Introduction
Top predators, such as some shark species, play a critical role and have been demonstrated to help maintain the balance within their respective food webs (Burkholder et al., Reference Burkholder, Heithaus, Fourqurean, Wirsing and Dill2013; Hussey et al., Reference Hussey, MacNeil, Siple, Popp, Dudley and Fisk2015). In some locations or even on a global scale, the retention of certain shark species is prohibited (e.g. Convention on International Trade in Endangered Species of Wild Fauna and Flora; Tolotti et al., Reference Tolotti, Filmalter, Bach, Travassos, Seret and Dagorn2015); however, in other locations, the increased demand and exploitation rates, both legal and illegal, raise concern about the health of certain shark populations (Shivji et al., Reference Shivji, Chapman, Pikitch and Raymond2005). Understanding the population structure, range, and abundance of a particular shark population can provide fisheries managers with essential information regarding the sustainable or unsustainable exploitation of that particular population (e.g. Chapman et al., Reference Chapman, Feldheim, Papastamatiou and Hueter2015; Pérez-Jiménez and Mendez-Loeza, Reference Pérez-Jiménez and Mendez-Loeza2015) and consequently can provide regulations to help restore the population to pre-existing levels (Hoffmann et al., Reference Hoffmann, Hilton-Taylor, Angulo, Böhm, Brooks, Butchart, Carpenter, Chanson, Collen, Cox, Darwall, Dulvy, Harrison, Katariya, Pollock, Quader, Richman, Rodrigues, Tognelli, Vié, Aguiar, Allen, Allen, Amori, Ananjeva, Andreone, Andrew, Ortiz, Baillie, Baldi, Bell, Biju, Bird, Black-Decima, Blanc, Bolaños, Bolivar-G, Burfield, Burton, Capper, Castro, Catullo, Cavanagh, Channing, Chao, Chenery, Chiozza, Clausnitzer, Collar, Collett, Collette, Fernandez, Craig, Crosby, Cumberlidge, Cuttelod, Derocher, Diesmos, Donaldson, Duckworth, Dutson, Dutta, Emslie, Farjon, Fowler, Freyhof, Garshelis, Gerlach, Gower, Grant, Hammerson, Harris, Heaney, Hedges, Hero, Hughes, Hussain, Icochea M, Inger, Ishii, Iskandar, Jenkins, Kaneko, Kottelat, Kovacs, Kuzmin, La Marca, Lamoreux, Lau, Lavilla, Leus, Lewison, Lichtenstein, Livingstone, Lukoschek, Mallon, McGowan, McIvor, Moehlman, Molur, Alonso, Musick, Nowell, Nussbaum, Olech, Orlov, Papenfuss, Parra-Olea, Perrin, Polidoro, Pourkazemi, Racey, Ragle, Ram, Rathbun, Reynolds, Rhodin, Richards, Rodríguez, Ron, Rondinini, Rylands, Sadovy de Mitcheson, Sanciangco, Sanders, Santos-Barrera, Schipper, Self-Sullivan, Shi, Shoemaker, Short, Sillero-Zubiri, Silvano, Smith, Smith, Snoeks, Stattersfield, Symes, Taber, Talukdar, Temple, Timmins, Tobias, Tsytsulina, Tweddle, Ubeda, Valenti, Paul van Dijk, Veiga, Veloso, Wege, Wilkinson, Williamson, Xie, Young, Akçakaya, Bennun, Blackburn, Boitani, Dublin, da Fonseca, Gascon, Lacher, Mace, Mainka, McNeely, Mittermeier, Reid, Rodriguez, Rosenberg, Samways, Smart, Stein and Stuart2010).
