Stygiomedusa gigantea (Browne, 1910) is one of the largest jellyfish in the world and possesses broad oral arms that may reach 10 m in length (Jarms and Morandini, Reference Jarms and Morandini2019). It is a bathypelagic species that, based on scattered records, is assumed to have a global distribution (Benfield and Graham, Reference Benfield and Graham2010; Nascimento et al., Reference Nascimento, Haddad and Nogueira Júnior2024). Although commonly reported from the Southern Ocean and the western US seaboard, there is only one record of this magnificent animal from Africa: a single specimen caught off Cabinda Province, Angola, from a depth shallower than 930 m (Repelin, Reference Repelin1967).
Here, we report on another specimen that was caught from the RV Mirabilis using a Super Gisund trawl net during a survey for deepsea red crab resources off northern Namibia (19.72°S, 11.39°E) on 11 August 2022. The net was towed for a period of 30 min at a speed of 1.6 ms−1, covering a distance of 2.89 km, and was disgorged on deck at ~16H57 local time. The bottom depth was 750 m, which is not unusual for this species (Benfield and Graham, Reference Benfield and Graham2010). The bottom temperature was 4.8°C, which is much warmer than that recorded from the depths in polar seas where many other specimens have been caught, but it is similar to that observed when captures have been made in temperate waters (Benfield and Graham, Reference Benfield and Graham2010).
Although the specimen was discarded after capture, there can be no doubt that it belongs to the genus Stygiomedusa (Figure 1). It is deep red/black in colour, possesses long strap-like oral arms and there are no marginal tentacles: the radial canals are pronounced and cream-coloured. Unfortunately, the specimen was not measured on deck, but the size of the jellyfish bell was approximated to be 45.6 cm in diameter (Supporting Figure 1).
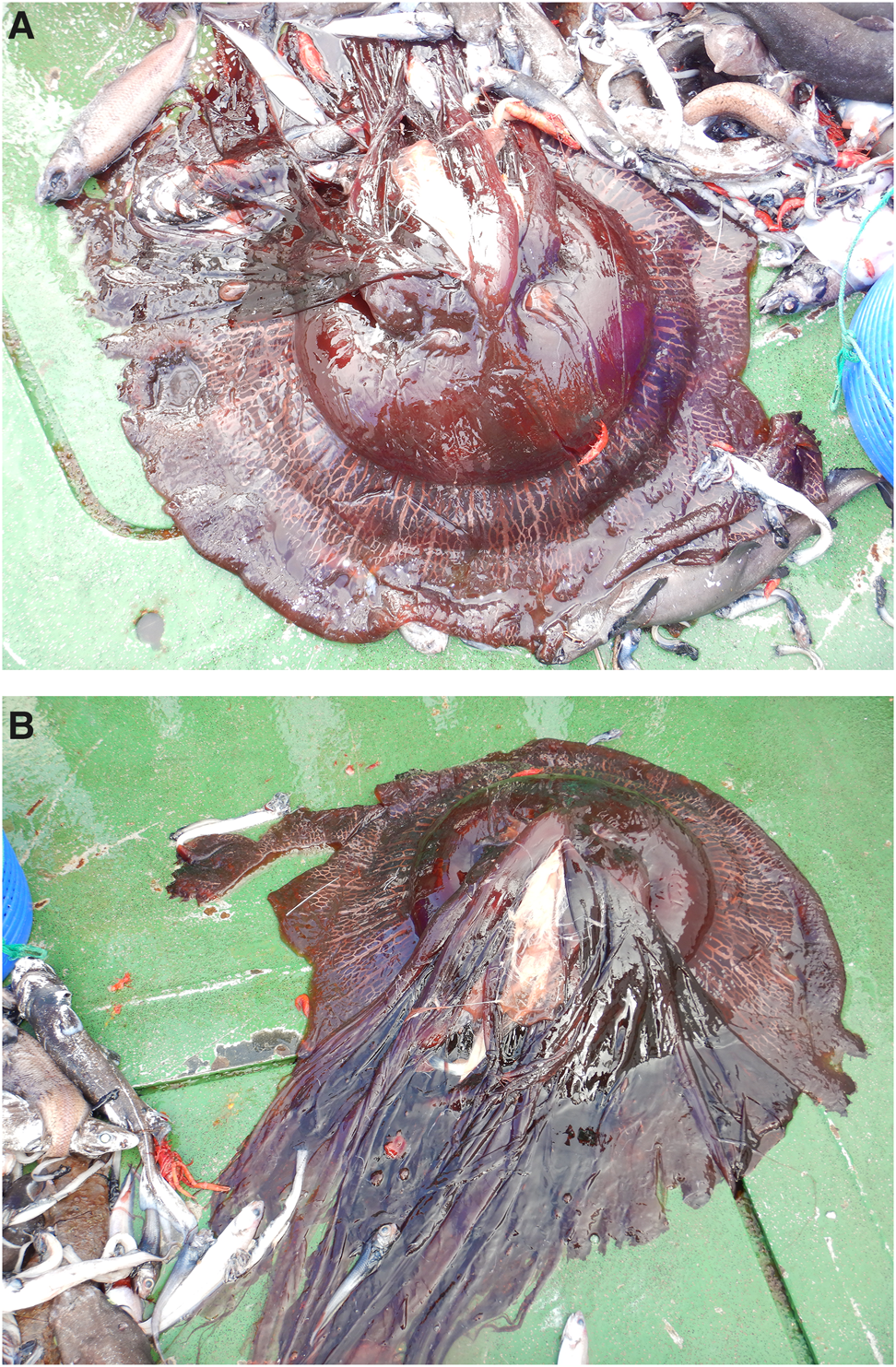
Figure 1. Photographs of a specimen of Stygiomedusa gigantea with an estimated bell diameter of 45.6 cm, on the deck of the RV Mirabilis in August 2022. Note the typical red/black colour, the broad oral arms and the network of anastomosing radial canals.
