Introduction
Monitoring has become a major tool of international ocean management programmes (e.g. within the framework of the European Marine Strategy Framework Directive and Habitats Directive). In the North Sea, the onset of marine monitoring programmes in the late 1960s (Rachor, Reference Rachor1977, Reference Rachor1980) has provided a wealth of quantitative data on the composition of benthic communities and their changes over time. In 2011, the German Federal Agency of Nature Conservation initiated an extensive programme to evaluate the environmental status of the seafloor habitats and to provide benthic species inventories in the German exclusive economic zone of the North Sea. Such an expansion of environmental monitoring and a manifold of research data led the North Sea to be among the most intensively studied and well-documented marine regions of the world (Zettler et al., Reference Zettler, Beermann, Dannheim, Ebbe, Grotjahn, Günther, Gusky, Kind, Kröncke, Kuhlenkamp, Orendt, Rachor, Schanz, Schröder, Schüler and Witt2018). At the same time, a multitude of physical and chemical anthropogenic influences have been impacting both pelagic and benthic North Sea environments for more than a century, while the introduction of new, potentially invasive species also exerts a significant pressure on coastal waters communities (Wiltshire et al., Reference Wiltshire, Kraberg, Bartsch, Boersma, Franke, Freund, Gebühr, Gerdts, Stockmann and Wichels2010; de Castro et al., Reference De Castro, Fileman and Hall-Spencer2017; Reise et al., Reference Reise, Buschbaum, Büttger and Wegner2017).
Within the context of marine conservation, neobiota (i.e. organisms occurring in an area that is not their native distribution region) have become a threat to marine biodiversity. About two species per year are anthropogenically introduced into German coastal waters (Buschbaum et al., Reference Buschbaum, Lackschewitz and Reise2012), where more than 150 neobiota have been registered, including data on their colonization history and invasive potential (Lackschewitz et al., Reference Lackschewitz, Reise, Buschbaum and Karez2022). Among them, 22 are polychaetes occurring regularly in brackish estuaries and, more rarely, in the open German Bight. Moreover, this basin has been experiencing a rapid rising of sea surface temperatures, which, together with an enhanced influx of oceanic water masses (Wiltshire et al., Reference Wiltshire, Kraberg, Bartsch, Boersma, Franke, Freund, Gebühr, Gerdts, Stockmann and Wichels2010), has contributed to promote the spreading of neobiota.
Among SE North Sea polychaete neobiota, we are here reporting the presence of Streptosyllis nunezi Faulwetter, Vasileiadou, Papageorgiou & Arvanitidis, Reference Faulwetter, Vasileiadou, Papageorgiou and Arvanitidis2008, which has been found in infaunal samples collected in 2015 and 2018 in the German Bight. The species is described and illustrated based on the German Bight individuals, and the reasons explaining its presence are discussed.
Materials and methods
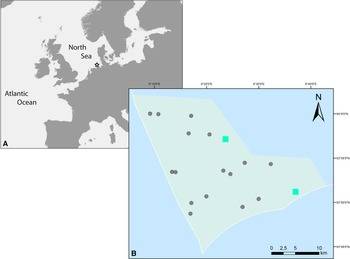
Infaunal samples were collected within a benthic community monitoring in subtidal sandbanks of the Borkum Reef Ground (SE North Sea; Figure 1A), protected according to the European Marine Habitats Directive. Ten stations (three replicates each) were sampled on each of two cruises of the research vessels ‘Uthörn’ (August 2015) and ‘Heincke’ (June 2018) with a van Veen grab (surface area: 0.1 m2, penetration depth: ≥10 cm). Water depth was recorded by a ship-based echo sounder. Sediment samples were sieved on board through a 1 mm mesh size sieve. All retained organisms were preserved in 4% formalin-seawater solution, sorted by species, and stored after taxonomic determination in 70% ethanol. Individual polychaetes were examined under a Leica M125 dissecting microscope. Diagnostic structures, such as parapodia and chaetae, were carefully dissected and viewed under 200- to 400-fold magnification on a microscopic slide with cover slip through an Olympus BX53F compound microscope for details.

Figure 1. (A) Location of the sampling area at Borkum Reef Ground in the southeastern North Sea. (B) Sampling stations inside the Borkum Reef Ground protected area; turquoise rectangles: occurrence of S. nunezi. In 2015, one individual occurred on the NW station; in 2018, one individual each occurred on the NW and on the SE station.
At each sampling station, a sub-sample of the top 6 cm of the sediment was taken with a cylindrical corer (diameter: 4.5 cm) and frozen on board at −20°C. The organic content of 40 g of the frozen sediments was determined as weight loss on ignition (drying: 60°C, 48 h; combustion: 500°C, 5 h). Grain size distributions were analysed following Wentworth (Reference Wentworth1922).