In the instance of white sharks (Carcharodon carcharias), there are nine known C. carcharias subpopulations: Southern-Western Australia (Bruce, Reference Bruce2016; McAuley et al., Reference McAuley, Bruce, Keay, Mountford, Pinnell and Whoriskey2017), Western North Atlantic (WNA, Skomal et al., Reference Skomal, Braun, Chisholm and Thorrold2017), Northeastern Pacific (NEP, Domeier and Nasby-Lucas, Reference Domeier and Nasby-Lucas2013), Eastern Australian and New Zealand (Bruce et al., Reference Bruce, Harasti, Lee, Gallen and Bradford2019), Mediterranean (Leone et al., Reference Leone, Puncher, Ferretti, Sperone, Tripepi, Micarelli and Tinti2020), South African (Kock et al., Reference Kock, O'Riain, Mauff, Meÿer, Kotze and Griffiths2013), Northwest Pacific (Tanaka et al., Reference Tanaka, Kitamura, Mochizuki and Kofuji2011), South American Atlantic (Cione and Barla, Reference Cione and Barla2008), and South American Pacific (Bustamante et al., Reference Bustamante, Vargas-Caro and Bennett2014; Figure 1). Due to its low rebound potential and current estimated population status, C. carcharias is listed as vulnerable on a global scale (Rigby et al., Reference Rigby, Barreto, Carlson, Fernando, Fordham, Francis, Jabado, Liu, Marshall, Pacoureau, Romanov, Sherley and Winker2019) according to the International Union for the Conservation of Nature Red List. The white shark is particularly vulnerable to exploitation since this species is characterized by having low fecundity, producing an average of 2–14 pups per litter (Francis, Reference Francis, Klimley and Ainley1996; Uchida et al., Reference Uchida, Toda, Teshima, Yano, Klimley and Ainley1996), slow growth (Wintner and Cliff, Reference Wintner and Cliff1999; Natanson and Skomal, Reference Natanson and Skomal2015), and late sexual maturity (Natanson and Skomal, Reference Natanson and Skomal2015), which is estimated to be >3.80 m total length (TL) for males and >4.50 m TL for females (Francis, Reference Francis, Klimley and Ainley1996; Pratt, Reference Pratt, Klimley and Ainley1996; Wintner and Cliff, Reference Wintner and Cliff1999). However, despite international protection of C. carcharias, this species exhibits transoceanic movements, making it susceptible to a variety of anthropogenic sources of mortality (e.g. Bonfil et al., Reference Bonfil, Meÿer, Scholl, Johnson, O'Brien, Oosthuizen, Swanson, Kotze and Paterson2005). While extensive tagging efforts have provided a baseline understanding of the movements of most C. carcharias