Introduction
The knowledge of native Brazilian Vermetidae species was updated by Spotorno et al. (Reference Spotorno, Tâmega and Bemvenuti2012), comprising the most recent inventory of these gastropods from Brazil. The authors provided a checklist with 16 species, most of them determined only in genus level, distributed into four genera: Dendropoma Mörch, Reference Mörch1861, Thylacodes Guettard, 1770, Thylaeodus Mörch, 1860 and Petaloconchus Lea, 1843.
In the 1970s, two species, Vermetus ( = Dendropoma) irregulare (d'Orbigny, 1842) and Petaloconchus varians (d'Orbigny, 1841), were commonly distributed along the Brazilian coast, the latter being the dominant species in the fossil reefs. According to previous studies, P. varians usually forms dense aggregations on the coast (Laborel, Reference Laborel and Taylor1977). Nevertheless, the species began to decline at the end of the 70s. It has been conjectured that the primary process of this reduction occurred by the synergic pressure of anthropogenic and biological variables (e.g. oil pollution, water turbidity and/or competition with Vermetus) (Laborel, op. cit). Recent studies have also indicated the recovery of the native P. varians population to the Southeastern coast, with high densities reported from rocky intertidal shores in Ilha Grande Bay (23°S) (Breves et al., Reference Breves, Széchy, Lavrado and Junqueira2017).
On the other hand, Eualetes tulipa (Russeau in Chenu, Reference Chenu1843) is the only non-native vermetid species known from Brazil. Despite the diagnosis not determining the type locality, E. tulipa was first recorded from the Pacific Coast, in Panamá Bay, which has been considered its probable centre of origin (Keen, Reference Keen1971; Spotorno-Oliveira et al., Reference Spotorno-Oliveira, Coutinho and Tâmega2018; Tan et al., Reference Tan, Loh and Ng2021).
In 2009, this vermetid was recorded in the cold-water environment of the northern Rio de Janeiro State (22°S). Thenceforth, E. tulipa has spread to warmer environments in the Tropical Brazilian Province, with previous reports at lower latitudes for the States of Ceará (3°S) and Rio Grande do Norte (6°S) (Spotorno-Oliveira et al., Reference Spotorno-Oliveira, Coutinho and Tâmega2018). As other calcifying sessile invertebrates, the vermetid dispersion strategy is likely to occur by rafting mechanisms, using drifting marine debris such as polystyrene, pet bottle, wood and coconut, fouling on ship hull, ballast water and also through current transport of mucus strands (Thiel and Gutow, Reference Thiel, Gutow, Gibson, Atkinson and Gordon2005; Breves and Skinner, Reference Breves and Skinner2014; Spotorno-Oliveira et al., Reference Spotorno-Oliveira, Coutinho and Tâmega2018). The pelagic short-lived larvae of E. tulipa may also contribute to the natural dispersion and introduction of the species in new areas, but data on the larvae competency have not been published yet (Spotorno-Oliveira et al., Reference Spotorno-Oliveira, Coutinho and Tâmega2018).
Eualetes Keen, Reference Keen1971 comprises only two valid species, but the type locality has not been assigned to either of them. Eualetes centiquadrus (Valenciennes, Reference Valenciennes and Petit-Thouars1846) is assumed to be restricted to the Pacific Ocean, including Colombia and West Mexico (Bieler and Petit, Reference Bieler and Petit2011). The congener, E. tulipa, has been gradually expanding its natural limits of distribution, being pointed out as introduced in some localities through the Indo-Pacific, such as the Hawaii Islands (Coles et al., Reference Coles, Kandel, Reath, Longenecker and Eldredge2006), India (Jebakumar et al., Reference Jebakumar, Nandhagopal, Ragumaran, Rajanbabu and Ravichandran2015), the Caribbean (Miloslavich and Penchaszadeh, Reference Miloslavich and Penchaszadeh1992; Miloslavich, Reference Miloslavich2018), the Southeast Asia (Tan et al., Reference Tan, Loh and Ng2021) and Southwestern Atlantic, as well (Spotorno-Oliveira et al., Reference Spotorno-Oliveira, Coutinho and Tâmega2018; Skinner et al., Reference Skinner, Tenório, Penha and Soares2019).
