiidae (51%), the Desmacididae (50%), the Tethyidae (37%), the Phellodermidae (25%), the Iotrochotidae (18%), the Isodictyidae (17%), the Tedaniidae (15%) and the Myxillidae (13%); and most of these take up sediments into the body as well as into their surface. Sediment-incorporating orders did not usually rely on other strategies, except for the tethyids, which are also known for developing external sediment crusts. External crusts appear to be uncommon in sponges, although in most orders at least some taxa occurred that had crusts. Specialist families employing crusts were the desmacidids and the tethyids (each 19%), the isodictyids (17%), the ancorinids (11%), the stelligerids and the irciniids (each 12%), and some tetillids, especially Cinachyrella. In the tetractinellids crusts often consisted of comparatively coarse material agglutinated to the outer surfaces (especially Stelletta and Geodia spp., see Appendices 2 and 3), which rarely occurred in other groups.
Anchoring strategies for the colonization of sediments were less common than sediment-incorporation. The Chondrosida stood out as the order with proportionally the most species using reinforcement of the basal parts by incorporation and agglutination (Table 1), and some isolated families also employed this approach: Isodictyidae (17%), Tethyidae (13%), Clionaidae (11%), Iotrochotidae (10%) and the genus Oceanapia.
Attachment to substrate buried in sediments appeared to be rare throughout the Porifera, and in none of the orders or families was a level of 10% of the species reached. However, 8% of the Phloeodictyidae, 7% of the Acarnidae and 5% of the Polymastiidae were commonly attached in this way. Especially bioeroding sponges seemed to benefit from this strategy, as several species of Cliona, Spheciospongia and Siphonodictyon are known to hide their main body volume in calcareous rock that can be buried in the sediments to a depth of around 10 cm, while fistules rise above the sediment surface (e.g. Rützler, Reference Rützler1971; Schönberg, Reference Schönberg2001 and personal observation on the Great Barrier Reef and at Okinawa).
Many of the above taxa may further improve anchoring by the development of rootlets, either by attaching them to stones or by spreading them into the sediments: the Clionaidae (10%), the Iotrochotidae (8%) and the Geodiidae (7%). Stalked forms such as in the Stylochordylae can also colonize sedimented areas by attaching rootlike basal parts to rock covered in sediments (13%), but they are more typical to occur on firm, unburied substrate.
Anchoring without the involvement of specialized spicules is mainly realized by psammobiotic species that can tolerate at least partial cover with sediments. This extreme adaptation is very rare in sponges, not spread through entire orders, and mostly occurs in only a few families: the Isodictyidae (17%), the Clionaidae (12%), the Ancorinidae (11%), the Phloeodictyidae (4%), the Desmanthidae and the Polymastiidae (each 3%), the Biemnidae and the Petrosiidae (each 2%). Psammobiotic ancorinids include some iconic and little-studied genera such as Disyringa and Tribrachium (see Fry & Fry, Reference Fry, Fry, Lévi and Boury-Esnault1979).
Anchoring with spicules is also rare in the Demospongiae and can either be realized by spicule bundles (Theneidae 33%, Tetillidae 24%) or by ring-like fringes of lateral spicules that may prevent sinking into the sediment (e.g. Polymastiidae 15%, especially Radiella). In the genus Thenea both strategies are effectively employed.
Apart from specialist groups, some taxa had only a low frequency of species in sediment relationships, but demonstrated their versatility by adapting several sediment-related strategies, including both reinforcement and anchoring behaviour: the Biemnida, the Clionaida, the Haplosclerida, the Suberitida and the Tetractinellida (Table 1). Some orders with low sediment interaction only accumulated sediments in or on their bodies, but showed no evidence of anchoring: the Agelasida, the Axinellida, the Bubarida (but with one rare occurrence of possible psammobiosis), the Desmacellida, the Scopalinida. For the Merliida, the Sphaerocladina and the Trachycladida no references were found that described their behaviour with respect to sediments.
The highest overall proportion of sediment-adapted sponges by class was found in the Hexactinellida, in which one entire order is represented by species anchoring in soft substrates with spicule tufts (Amphidiscosida), and many lyssacinosid species contribute (Table 1, Appendix 3). Some other groups or species were able to exist on soft sediments with the help of rootlets, which mostly have to be attached to hard substrate that may later become embedded in sediments. Nevertheless, hexactinellids are not known for any other sediment relationships than anchoring and tall growth (elongated body shape, pronounced stalks), removing their inhalant areas from risks associated with resuspended sediments, and are thus very limited with regards to adaptations to sediments.