populations (e.g. Skomal et al., Reference Skomal, Braun, Chisholm and Thorrold2017; Bruce et al., Reference Bruce, Harasti, Lee, Gallen and Bradford2019), there remains uncertainty as to the structure, size, and range of most subpopulations, particularly in the Northwest Pacific population (Christiansen et al., Reference Christiansen, Lin, Tanaka, Velikanov, Mollet, Wintner, Fordham, Fisk and Hussey2014).
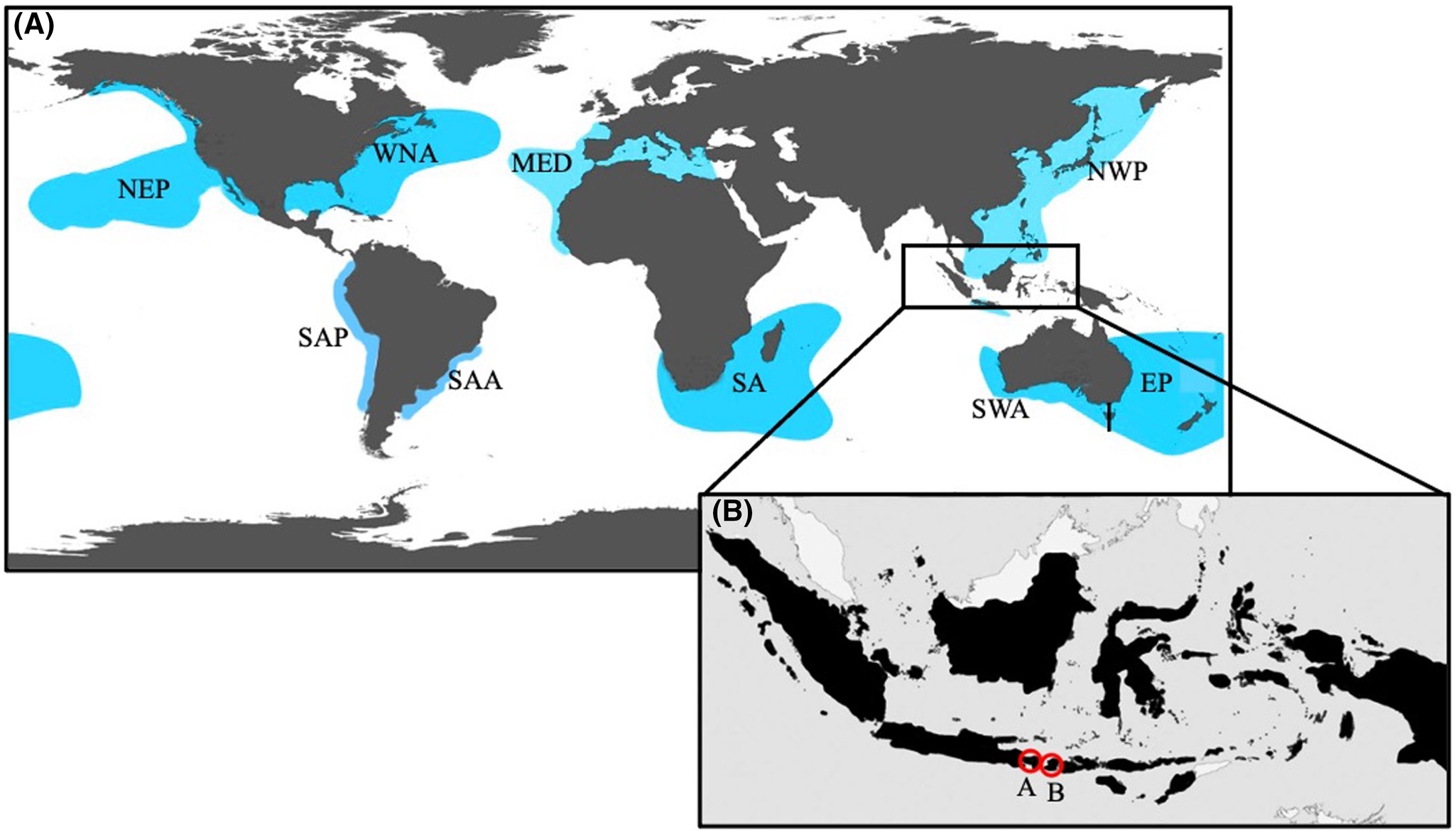
Figure 1. (A) Map illustrating the relative locations of white shark (C. carcharias) populations (modified from Huveneers et al., Reference Huveneers, Apps, Becerril-García, Bruce, Butcher, Carlisle, Chapple, Christiansen, Cliff, Curtis and Daly-Engel2018). The populations are: Northeastern Pacific (NEP), Western North Atlantic (WNA), Mediterranean (MED), South African (SA), Northwest Pacific (NWP), Southern-Western Australian (SWA), Eastern Australian and New Zealand (EA), South American Pacific (SAP), and South American Atlantic (SAA). The rectangular region highlights the location of Indonesia. (B) Map representing Indonesia (area in black), as well as the white shark (C. carcharias) sightings within the region: (A) Nusa Penida in Bali, Indonesia (8°44′S; 115°32′E; 5.0 m female) and (B) Lombok, Indonesia (08°51′S; 118°18′E; 6.0 m male).
In recent years, sparse sightings of C. carcharias have occurred within Indonesian waters (Fahmi and Dharmadi, Reference Fahmi and Dharmadi2014; Coleman, personal communication, 2019). More specifically, in 2019 within Nusa Penida in Bali, Indonesia (8°44′S; 115°32′E; Figure 1A), scuba divers encountered an approximately 5.0 m TL female C. carcharias (Figure 2A). In 2013, a ca. 6.0 m TL male C. carcharias (Figure 2B) was captured by demersal longline fishermen off Dompu in West Nusa Tenggara, Indonesia (08°51′S; 118°18′E; Figure 1B). Although multiple C. carcharias subpopulations have been identified in the waters surrounding Indonesia, including the Northwest Pacific, the Southern-Western Australian, and Eastern Australian and New Zealand, there exists no record of any tagged sharks associated with these stocks travelling into these tropical waters. Therefore, using a sample collected from the upper lateral tooth of the C. carcharias captured in the Lesser Sunda region and comparing it to the publicly available mitochondrial DNA (mtDNA) database (GenBank®; Bethesda, MD, USA), the present study aimed to determine which subpopulation this C. carcharias may be most closely related to, thus suggesting site of origin.