Jarms and Morandini (Reference Jarms and Morandini2019) define S. gigantea (in part) as having rhopalar radial canals that are ‘simple proximally for one quarter of their length before anastomosing in a net-like fashion…[while]…inter-rhopalar radial canals are simple for half their length before joining the anastomoses of the rhopalar radial canals’. This disagrees with the observations here, and clear anastomoses between all radial canals can be seen throughout their length (Figure 1). While this resembles the partial abnormality noted by Russell and Rees (Reference Russell and Rees1960) of an adult caught measuring 50 cm across the bell in the Bay of Biscay, it is in full agreement with the observations of Harbison et al. (Reference Harbison, Smith and Backus1973) of a specimen with a bell diameter of 140 cm collected in the mid North Atlantic. And it agrees too with the description of Stygiomedusa stauchi made by Repelin (Reference Repelin1967) of an individual caught (75 cm bell diameter) off the mouth of the Congo River, and of a specimen investigated by Cornelius (Reference Cornelius1973) from off Cape Horn.
The pattern and arrangement of radial canals in (e.g.) Rhizostomeae are commonly used to diagnose genus identity, in part (Jarms and Morandini, Reference Jarms and Morandini2019). And while Cornelius (Reference Cornelius1972) might have considered them useful to separate what were then regarded as two species of Stygiomedusa (Stygiomedusa fabulosa Russell, Reference Russell1959; S. stauchi Repelin, Reference Repelin1967), Harbison et al. (Reference Harbison, Smith and Backus1973) suggested that differences in ‘…radial canal patterns are in all likelihood due solely to ontogenetic reasons.’ With access to additional material, Cornelius (Reference Cornelius1973) revised his earlier thoughts, noting that hitherto collected specimens represented a continuous morphological series between those displaying ‘unbranched interrhopalar canals and rhopalar canals with a simple ‘back-to-back B’ anastomosis as in the holotype of S. fabulosa (Russell and Rees, Reference Russell and Rees1960) to [those with] a completely reticulated condition as in the holotype of S. stauchi (Repelin, Reference Repelin1967)’. The specimen reported here is of a similar size to that reported by Cornelius (Reference Cornelius1973) with full anastomoses but is much smaller than those reported by Harbison et al. (Reference Harbison, Smith and Backus1973) or Repelin (Reference Repelin1967) in a similar condition. This suggests that ontogeny may not explain the pattern of reticulation in Stygiomedusa, but in the absence of either the specimen itself or molecular material, we must concur with conventional wisdom. That said, we urge colleagues to pay attention to the pattern of radial canals in future and to also try and collect samples for DNA analysis.
The diversity of form amongst species within the paraphyletic family Ulmaridae (Bayha et al., Reference Bayha, Dawson, Collins, Barbeitos and Haddock2010; Lindsay et al., Reference Lindsay, Grossmann, Montenegro and Morandini2023) is unrivalled among Discomedusae. Some species lack marginal tentacles (Deepstaria, Stygiomedusa, Tiburonia), some have greatly elongated oral arms (Stygiomedusa) and others possess short oral arms that do not extend beyond the bell margin (Deepstaria). Although we know little about the life-cycle of most species, we can assume that the majority are metagenetic, though one (Stygiomedusa) is considered to be viviparous (Russell, Reference Russell1959). Uniquely among Discomedusae, the family Ulmaridae includes a number of deep-water species. These are distributed among six subfamilies (Table 1). At present, each of these subfamilies is represented by a single genus, all of which are monotypic except for Deepstaria, which is considered to have two species.