Deeply involved in the organic matter flow, being also sensitive to climate changes, hydrodynamics and turbidity (Colombo et al., Reference Colombo, Costa, Aleo, Tramati, Mazzola, Vizzini, Pergent-Martini and Brichet2009; Soares et al., Reference Soares, Meirelles and Lemos2010), these cemented odd gastropods are promptly recognized by the irregular, untwisted teleoconch (the adult shell), while the embryonic, as well as the larval shell, follow the regular coiled pattern. After attaching to the substrate, the juvenile rapidly develops into a linear shell structure, with the aperture of the tubes commonly projecting upwards. Regarding feeding habits, vermetids are filtering ‘worm-like’ molluscs that act as scavengers capturing organic particles through a mucus net (Kappner et al., Reference Kappner, Al-Moghrabi and Richter2000). In this way, long shell-like tubes have a double action by (1) preventing the vermetid from overgrowth by corals, and (2) keeping free access for the mollusc to feed on food particles (Kappner et al., Reference Kappner, Al-Moghrabi and Richter2000).
Finally, the vermetids have essential reproductive strategies (e.g. capsular lecithotrophic development, intracapsular adelphophagy, female long-term sperm storage, reproduction by self-fertilization and parthenogenesis) and diverse dispersion mechanisms to succeed in a foreign environment. As Strathmann and Strathmann (Reference Strathmann and Strathmann2006) supported, ‘Any of these reproductive traits would aid founding of a population by a dispersed individual and invasive spread of this species’ (p. 105).
In the present study, we record E. tulipa for the first time from the Bahia State (12 oS), occurring syntopically with Tubastraea in a benthic fouling community, located in a port area. An anatomical and morphological description of Eualetes exemplars from Bahia is provided.
Material and Methods
Study area
This study is part of a major project intending to assess the diversity, density and dispersion of sun corals in the Todos-os-Santos Bay, Bahia State (BA). Sampling was carried out in August 2019 by scuba diving at the ‘Terminal Turístico Náutico da Bahia’ – TTNB (12°58′00.0″S/38°31′31.5″ W) (Figure 1). The area is a private boat dock used for navigators and travellers from all over the world. A benthic fouling community, majorly composed of sun cup corals, sponges, oysters, barnacles, hydroids, octocorals and ascidians, is observed attached to the decks of the dock, in an upside-down position, and vertically along the pillars – from the surface to a depth of approximately 3–5 m. The internal deck structure is covered by fiberglass, on which the community has established. The Todos-os-Santos Bay is Brazil's second-largest navigable bay, with a maximum area of 1223 km2 and an average depth of 9.8 m (Cirano and Lessa, Reference Cirano and Lessa2007). Because of the scenic beauty, the pristine ecosystems, and the extraordinary biodiversity, it was designated an Environmental Preservation Area in 1999 (State decree no. 7.595 5th June 1999) (Hatje and Andrade, Reference Hatje and Andrade2009).

Figure 1. Map of the study area. (A) Terminal Turístico Náutico da Bahia (TTNB). (B) Numbers indicating the chronological order of appearance of Eualetes on the Brazilian coast: 1 = Ceará State, 2 = Rio Grande do Norte State, 3 = Rio de Janeiro State, 4 = Bahia State.
Sampling
Quadrats were adopted as a protocol for collecting data and samples. Four quadrats (50 × 50 cm) were systematically placed in three different areas considering the densities of the Tubastraea spp. colonies, i.e., cover varying between 1 and 20% (low density), 40 and 60% (medium density) and 80 and 100% (high density). Considering they were never replaced at the same point, a total of 12 quadrats were obtained. As a rule, the coral cover was measured by the frequency of the occurrence of the colonies in the quadrat grids, following the cover categories previously established, being all sessile organisms inside quadrats removed and placed into plastic bags individually. To avoid skeleton and shell fragmentation, spatula, hammer and chisel were carefully used during the process. A total of 44 vermetids were selected for analysis.
Analysis
In the laboratory, animals were sorted out, being the samples of E. tulipa separated and preserved in an ethanol solution of 70%. All 44 specimens were separated according to the quadrat, considering the three Tubastraea densities. After that, all the specimens were measured and the epibiont fauna in each exemplar was identified and counted, bryozoans were not counted by the complex disposition of these organisms in the shell surface, being classified by presence and absence of colonies in vermetiid shells. An infographic with all the collected information was developed.