Figure 2. Recent and documented white shark (C. carcharias) sightings within Indonesian waters. (A) An estimated 5.0 m TL female C. carcharias sighted by divers at Nusa Penida in Bali, Indonesia in 2019 © Two Fish Divers. (B) A 6.0 m TL male C. carcharias captured by fishermen in eastern Lombok, Indonesia in 2013 © Fahmi and Dharmadi (Reference Fahmi and Dharmadi2014). (C) Upper lateral tooth collected from the white shark C. carcharias landed in eastern Lombok, Indonesia in 2013. © Fahmi and Dharmadi (Reference Fahmi and Dharmadi2014).
Methods
In 2013, a C. carcharias was captured by demersal longline fishermen and brought to a fishing market where scientists were able to collect both photographic evidence and one upper lateral tooth (Fahmi and Dharmadi, Reference Fahmi and Dharmadi2014; Figure 2C). At the Centre Laboratory of Sequencing, National Research and Innovation Agency in West Java, Indonesia from February to April 2023, DNA was extracted from the 10-year-old upper lateral tooth using a DNeasy Tissue Kit (Qiagen™) and the addition of ethylenediaminetetraacetic acid to the incubation stage (modified from Swig and Collier, Reference Swig and Collier2021). A polymerase chain reaction (PCR) was used to amplify about 500 bp of the mtDNA D-loop region. The two primers for the D-loop region were: GWDL-L (TTG ACG CGA TCA AGG ACG AA) and GWDL-H (CAA ACA TCC ATT TGG CCT TC) (Pardini et al., Reference Pardini, Jones, Scholl and Noble2000). A total volume of 24 μl included 12.5 μl MyTaq™ HS Red Mix PCR kit, 1 μl of the 5 μM pre-mixed forward and reverse primers (IDT™), 3 μl of a standardized amount (10–15 ng μl−1) of DNA, and 7.5 μl of sterile water. The PCR profile included a 1 min initial denaturing step at 95°C, 40 cycles at 95°C for 15 s, 55°C for 30 s, 72°C for 1 min, and a 5 min final extension step at 72°C (Tanaka et al., Reference Tanaka, Kitamura, Mochizuki and Kofuji2011). The negative control (Borneo river shark [Glyphis fowlerae]) was used to validate the GWS primer. To confirm species, two additional primers were run (i.e. Elas02; Taberlet et al., Reference Taberlet, Coissac, Hajibabaei and Rieseberg2012 and Leray-XT; Wangensteen et al., Reference Wangensteen, Palacín, Guardiola and Turon2018) by following the PCR mix and PCR profile (Prasetyo et al., Reference Prasetyo, Murray, Agung, Sales, McDevitt and Mariani2023). After PCR, each replicate was visually examined on a 1.2% agarose gel and stained with GelRed® Nucleic Acid Gel Stain. Each well received 2 μl of the sample and a 100 bp ladder from Invitrogen™ was included in the gel for reference.
PCR products are then purified using a QIAquick PCR Purification Kit (Qiagen). The purification products were then Sanger sequenced using the Genetic Analyzer 3500 (ABI™) at the Sequencing Centre, Genomic Laboratory, National Research and Innovation Agency, Bogor, Indonesia. All positions containing gaps and missing data were eliminated from the dataset (‘Complete deletion’ option). After trimming and quality control have been conducted, the sequences (GenBank accession no. PP078820) were then aligned using software UGENE (Okonechnikov et al., Reference Okonechnikov, Golosova, Fursov and Team2012) and compared with the National Center for Biotechnology Information (NCBI) sequences for the Japanese (GenBank accession nos. AB598394–AB598397) (Tanaka et al., Reference Tanaka, Kitamura, Mochizuki and Kofuji2011) and Australian–New Zealand (GenBank accession nos. KY067571–KY067590) subpopulations. To further examine the phylogenetic tree, maximum-likelihood was constructed using the neighbour-joining method with the Kimura-2 model for 1000 bootstrap replications in MEGA-X with default parameters. To improve the clarity of the phylogenetic tree, FigTree v1.4.4 and Inkscape were used.