Some bathymetric patterns were recognized in the way sponges anchored themselves in sediments (Table 2). Shallow-water sponges mostly employed rootlets, agglutination, incorporation and attachment to buried objects, and can be found in settings with coarse as well as with fine sediments. Demosponges anchoring themselves with megascleres were reported from any water depth but appeared to be most common in intermediate depths on the continental shelf. Anchoring with spicules is most common in the deep sea and was mostly represented by hexactinellids.
Table 2. Summary of sponge anchoring strategies and their bathymetry. Most data are available from shallow depths between 0 and 100 m, mostly only to 20 m. See text and Appendices for references.

Challenges and relevance for environmental assessment and monitoring
We still do not know enough about the tolerance levels and responses of sponges to sedimentation and turbidity in order to generate adequate recommendations for environmental assessment and monitoring. Moreover, many surveys are conducted by only addressing functional guilds (e.g. filter feeders). Where Porifera are noted, they are commonly lumped together as one group and usually represent only a few large, conspicuous species (e.g. Al-Zibdah et al., Reference Al-Zibdah, Damhoureyeh and Badran2007; Bridge et al., Reference Bridge, Done, Beaman, Friedman, Williams, Pizarro and Webster2011). In more detailed approaches the data analyses increasingly rely on morphologies or where sampling is possible, on species counts, biodiversities and abundances (e.g. Schönberg & Fromont, Reference Schönberg and Fromont2012; Przeslawski et al., Reference Przeslawski, Alvarez, Battershill and Smith2014). However, we have virtually no knowledge about the biology of the sponges that are reported, especially when collections retrieve a high percentage of undescribed material that naturally is not kept alive. In order to fully understand responses of habitat-forming sponge communities to suspended particle concentrations, scouring and sediment deposition, we need more information, e.g.: What morphologies occur, how different species feed, which species are photosynthetic, how are they naturally adapted to sediments, and what are their vulnerabilities to sediment pressures? The World Porifera Database presently recognizes 8637 valid and described species of sponges (Van Soest et al., Reference Van Soest, Boury-Esnault, Hooper, Rützler, De Voogd, Alvarez, Hajdu, Pisera, Manconi, Schönberg, Janussen, Tabachnick, Klautau, Picton, Kelly, Vacelet, Dohrmann and Díaz2015), and understanding the biology of a representative subset would require an immense amount of research effort. When concentrating on a restricted area, this effort may potentially be pared down. For example, only some 50 valid species are listed on the World Porifera Database for Western Australia (Van Soest et al., Reference Van Soest, Boury-Esnault, Hooper, Rützler, De Voogd, Alvarez, Hajdu, Pisera, Manconi, Schönberg, Janussen, Tabachnick, Klautau, Picton, Kelly, Vacelet, Dohrmann and Díaz2015), an area in which the biology of sponges is not adequately studied. Of these 50 reported species maybe fewer than 10% may be common or occurring at several sites and thus representative (Schönberg & Fromont, Reference Schönberg and Fromont2012). Five species could easily be subjected to a number of physiological and ecological studies, generating data for the most common sponges of Western Australia and assisting environmental protection agencies to generate suitable recommendations for habitats in waters that are often sponge-dominated (Heyward et al., Reference Heyward, Fromont, Schönberg, Colquhoun, Radford and Gomez2010; Schönberg & Fromont, Reference Schönberg and Fromont2012). However, it is not that easy, because many more species exist in Western Australia that are only registered as operational taxonomy units (distinction between species, but no full identification), i.e. we have neither a publicly available taxonomic description for them nor an understanding of their biology (e.g. Hooper et al., Reference Hooper, Hall, Ekins, Erpenbeck, Wörheide and Jolley-Rogers2013). Schönberg & Fromont (Reference Schönberg and Fromont2012) estimated that over 500 species may exist on the shelf along Ningaloo Reef, but in reality many more species exist in north-western Australia (Fromont et al., unpublished technical report). The dichotomy between described species and true local diversities can thus be crippling with respect to science, management and conservation efforts (e.g. Hooper et al., Reference Hooper, Hall, Ekins, Erpenbeck, Wörheide and Jolley-Rogers2013). Considering the vast diversity of biological responses in sponges and the variability that can occur even within the same species, at this stage predictions or recommendations with regards to responses of sponges to sediments need to remain simple and preliminary and will have to be regarded with caution.
Shallow-water habitats in which sediment damage to sponges would be expected to be negligible may include (Schönberg & Fromont, Reference Schönberg, Fromont, Radford and Ridgway2014):
• Where sediment settles out: Areas with a high proportion of erect and endopsammic sponges that can avoid smothering or are already highly adapted to live with sediments (e.g. tube-shaped- and stalked sponges, other erect forms, fistular sponges such as Oceanapia and Siphonodictyon spp.);
• Where coarser sediments are resuspended: Areas dominated by sediment-incorporating sponges (e.g. keratose sponges, myxillinids) – but they may still suffer when fine sediments are resuspended;
• Where finer sediments are resuspended: Areas with a high percentage of sponges with external sediment crusts (e.g. Cinachyrella spp.).