For taxonomical analyses and morphometrics, four individuals were dissected and drawn under a stereoscopic microscope (Zeiss Axio 1.6) with camera lucida. By comparing radula and shell characteristics, individuals were identified based on Spotorno-Oliveira et al. (Reference Spotorno-Oliveira, Coutinho and Tâmega2018) and Skinner et al. (Reference Skinner, Tenório, Penha and Soares2019). The shells were photographed and measured with a Mitutoyo digital caliper. The epibionts were counted, morphotyped and identified to the lowest possible taxonomic level. For scanning electron microscopy, the radula was removed from dissected specimens and mounted on aluminium pin stubs, previously covered with a double-sided sticky tape, sputter-coated with 35 nm of gold in a Denton Vacuum Desk IV ion coater and examined through a Jeol JSM-6390LV, located at the Oswaldo Cruz Foundation (FIOCRUZ-BA).
Results
Systematics
CLASS GASTROPODA Cuvier, 1795
ORDER LITTORINIMORPHA Golikov & Starobogatov, 1975
FAMILY VERMETIDAE Rafinesque, 1815
Genus Eualetes Keen, Reference Keen1971
Eualetes tulipa (Rousseau in Chenu, Reference Chenu1843)
(Figures 2–4)

Figure 2. (A) The benthic community settled upside down on a port pier in the Todos-os-Santos Bay. (B) Eualetes vermetids (arrows) occurring sintopically with Tubastraea corals. Mucus threads (Mu) are observed around the vermetid tube shell. (C) Eualetes tulipa shell growth patterns. Teleoconch with piled-up whorls and apertures close to the substrate. (D) Teleoconch expanded towards forming elongated cylindrical tubes with high apertures, densely covered by epibionts. (E, F) Polyp of Tubastraea epibiotic of Eualetes tulipa shell. Scale bars: 2 cm (A–D); 1 cm (E, F).
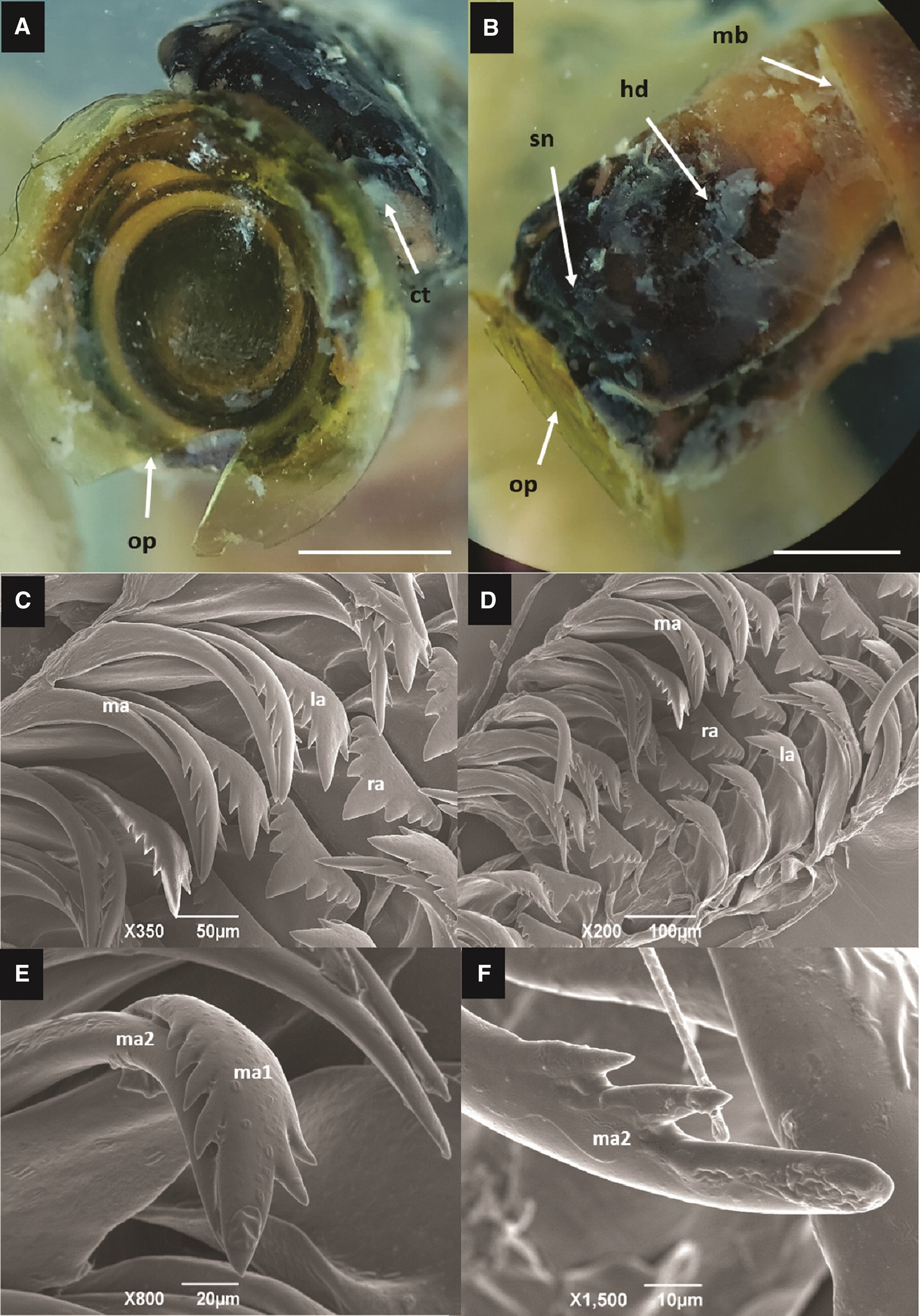
Figure 3. Eualetes tulipa (A) Front view of the corneous operculum. Scale bar = 3 mm. (B) Lateral view of the head. op, operculum; ct, cephalic tentacles; sn, snout; hd, head; mb, mantle border. Scale bar = 3 mm. (C, D) Radula taenioglossate, consists of a central and robust tooth (rachidian tooth), a pair of lateral teeth and two pairs of marginal ones. (E, F) Marginal teeth slender and fused, the external tooth (less serrated), with a main well-marked cusp, and only two lateral cusps. ma, marginal teeth; ma1, marginal inner tooth; ma2, marginal outer tooth; la, lateral tooth; ra, rachidian tooth.