Results
The modified DNA extraction protocol successfully resulted in the DNA extraction from the 10-year-old upper lateral tooth. However, the DNA concentration was very low by 7.75 ng μl−1. Despite the low DNA concentration, the three primer sets successfully amplified the targeted sequence. A short-targeted sequence was chosen to anticipate fragmented DNA extracted from the tooth (GenBank accession no. PP078820; Figure 3).

Figure 3. Gel electrophoresis image of the amplified sequence associated with the 6.0 m TL white shark (C. carcharias) captured in 2013 by demersal longline fishermen off of eastern Lombok, Indonesia. The 100 bp DNA Ladder by Geneaid™ was used (lane 1). Experiments were conducted using three distinct primer sets: Leray-XT (Wangensteen et al., Reference Wangensteen, Palacín, Guardiola and Turon2018), Elas02 (Taberlet et al., Reference Taberlet, Coissac, Hajibabaei and Rieseberg2012), and GWS (Pardini et al., Reference Pardini, Jones, Scholl and Noble2000). Each primer set was designed to target specific regions, namely cytochrome c oxidase I region (COI; lane 2), 12S ribosomal RNA region (12S; lane 3), and D-loop region (D-loop; lane 4), respectively. The Leray-XT and 12S primers amplified sequences were used to confirm the identification of species, whereas the GWS primer sequences were utilized for further investigation. The negative control (Borneo river shark [G. fowlerae]) was used to validate GWS primer (lane 5).
Sanger sequencing confirmed that the tooth was from a C. carcharias based on three different primers (Table 1). Moreover, the neighbour-joining tree associated with the mtDNA in the D-loop region (about 400–500 bp) revealed that the shark has a closer or nested clade with the Australian subpopulations rather than the Japanese subpopulation (Figure 4) with a bootstrap value at 93%. Although these results suggest the site of origin for this individual C. carcharias is from the Australian subpopulations, the available GenBank database does not differentiate between the two subpopulations (i.e. Southern-Western and Eastern Australian and New Zealand). Therefore, determining the specific Australian subpopulation that this C. carcharias was most closely associated with was not possible.
