Introduction
Crustaceans commonly known as mud or ghost shrimps belong to the infraorders Axiidea and Gebiidea, both previously treated as thalassinids, have body forms adapted for burrowing in marine, primarily soft-bottom intertidal or subtidal (<200 m) areas (Dworschak, Reference Dworschak2005). They show symbiotic associations with organisms that inhabit the burrows, among which are endoparasites, e.g. bacteria of genus Vibrio and Pseudomonas, cestodes and rhizocephalan barnacles, and ectoparasites such as copepods and bopyrid isopods (Dworschak et al., Reference Dworschak, Felder, Tudge, Schram and von Vaupel2012).
Axiideans and gebiideans are the fourth largest group of decapods, after caridean shrimps, anomurans and brachyurans, parasitized by bopyrid isopods (Boyko et al., Reference Boyko, Williams and Shields2017). Most bopyrids of axiideans and gebiideans are ectoparasites, i.e. the female and dwarf male dwell in the hosts' branchial chamber, producing a characteristic bulge, or they can be found between the host's pleopods (Phyllodurus Stimpson, Reference Stimpson1857), or in a single species, Axiophilus mirabiledictu (Markham and Dworschak, Reference Markham and Dworschak2005), endoparasitically (Markham and Dworschak, Reference Markham and Dworschak2005). The subfamilies Keponinae and Pseudioninae comprise species that parasitize both shrimp infraorders whereas Phyllodurinae is known only from hosts in Gebiidea (Boyko et al., Reference Boyko, Williams and Shields2017). In Mexico one species of Phyllodurinae and five species of Pseudioninae have been recorded parasitizing burrowing shrimps, of them only the pseudionines Orthione furcata (Richardson, Reference Richardson1904), Progebiophilus upogebiae (Hay, Reference Hay1917) and Robinione overstreeti (Adkison and Heard, Reference Adkison and Heard1995) are currently known to occur along the Mexican Atlantic coast (Román-Contreras, Reference Román-Contreras, Álvarez and Rodríguez-Almaraz2008; Romero-Rodríguez and Álvarez, Reference Romero-Rodríguez and Álvarez2021).
Examination of a pair of bopyrid isopods found in the branchial chamber of a ghost shrimp from Veracruz, Mexico, revealed that the parasite's characteristics are similar to those of the pseudionine genus Ionella Bonnier, Reference Bonnier1900 which currently includes four species (Boyko et al., Reference Boyko, Bruce, Hadfield, Merrin, Ota, Poore and Taiti2023), all of them reported from the Pacific Ocean (Bonnier, Reference Bonnier1900; Shiino, Reference Shiino1964a, Reference Shiino1964b; Danforth, Reference Danforth1970; Markham, Reference Markham and Crosnier1994). The aims of this study are to provide a description of the pair of bopyrids examined and to report for the first time a species of Ionella from the Atlantic.
Materials and methods
A single burrowing shrimp bearing a pair of bopyrid isopods was provided by Dr José Luis Villalobos, it was collected manually using a yabby pump on sandy substrate in the intertidal zone of Montepío beach, Veracruz, Mexico. A map and a general description of this area were provided by Hernández et al. (Reference Hernández, Álvarez and Villalobos2010).
The specific host identity, size (carapace length, CL), sex (based on the position of the gonopores on the pereopods) and the shape of first pleopods were determined according to Dworschak et al. (Reference Dworschak, Felder, Tudge, Schram and von Vaupel2012) and Hernáez et al. (Reference Hernáez, Windsor, Paula and Santana2020). The parasites were gently removed from the host to record their total lengths (TL), measured from the anterior margin of the first pereomere of the longer side to the posterior margin of the pleon (Romero-Rodríguez and Álvarez, Reference Romero-Rodríguez and Álvarez2020). The embryos of the brood mass of the female parasite were counted; ten embryos were randomly selected in order to measure their width (d1), length (d2) and to calculate their volume (V) using the formula V = π(d1)2 × (d2)/6 (Cericola and Williams, Reference Cericola and Williams2015; Romero-Rodríguez and Álvarez, Reference Romero-Rodríguez and Álvarez2020). Measurements were made to the nearest 0.1 mm using an ocular micrometer attached to a compound microscope. Drawings made with a camera lucida were used to construct figures using Adobe Illustrator. Digital photographs of the parasites were taken with a Leica DFC490 camera mounted on a Leica Z16APOA stereomicroscope provided with the Leica Application Suite version 4.3.0. Both parasites and host are deposited in the Colección Nacional de Crustáceos (CNCR), housed at the Instituto de Biología of the Universidad Nacional Autónoma de México (UNAM).