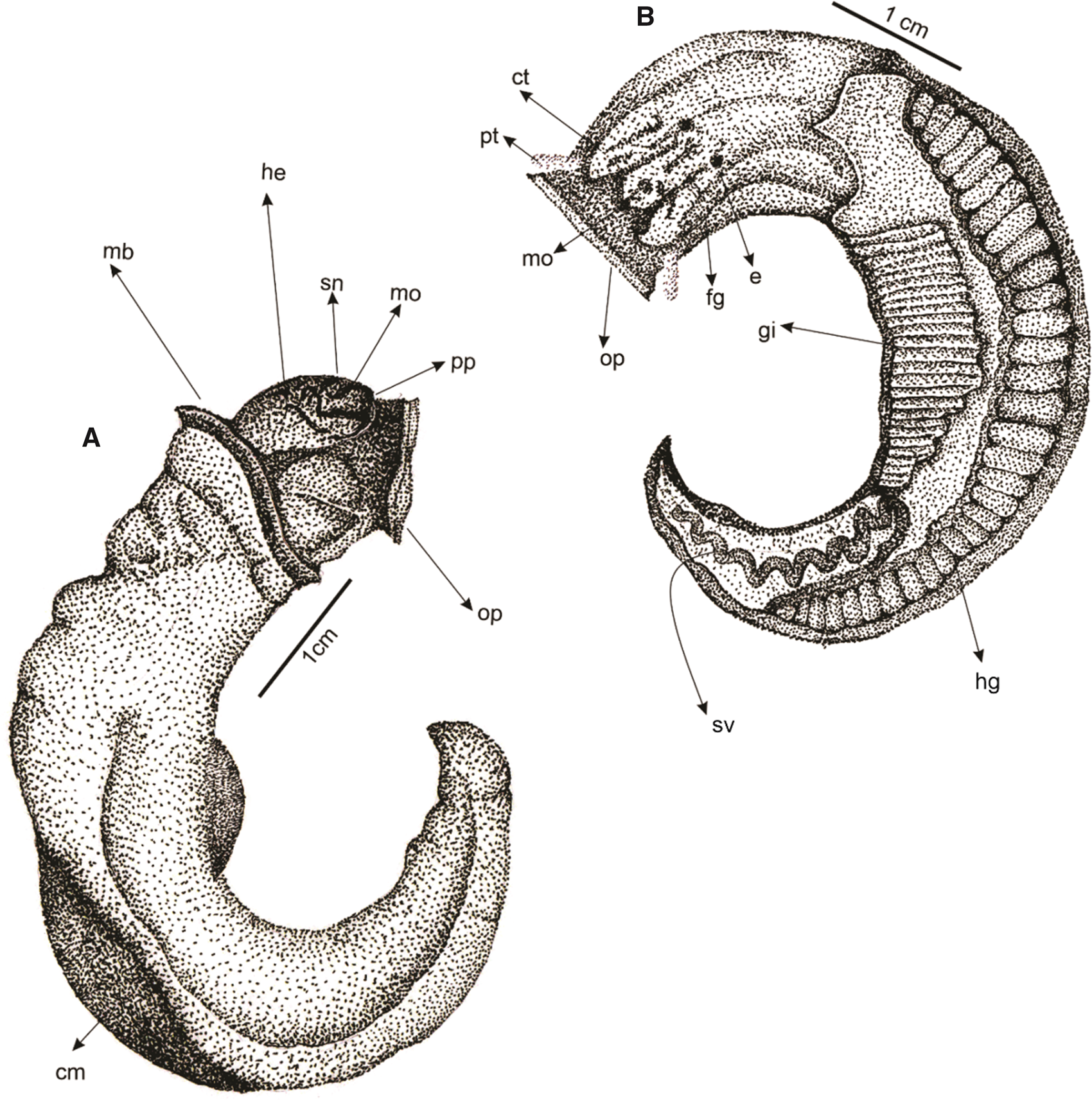
Figure 4. Scheme of the external morphology and internal anatomy of the vermetid Eualetes tulipa. (ct, cephalic tentacles; cm, columellar muscle; e, eye; fg, foot groove; gi, gill; he, head; hg, hypobranchial gland; mb, mantle border; m, mouth; op, operculum; pp, propodial pad; pt, pedal tentacle; sn, snout; sv, seminal vesicle).
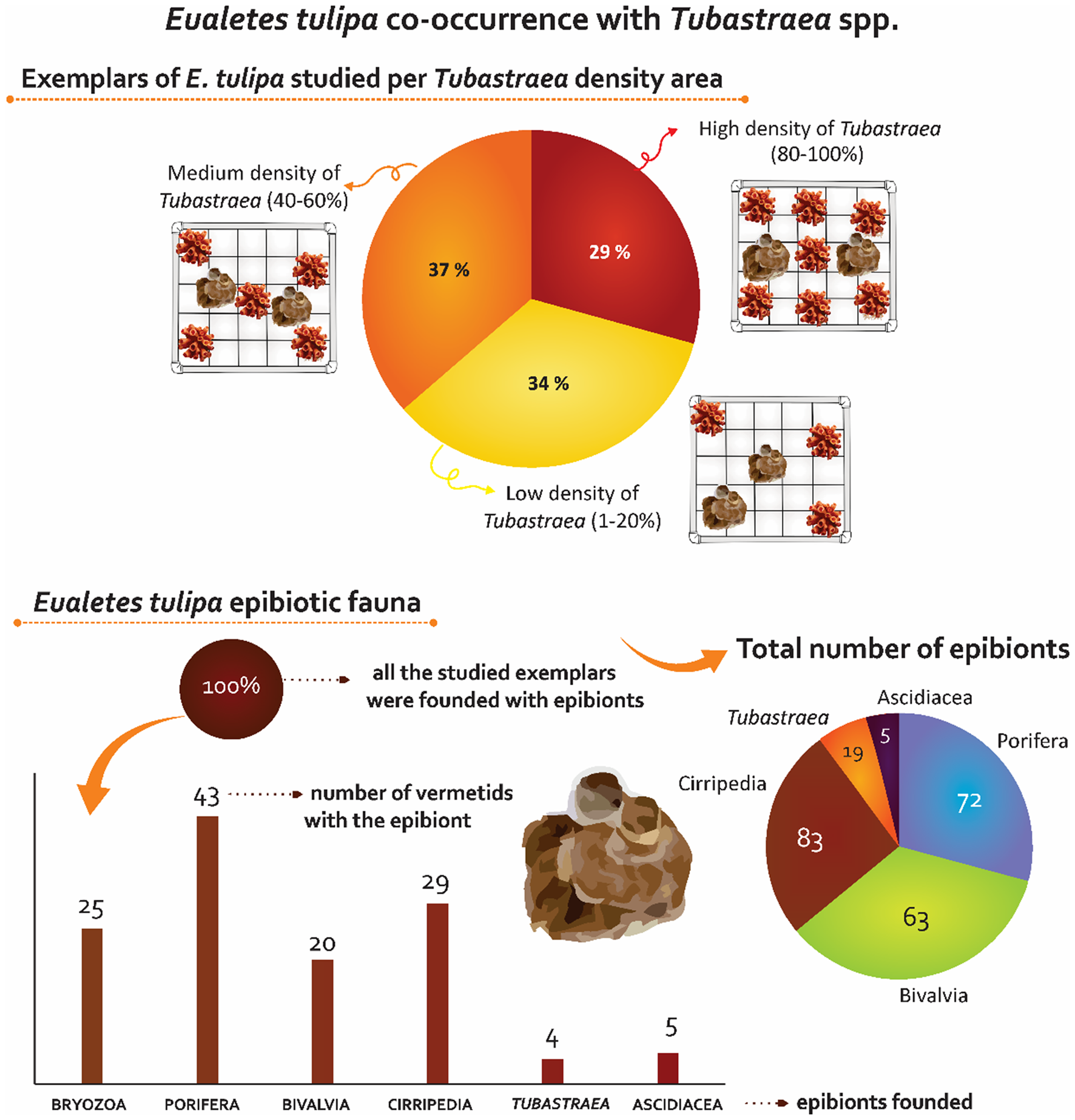
Figure 5. Infographic of Eualetes tulipa exposing the co-occurrence of the vermetid with Tubastraea and its epibionts diversity.
Examined material
Four specimens. Terminal Turístico Náutico da Bahia – TTNB, Todos-os-Santos Bay, Bahia State, Brazil, (12°58′00.0″ S/38°31′31.5″ W), depth 3–5 m, associated with the benthic fouling community, 30th august, 2019, MZUSP 159664.
Description
Teleoconch irregularly coiled, periostracum varying in a single individual from reddish-brown to greyish in early whorls, to light-yellow to white on the bottom, opercular aperture diameter ranging from 1.73 to 9.43 mm (mean diameter = 6.12 mm). Adult shells densely covered by cementing epibionts. Head pronounced. Snout cylindrical. Mouth semicircle shaped, with a horizontal notch on the anterior margin snout. Eyes rounded and small, inserted in the cephalic tentacle bases. Operculum corneous, thin, with a translucent border and dark centre. Average diameter about 7.1 mm. Radula taenioglossate (radular formulae: 2-1-1-1-2). Pallial cavity occupying about ⅓ of the soft parts length, while the visceral mass about ⅔ of total length. Gills with three leaflets per mm; leaflets cylindrical, dark-grey digestive gland prevailing over the visceral mass. Gonad beneath the digestive gland, ventrally positioned, forming a distal, thin and spiral tail.
Remarks
The analysed exemplars of E. tulipa from Todos-os-Santos Bay differ from the specimens described from Brazil by showing teleoconch opercular aperture varying from 1.73 to 9.43 mm, while previous studies described shell aperture ranging 5.22–14.07 mm in specimens recorded from Ceará, Rio Grande do Norte and Rio de Janeiro States (Spotorno-Oliveira et al., Reference Spotorno-Oliveira, Coutinho and Tâmega2018).