Indicators that suggest sediment stress in sponges related to patterns recognized during the present literature review may include:
• A high percentage of incorporation of sediments into sponges that are not typically known to do this (e.g. Calcarea, Homoscleromorpha, Hexactinellida, Polymastiida, Suberitida, Trachycladida);
• Finding sediments incorporated in the tissues in species that predominantly incorporate into spongin;
• Occurrence of sediments in body parts of sponges that are vital to their function and survival and that are usually free of sediments (pore areas, choanocyte chambers, canal system);
• A high percentage of incorporation of very fine sediments in sponges;
• A large number of sponges with their outer surfaces covered with sediments, especially species that usually have clean surfaces;
• Higher than usual evidence of necrosis that may have been caused by smothering or clogging, and disease as sponges fail to keep surfaces clean.
For respective studies micro-computed tomography of tissue samples could be employed for qualitative and quantitative studies. This has been trialled for Great Barrier Reef sponges (Büttner & Siebler, Reference Büttner and Siebler2013; Figure 4) and is presently further developed by Strehlow et al. (personal communication).
CONCLUSIONS ON SPONGE-SEDIMENT RELATIONSHIPS
Not all sponges suffer from effects of sedimentation and turbidity, and about 10% of all marine sponges are equipped for such conditions by having specific morphological adaptations, which is often recognized in their scientific names. Thereby sponges can live without harm in sediment-dominated areas, anchoring and elevating themselves above the soft substrate or being buried within. Sediments are used by many sponges to their advantage by reinforcing body structures and gaining shelter from potentially harmful environmental conditions and spongivory. Such behaviour is thought to save energy and to create selective advantages. Recognizing taxa with respective adaptations and tolerances will be important for assessment of anthropogenic disturbances, management and conservation.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0025315415001411.
ACKNOWLEDGEMENTS
This publication is part of a literature review for project 6.1 on dredging effects on north-western Australian filter feeders administered through the Western Australian Marine Science Institution, led by R. Jones, R. Masini, C. Sim and K. Crane and is co-funded by The Australian Institute of Marine Science and Chevron Australia. This work would have been impossible without the assistance of library staff at the Australian Institute of Marine Science and the University of Western Australia. Various sponge colleagues provided even more literature that was not easily accessible, discussed some ideas with me from their angle of expertise or helped with some difficult taxonomic issues, which was very much appreciated: R. van Soest, N. Boury-Esnault, P. Cárdenas, F. Hoffmann and A. Pisera. Photographs of hexactinellid sponges were taken at the Western Australian Museum, while H. Reiswig, D. Janussen and P. Cárdenas sent me more photographs by email, and C. Goecke the Senckenberg collection details (I acknowledge the Senckenberg Museum Frankfurt for use of this material). Information on Western Australian sponges was derived from the following projects: Ningaloo data – Predicting biodiversity using biological and physical surrogates, The Marine Biodiversity Hub, Commonwealth Environment Research Facilities Program (CERF); Kimberley data – Sponge trophodynamics in NW Australia, Patterns and processes in tropical marine biodiversity, Australian Institute of Marine Science; data from Onslow – Defining thresholds and indicators of filter feeder responses to dredging-related pressures, Project 6.3 of the Western Australian Marine Science Institute Dredging Node; all other data – Monitoring and detecting changes in bioerosion, Sustainable use of NW marine ecosystems, Australian Institute of Marine Science. J. Fromont and O. Gomez identified species from Western Australia (as well as participants of the CERF workshop for specimens from Carnarvon Shelf, see Schönberg et al., Reference Schönberg, Fromont, Gomez, Alvarez, Battershill, Goudie, Pisera, Sorokin, Sutcliffe and Case2012). The crew of RV Solander and staff at the research stations at Orpheus and One Tree Island, colleagues and field assistants are acknowledged for field support. Skeletal sections of sponges and respective images were made by S. Tecchiato at the Western Australian Museum and digitalized at the Centre for Microscopy, Characterisation and Analysis (CMCA) at the University of Western Australia with the Scanscope digital slide scanner, assisted by P. Rigby. E. Büttner and F. Siebler tested the use of microcomputer tomography for various aspects of visualizing and quantifying sponge biology, also at the CMCA, assisted by T. Abel. E. Voultsiadou checked the translations of the Greek names making some helpful suggestions. A. Martins Sequeira, H. Reiswig and P. Cárdenas read the manuscript before submission and helped in improving it.