Results
S. nunezi was collected in 2015 (one individual) and in 2018 (two individuals from two stations) (Figure 1B) at 22–27 m depth from moderately well-sorted (graphic standard deviation: 0.55–0.62) fine sediments (median grain size: 343.0–379.4 μm) with low organic content (0.2–0.3%).
Taxonomic account
Syllidae Grube, 1850
Anoplosyllinae Aguado & San Martín, 2009
Streptosyllis Webster & Benedict, 1884
Type species: Streptosyllis arenae Webster & Benedict, 1884
Streptosyllis nunezi Faulwetter, Vasileiadou, Papageorgiou & Arvanitidis, Reference Faulwetter, Vasileiadou, Papageorgiou and Arvanitidis2008
Figure 2
Streptosyllis nunezi Faulwetter et al. (Reference Faulwetter, Vasileiadou, Papageorgiou and Arvanitidis2008): pp. 5–10, Figures 4–6; Musk et al. (Reference Musk, Faulwetter and McIlwaine2016): pp. 5–7, Figure 2.
Description
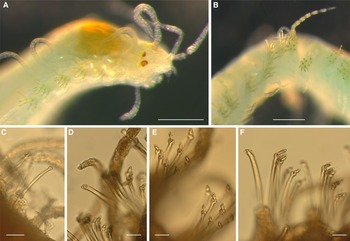
All specimens incomplete, 6–7 mm long and 0.5–0.6 mm of maximum width for 52–69 chaetigers. Bodies pale yellowish when preserved.
Prostomium anteriorly rounded, with poorly preserved palps non-visible in dorsal view, visible as rounded lobes in ventral view. Antennae slender, smooth; median antenna about six times as long as prostomium and twice as long as lateral antennae; lateral antennae about three times as long as the prostomium. Eyes large, reddish, anterior pair about twice as large as posterior pair, with two small anterior eyespots (Figure 2A). Proventriculum extending through 6–8 chaetigers, with about 55 muscle cell rows. Peristomium indistinct, with two pairs of smooth tentacular cirri about as long as lateral antennae. Parapodia conical, with dorsal cirri about four times as long as the parapodial lobe, smooth or pseudoarticulated with bright yellowish granular inclusions (Figure 2B); ventral cirri smooth, non-articulated, digitiform, as long as parapodial lobe. Single dorsal simple chaeta distally surrounded by cup-shaped hyaline hood, present from chaetiger 1 (Figure 2C), smooth in most anterior segments, with 2–4 blunt teeth beneath hood at midbody (Figure 2D). Anterior chaetigers with 5–6 short-bladed compound chaetae (Figure 2E), then up to 13 short-bladed and 2–6 long-bladed compound chaetae (Figure 2F); blades entirely or terminally surrounded by hyaline hoods, evenly rounded on short blades, forming teeth-like structures on long blades; shaft teeth also covered by rounded hyaline hoods (Figure 2E, F). Aciculae distally knobbed, with somewhat wider knobs and slightly wider shafts in chaetigers 2–5. Pygidium not seen.

Figure 2. S. nunezi. (A) anterior end; (B) middle segments with dorsal cirrus; (C) simple chaeta, chaetiger 4; (D) simple chaeta, about chaetiger 35; (E) two fascicles of short-bladed compound chaetae, anterior segments; (F) two fascicles with long-bladed chaetae, midbody segments; scales: 200 μm in (A) and (B), 20 μm in (C–F).
Remarks
The morphology of the specimens from the German Bight corresponds well with the morphology of the specimens from the UK, the Canary Islands, and Crete, which is the type locality (Faulwetter et al., Reference Faulwetter, Vasileiadou, Papageorgiou and Arvanitidis2008). However, the body size of our specimens was somewhat larger (6–7 mm, 52–69 chaetigers in incomplete individuals) than the types, up to 2.5 mm for 33 chaetigers in Faulwetter et al. (Reference Faulwetter, Vasileiadou, Papageorgiou and Arvanitidis2008), and the UK specimens, 5 mm for 64 chaetigers in Musk et al. (Reference Musk, Faulwetter and McIlwaine2016). Likewise, the proventriculum was also slightly larger, extending over 6–8 chaetigers in our specimens, instead of over 6 in the UK specimens and over 4–5 in the types. Whether these differences may be species-specific or just a variation of the same species along its range of distribution certainly merits further studies.
In the North Sea, Streptosyllis is represented by Streptosyllis arenae and Streptosyllis websteri Southern, 1914 (Hartmann-Schröder, Reference Hartmann-Schröder1996). S. nunezi differs from S. arenae in having the shaft teeth covered by hyaline hoods (not covered in S. arenae), the blade hoods having 1–2 lateral tips (smooth in S. arenae), and the strongly serrated dorsal simple chaetae (smooth in S. arenae). The species differs from S. websteri in aciculae in chaetigers 2–5 being only slightly thicker than in subsequent chaetigers (aciculae of chaetigers 2–5 much thicker and distally distinctly knobbed in S. websteri), compound chaetae with hoods on blades and shafts (absent in S. websteri), and simple chaetae with 2–4 blunt teeth in midbody segments (finely serrated in S. websteri).