Opercular aperture is a variable measure, considering the previous records of E. tulipa around the world, according to Skinner et al. (Reference Skinner, Tenório, Penha and Soares2019), the aperture size may reflect the population density. Organisms from Rio de Janeiro show 17 mm of aperture, India and Venezuela specimens have 15 and 12 mm of aperture, respectively, whereas organisms from Hawaii show 8 mm of aperture (Miloslavich and Penchaszadeh, Reference Miloslavich and Penchaszadeh1992; Jebakumar et al., Reference Jebakumar, Nandhagopal, Ragumaran, Rajanbabu and Ravichandran2015; Skinner et al., Reference Skinner, Tenório, Penha and Soares2019).
Recent records of the vermetid from Singapore described populations with 11 mm of shell aperture (Tan et al., Reference Tan, Loh and Ng2021). Populations of E. tulipa from Rio de Janeiro are also considered well-established and more than 50% of studied individuals show shell aperture of more than 8 mm (Skinner et al., Reference Skinner, Tenório, Penha and Soares2019). The specimens here analysed have a lower aperture average of 6.12 mm, which could reflect the low age of the observed organisms.
Ecologic notes. Eualetes tulipa was found in all densities of Tubastraea, 16 of 44 studied individuals were found in low density of Tubastraea (1–20%), 17 individuals were found in medium density (20–40%), and 13 individuals were found in high density (80–100%) (Figure 4). The measure of the shell aperture showed low variance, in low densities of Tubastraea, the vermetiids showed 6.33 mm of shell aperture on average, in medium densities, 5.42 mm on average, in high densities, the exemplars showed 6.49 mm of shell aperture on average.
All the specimens of E. tulipa were densely covered by epibionts of six Phyla, which are Porifera (Desmospongiae), Bryozoa, Arthropoda (Cirripedia), Mollusca (Bivalvia), Chordata (Ascidiacea) and Cnidaria (Tubastraea spp.) (Figure 5). A total of 73 sea sponges were found attached to 43 vermetiid specimens. Bivalvia molluscs were found in epibiotic with 20 vermetiids, a total of 83 individuals were counted. Bryozoa colonies were found in 25 vermetiid specimens (Figure 4). Five exemplars of Ascidiacea were found in five vermetiid shells, being one of the less abundant epibiotic taxa associated with E. tulipa (Figure 5).
In this study, Tubastraea colonies were found for the first time as epibiotic fauna of E. tulipa. The sun coral colonies ( = 5) and primary polyps ( = 14) were found in epibiosis with five vermetid shells, three of them collected from a high-density Tubastraea cover while the others, in medium density (Figures 2E, F and 5). Up to now, E. tulipa has been only reported to TTNB, an artificial environment with biofouling influenced by the establishment of sun corals.
Discussion
In the Southwestern Atlantic, the number of exotic marine gastropods increased south and northwards, from cold to warmer waters, during the last 5 years. Gernet et al. (Reference Gernet, Belz, Baggio, Birckolz, Santos, Simone, Abate and Metri2019) recorded a member of the scavenger family Nassaridae, Nassarius foveolatus (Dunker, Reference Dunker1847), to Paraná State (25°S). A year later, Spotorno-Oliveira et al. (Reference Spotorno-Oliveira, Lopes, Larroque, Monteiro, Dentzien-Dias and De Souza Tamega2020) reported the bivalve predator, Rapana venosa (Valenciennes, Reference Valenciennes and Petit-Thouars1846), to Cassino Beach (Rio Grande do Sul State, 32°S). In 2023, another predator gastropod, Indothais lacera (Born, Reference Born1778), was identified in several localities in the TSB (12 oS) (Pedro et al., Reference Pedro, Salvador and Simone2023). In the same area, E. tulipa was for the first time observed co-occurring with Tubastraea colonies, alerting for a new non-indigenous species introduced in a protected environmental unit.
Milazzo et al. (Reference Milazzo, Rodolfo-Metalpa, Chan, Fine, Alessi, Thiyagarajan, Hall-Spencer and Chemello2014) attest that the ‘vermetids form reefs in sub-tropical and warm-temperate waters that protect coasts from erosion, regulate sediment transport and accumulation, serve as carbon sinks and provide habitat for other species’ (p. 1). Vermetid molluscs are also biological indicators, playing a role in the proxies to reconstruct paleoenvironments by estimating sea-level changes (Suguio et al., Reference Suguio, Barreto, Oliveira, Bezerra and Vilela2013). Indeed, the impacts of E. tulipa on native reef communities are not fully comprehended. The species was analysed in the Caribbean, and it was suggested a negative influence on benthic marine populations caused by space competition with Dendropoma corrodens (d'Orbigny, 1841) (Miloslavich et al., Reference Miloslavich, Klein and Penchaszadeh2010). Moreover, Shima et al. (Reference Shima, Phillips and Osenberg2013) also pointed out the deleterious effects of E. tulipa on the growth and survival of neighbouring coral colonies.