Table 1. BLAST result using the NCBI for the 6.0 m TL male white shark (C. carcharias) captured in eastern Lombok, Indonesia
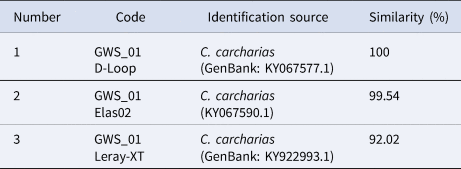
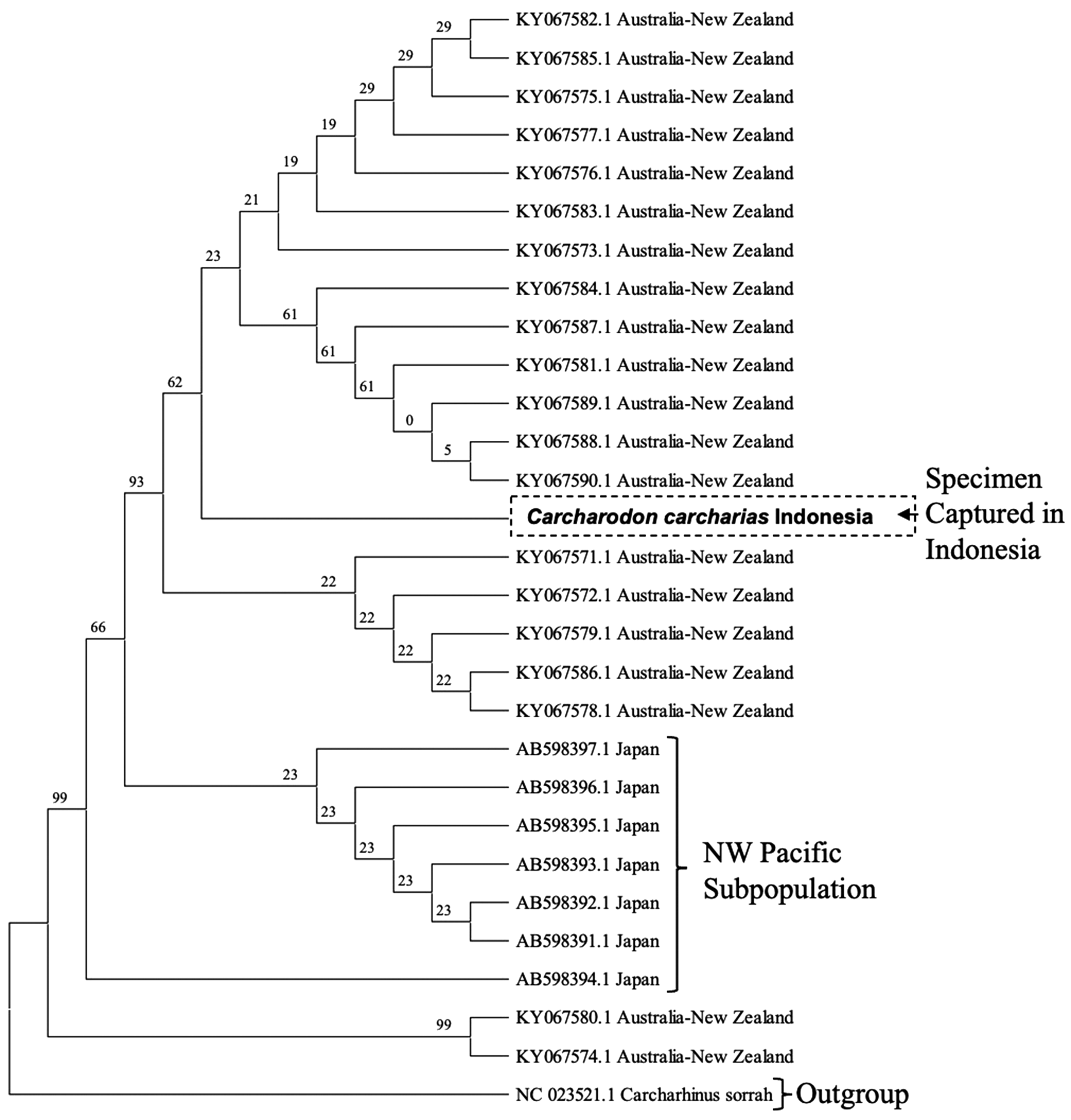
Figure 4. Genetic relationship of 29 white sharks (C. carcharias) from the Northwest Pacific (Japan) and Australia–New Zealand subpopulations in comparison to the Indonesian specimen using the D-loop region inferred using the neighbour-joining method with the Kimura-2 model for 1000 bootstrap replications. There was a total of 412 positions in the final dataset. Bootstrap support values are shown near internal nodes.
Discussion
Understanding the migratory habits of marine top predators is important as it provides critical information about stock structure and potential threats that may negatively impact population health and abundance. Using the present mtDNA analysis of the D-loop region, this study is the first to demonstrate at least one site of origin (e.g. from Australia) for C. carcharias that enter Indonesian waters. While it is currently uncertain as to whether this shark came from the Southern-Western Australian (e.g. Bradford et al., Reference Bradford, Patterson, Rogers, McAuley, Mountford, Huveneers, Robbins, Fox and Bruce2020) or Eastern Australian and New Zealand (e.g. Spaet et al., Reference Spaet, Butcher, Manica and Lam2022) C. carcharias subpopulation, this study provides an initial step to further understanding the true range of this highly migratory top predator. Presently the range associated with both Australian subpopulations has not been demonstrated to extend into Indonesian waters (e.g. Spaet et al., Reference Spaet, Patterson, Bradford and Butcher2020); however, the Eastern Australian and New Zealand subpopulation has been documented as far north as Papua New Guinea (PNG; Spaet et al., Reference Spaet, Patterson, Bradford and Butcher2020). Therefore, the present study suggests a range extension for Australia-associated C. carcharias. Although it is likely that the documented C. carcharias encounters from the present study are associated with the Eastern Australian and New Zealand subpopulation due to the proximity of Indonesia to PNG, due to the lack of tangible satellite telemetry or genetic data it is uncertain which subpopulation this range extension pertains to.