Table 1. The date of first designation of the different subfamilies and genera in the family Ulmaridae
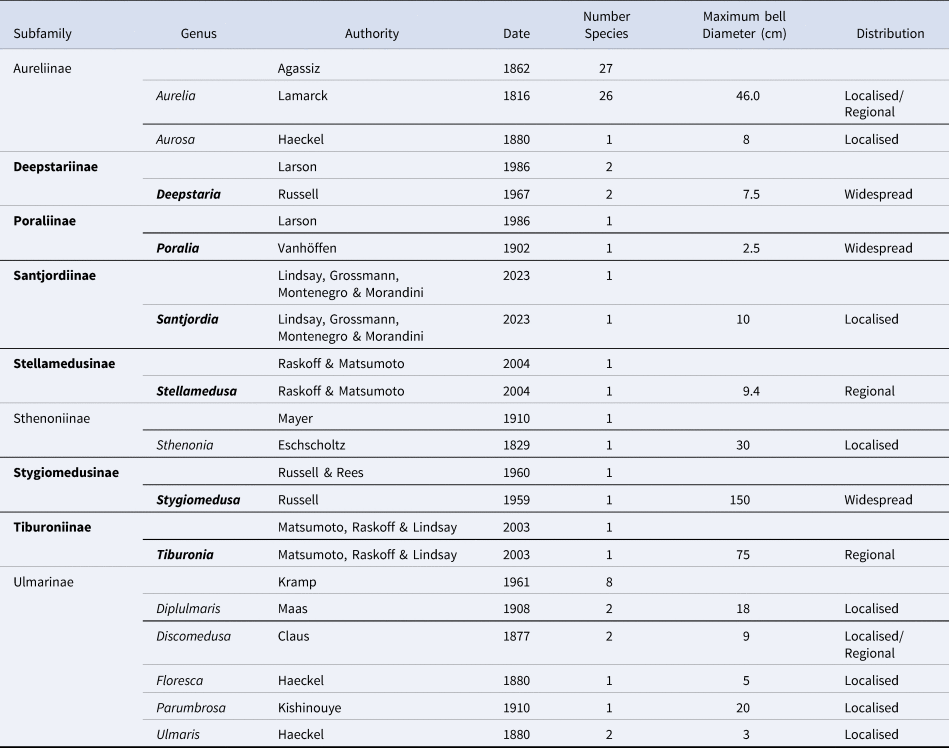
Also shown are the number of species, the maximum bell diameter of species and known distribution. Taxa in bold typeface, meso- or bathypelagic. Data from WoRMS (2024) and Jarms and Morandini (Reference Jarms and Morandini2019).
Within the Ulmaridae, genera that are meso-or bathypelagic have a more recent date of first description (mean = 1976, Table 1) than those with a shallower bathymetric distribution (mean = 1873, Table 1) (ANOVA F 1,12 = 25.34, P < 0.001, Table 1). While this can be explained, in part, as a reflection of recent advances in our ability to explore the deep-sea, it also mirrors an increase in effort to sample an environment in which such species are uncommon or rare (Lindsay et al., Reference Lindsay, Grossmann, Montenegro and Morandini2023). The date of first description is generally related to geographic distribution (e.g. Gibbons et al., Reference Gibbons, Richardson, Angel, Buecher, Esnal, Fernandez Alamo, Gibson, Itoh, Pugh, Boettger-Schnack and Thuesen2005), with taxa having an earlier date of description being more widespread. The data presented in Table 1 clearly refute that idea, at the level of the genus or the subfamily level, because the more recently described species have generally wider, or assumed wider, distributions than earlier-described taxa!
Supplementary Material
The supplementary material for this article can be found at https://doi.org/10.1017/S0025315424000833
Acknowledgements
MJG would like to thank the Namibian Ministry of Fisheries and Marine Resources and the National Marine Information and Research Centre in Swakopmund for their ongoing support of his research into regional jellyfish. The comments of the two anonymous reviewers have greatly helped to clarify the text and arguments posited here, for which we are grateful.
Author Contributions
MJG conceived the idea for the work based on photographs taken by EM and LK. All authors contributed to the writing and data analysis, and all authors have reviewed the manuscript.
Financial Support
MJG would like to thank the NRF and the University of the Western Cape for their support of his research, while EM and LK acknowledge the Ministry of Fisheries and Marine Research.
Competing interest
None.
Ethical Standards
Not applicable.
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article.