From warm waters in the Northeast to colder environments in the Southeast, the exotic E. tulipa is expanding its distribution along the Brazilian coast (Figure 6). Considering the dispersion of the sun corals through the Todos-os-Santos Bay in the last decade (Sampaio et al., Reference Sampaio, Miranda, Maia-Nogueira and Nunes2012), the presence of another exotic carbonate-producing organism associated with a biofouling community dominated by Tubastraea is an important record.

Figure 6. Distribution of Eualetes tulipa, focusing on records from Brazil. Based on Spotorno-Oliveira et al. (Reference Spotorno-Oliveira, Coutinho and Tâmega2018) and Tan et al. (Reference Tan, Loh and Ng2021).
The exotic E. tulipa is expanding its distribution along the Brazilian Coast (Figure 6). Regarding previous dispersion of the sun corals across the warm waters of the TSB (Sampaio et al., Reference Sampaio, Miranda, Maia-Nogueira and Nunes2012), the presence of another exotic carbonate-producing organism associated with Tubastraea biofouling communities must be evaluated. Although the real risks of the E. tulipa on native fauna have not been measured yet, most records of this gastropod from Brazil occurred in localities under anthropic pressure, majorly in areas of considerable vessel traffic and touristic activities (Figure 6). The co-occurrence of Eualetes and Tubastraea in artificial substrates of an anthropized environment may suggest a facilitation scenario (Tyrrell and Byers, Reference Tyrrell and Byers2007).
Because of the coverage of epibionts (e.g. algae, sponges, ascidians, bivalves, sun corals), this vermetid has a cryptic-like habit. Indeed, it may justify the few records of the species have been done over a relatively wide time interval (Spotorno-Oliveira et al., Reference Spotorno-Oliveira, Coutinho and Tâmega2018). In turn, overgrowth interactions do not affect these operculate snails that live inside of a calcareous tube-like shell. Due to the high shell plasticity, E. tulipa can change the direction of the opercular aperture, projecting it upwards, spreading the mucus net over the neighbourhood, and being capable of capturing food in all directions (Rezende et al., Reference Rezende, Spotorno-Oliveira, D’ávila, Maia and De Oliveira2021).
In a globalized world, the introduction and overspread of exotic species from the Indo-Pacific to the Atlantic Ocean become recurrent, and some of the most dispersive species will be sooner considered cosmopolitan. Undoubtedly, introduced organisms may represent a risk to local biodiversity, being also involved in facilitation processes in artificial substrates (Tyrrell and Byers, Reference Tyrrell and Byers2007). Therefore, biomonitoring programs are highly necessary to consolidate conservation and management policies to prevent bioinvasion impacts along the Brazilian Coast. Finally, treefold research approaches, based on taxonomy, ecology and reproductive biology are expected to provide relevant data on the establishment and interaction of exotic species worldwide.
Acknowledgments
We thank Dr Wagner Magalhães (IBIO/UFBA) for the field images and the LABIMAR team for sampling support. Chico Mendes Institute for Biodiversity Conservation (ICMbio) provided the collecting permission (SISBIO No 15161-1).
Author contributions
J. A. and S. S. conceived and designed the research with the support of E. N. and R. J. E. N. and R. J. coordinated the lab and fieldwork. All authors wrote the manuscript. All authors read and approved the manuscript.
Financial support
This study is part of the project ‘Assessment and research of sun coral in Todos os Santos Bay’, a cooperation agreement between UFBA and PETROBRAS (No 5850.0107361.18.9) regulated by RD&I investments clauses of Brazilian Agency of Petroleum, Natural Gas and Biofuels (ANP Resolution 05/2015).
Competing interest
None.
Ethical standards
No animal testing was performed during this study. All necessary permits for sampling have been obtained by the authors from the competent authorities and are mentioned in the Acknowledgements.
Data availability
Data will be made available on request.