Furthermore, while little is known about the migratory patterns of the Northwest Pacific C. carcharias stock (Christiansen et al., Reference Christiansen, Lin, Tanaka, Velikanov, Mollet, Wintner, Fordham, Fisk and Hussey2014), DNA analyses suggest that this is a reproductively isolated stock (Tanaka et al., Reference Tanaka, Kitamura, Mochizuki and Kofuji2011). More specifically, Tanaka et al. (Reference Tanaka, Kitamura, Mochizuki and Kofuji2011) demonstrated through the use of mtDNA analyses that the Northwest Pacific C. carcharias form a monophyletic clade and exhibit unique life-history characteristics in comparison to other C. carcharias stocks. Therefore, although likely a rare occurrence, C. carcharias that utilize Indonesian waters are likely only from one or both of the Australia-associated stock (i.e. Southern-Western Australian and Eastern Australian and New Zealand) or if they are from the Northwest Pacific, they are not utilizing this region for mating purposes as this would be evidenced through previous DNA analyses (e.g. Tanaka et al., Reference Tanaka, Kitamura, Mochizuki and Kofuji2011).
Although it is uncertain if all C. carcharias that utilize Indonesian waters are from the Australian subpopulations (e.g. Southern-Western Australian and Eastern Australian and New Zealand) or from the Northwest Pacific subpopulation, it is further uncertain as to why, how frequently, and for what duration they are utilizing these tropical waters. It is not uncommon for C. carcharias to utilize tropical waters as they have a global distribution in temperate, subtropical, and tropical seas (Bonfil et al., Reference Bonfil, Meÿer, Scholl, Johnson, O'Brien, Oosthuizen, Swanson, Kotze and Paterson2005, Reference Bonfil, Francis, Duffy, Manning and O'Brien2010; Duffy et al., Reference Duffy, Francis, Manning, Bonfil and Domeier2012; Skomal et al., Reference Skomal, Braun, Chisholm and Thorrold2017). However, research illustrates that C. carcharias movement patterns have been suggested to be correlated with a variety of both biotic and abiotic variables, including reproductive behaviour (e.g. mating or parturition; Domeier, Reference Domeier and Domeier2012), water temperature (e.g. Skubel et al., Reference Skubel, Kirtman, Fallows and Hammerschlag2018), and prey (Nasby-Lucas et al., Reference Nasby-Lucas, Dewar, Lam, Goldman and Domeier2009). While all three are plausible explanations for their presence within Indonesian waters, insufficient data make it difficult to make any sound conclusions.
Indonesia is considered one of the world's largest shark fin exporters in the world (Okes and Sant, Reference Okes and Sant2019; FAO, Reference FAO2022) with serious challenges on managing fisheries and its trade (Prasetyo et al., Reference Prasetyo, McDevitt, Murray, Barry, Agung, Muttaqin and Mariani2021). While C. carcharias may not be commonly utilizing these waters, the potential anthropogenic threats are a cause for concern. Therefore, future research should aim at distinguishing what subpopulation(s) these Indonesian C. carcharias originated from, with a focus on satellite tagging efforts that may shed light on the potential ecological importance of Indonesia's waters to this top predator.
Acknowledgements
We acknowledge the facilities, scientific, and technical support from the Sequencing Centre, Genomic Laboratory at the National Research and Innovation Agency (BRIN).
Author contributions
C. P. O., A. P. P., M. S., and Fahmi all formulated the research question and assisted with writing the manuscript. Fahmi provided the white shark sample. C. P. O., A. P. P., and Fahmi helped design the study, whereas A. P. P. carried out the study, analysed the data, and interpreted the findings.
Financial support
This work was partially supported by O'Seas Conservation Foundation, Inc.; Discovery Channel, Inc. and Talesmith TV; and Mrs. Uli Pandjaitan.
Competing interests
None.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.