Discussion
The benthic invertebrate communities of the German Bight have repeatedly been studied over the past 100 years (Hagmeier, Reference Hagmeier1923; Salzwedel et al., Reference Salzwedel, Rachor and Gerdes1985; Neumann et al., Reference Neumann, Reiss, Ehrich, Sell, Panten, Kloppmann, Wilhelms and Kröncke2013a). S. nunezi was described in the early 20th century and, thus, may have been misidentified in previous studies. Syllids commonly prefer hard substrata, often living on sessile epifauna and macrophytes, and are rarer in soft sediments (Kohn and Lloyd, Reference Kohn and Lloyd1973; Bone and San Martín, Reference Bone and San Martín2003; Jumars et al., Reference Jumars, Dorgan and Lindsay2015). Extensive previous assessments of the Borkum Reef Ground infauna did not reveal a single syllid (Dörjes, Reference Dörjes1977), while the ICES report on North Sea benthos mentioned five species from 1999 to 2007, including S. websteri, three Exogone and one Eusyllis (Rees et al., Reference Rees, Eggleton, Rachor and Vanden Berghe2007), and the annotated check list of the macrozoobenthic species in German waters reported 25 species from 12 genera, none of them belonging to Streptosyllis and being mostly associated with hard bottoms (Zettler et al., Reference Zettler, Beermann, Dannheim, Ebbe, Grotjahn, Günther, Gusky, Kind, Kröncke, Kuhlenkamp, Orendt, Rachor, Schanz, Schröder, Schüler and Witt2018). Therefore, our finding of three specimens of S. nunezi in 2 different years suggests a recently established, non-numerous, population that constitutes the first report of S. nunezi for the SE North Sea.
S. nunezi may have thermophilic affinities, as suggested by its original description from sandy sediments in two subtropical regions, the Mediterranean Sea and the Canary Islands (eastern subtropical North Atlantic) (Faulwetter et al., Reference Faulwetter, Vasileiadou, Papageorgiou and Arvanitidis2008). It was later reported from the W English Channel, including the North Sea off the Humber region, where it was considered as part of the resident fauna rather than a recent introduction (Musk et al., Reference Musk, Faulwetter and McIlwaine2016). Although we cannot discard S. nunezi as being a native species in our study area, we alternatively suggest that it may have entered the North Sea from the southwest through the English Channel. This way, the German Bight records could more likely result from a progressive extension of the species distribution into the temperate waters of the southern North Sea. However, the northern North Sea population could have also arrived along with the Faire Isle current between Orkney and Shetland, as suggested for the recent expansion of the angular crab, Goneplax rhomboides (Linnaeus, 1758), into the North Sea (Neumann et al., Reference Neumann, de Boois, Kröncke and Reiss2013b).
Our finding of S. nunezi in the SE North Sea confirms the key role of extensive monitoring programmes (e.g. those carried out within the framework of international environmental legislation) in facilitating the assessment of regional biodiversity and its potential changes. On the other hand, the long geographical distances between the type location and the more recent northern Europe sites point out the interest of carrying out further molecular analyses, which could either allow evaluating the inter-population connectivity and possible introduction pathways or reveal the existence of cryptic species. This is a more and more common trend in modern integrative taxonomy, particularly among syllids (e.g. Álvarez-Campos et al., Reference Álvarez-Campos, Giribet and Riesgo2017; Aguado et al., Reference Aguado, Capa, Lago-Barcia, Gil, Pleijel and Nygren2019), which could be an alternative explanation of the differences observed between the morphology of the type specimens and those from northern Europe that would allow discarding possible introductions.
Data
Data will be made available on request.
Acknowledgements
The authors express their gratitude to the crews of R/V ‘Heincke’ and R/V ‘Uthörn’ for their assistance with the sampling at sea. Special thanks are extended to Karin Meißner from the Senckenberg Institute for her generous assistance with the microphotography and to the two anonymous reviewers, whose valuable comments significantly enhanced the quality of the manuscript. This publication is dedicated to Captain Hans-Carl ‘Charly’ Lührs who has supported our work for many years with great enthusiasm, expertise, and collegiality.
Author contribution
BE: investigation and data collection, data analysis and interpretation, writing – original draft. MG: investigation and data collection, data analysis and interpretation, review and editing. JB: sample design and methodology, figures and maps, review and editing. KH: research conceptualization, review and editing. LG: funding acquisition, research conceptualization, sample design and methodology, writing original draft.
Financial support
The study leading to this publication was funded by the German Federal Agency for Nature Conservation (grant nos. 53202 and 3519532201). The authors acknowledge support by the Open Access publication fund of Alfred-Wegener-Institut Helmholtz-Zentrum für Polar- und Meeresforschung.
Competing interests
None.
Ethical standards
All research pertaining to this article did not require any research permit(s).