Results
Suborder Epicaridea Latreille, Reference Latreille1825
Family Bopyridae Rafinesque, Reference Rafinesque1815
Subfamily Pseudioninae Codreanu, Reference Codreanu1967
Genus Ionella Bonnier, Reference Bonnier1900
Ionella fimbriata sp. nov.
(Figures 1–3; Tables 1–3)
[urn:lsid:zoobank.org:pub:D8265FC1-EC63-4021-B8DA-BD98D25E4693]
Material examined
Holotype
One ovigerous female 7.0 mm TL, within the right branchial chamber of Neocallichirus grandimana (Gibbes, Reference Gibbes1850) male of 16.68 mm CL, J.L. Villalobos det. host, Montepío beach, San Andrés Tuxtla, Veracruz, Mexico (18°38′41.04″N 95°05′50.02″W), 18 August 2022, J.L. Villalobos et al. coll., CNCR-37084.
Allotype
One male 3.08 mm TL, same collection data as for holotype, CNCR-37084.
Etymology
The name is from the Latin fimbriatus (fringed), alluding to the appearance of the female pereopods with intricate elongate cuticular extensions on margins of bases and ischia, resembling fringes.
Diagnosis
Female: body white in colour, lacking pigmentation and eyes, oval in shape, nearly symmetrical, seven pereomeres distinct and laterally wider in medial region, seven pairs of pereopods increasing in size posteriorly with elongate cuticular extensions on upper margin of bases and lower margin of ischia, marsupium tightly closed, six pleomeres distinct dorsally but 1–5 fused laterally, five pairs of triangular lateral plates with tuberculate margins, five pairs of pinnate biramous pleopods bearing tuberculate projections, uropods uniramous. Male: whitish in colour with minute setae scattered on dorsal and ventral surfaces of pereomeres. Head semicircular, distinct from pereon, pereomeres larger posteriorly; pleomeres narrower than pereomere 7, recurved posteriorly, final pleomere small, semicircular; five pairs of biramous globose pleopods, one pair of uniramous uropods.
Type locality
Montepío beach (18°38′41.04″N 95°05′50.02″W), intertidal zone, San Andrés Tuxtla, Veracruz, Mexico.
Description
Holotype. Ovigerous female (Figures 1A, B & 2A–L), body length 7.0 mm, maximal width 11.54 mm at pereomere 5, head length 1.96 mm, head width 2.66 mm, pereon length 2.0 mm, pleon length 2.56 mm, pleon width 9.80 mm. Head rounded with semi-triangular anterolateral projections, two slight semi-square anterodorsal depressions. Anterior margin nearly straight, frontal lamina recurved backwards. Oral cone protruding on anteromedial margin. Posterior margin rounded, distinct from first pereomere. Eyes absent (Figures 1A & 2A). Antennae separated from each other. Antennule short, three-segmented, basal segment largest and rounded, second segment tapering, distal one smaller, bearing fine distal setae (Figure 2C). Antenna long, six-segmented, exceeding lateral margins of head, basal segment largest, subsequent segments gradually decreasing in size, distal segment tiny, bearing fine apical setae (Figure 2C). Maxilliped lacking palp, surface smooth, anterior segment large, suboval, margin densely lined with setae; posterior segment smaller, rounded triangular in shape, plectron truncated, bearing setae on upper distal margin (Figure 2D). Barbula with one stout, acute, falcate projection on each side, medial margin bearing short rounded projections of variable sizes, dorsomedial region protruding as conspicuous rounded bulge (Figure 2E).

Figure 1. Ionella fimbriata sp. nov. (CNCR-37084) parasite of Neocallichirus grandimana in Veracruz, Mexico: (A) ovigerous female, holotype, dorsal view, pleomeres 2–4 medially damaged; (B) same, ventral view, white arrow indicating male attached on pleopods; (C) male, allotype, dorsal view; (D) same, ventral view, left pereopod 5 damaged. Scale bars: A–B 1.0 mm, C–D 0.5 mm.

Figure 2. Ovigerous female of Ionella fimbriata sp. nov., holotype (CNCR-37084), parasite of Neocallichirus grandimana in Veracruz, Mexico. (A) habitus, dorsal view, pleomeres 2–4 medially damaged; (B) same, ventral view; (C) antennule and antenna; (D) maxilliped; (E) barbula; (F) right pereopod 1; (G) right pereopod 7; (H) right oostegite 1, dorsal view; (I) same, ventral view; (J) detail of pleomere 6 and uropods; (K) left pleopod 1; (L) same, lateral view. Scale bars: A, B, D, F–I, K–L 1.0 mm, C, E, J 0.5 mm.
Pereon broadly rounded, of seven distinct pereomeres, all wider laterally than in central region. Pereomeres 2–5 folded up producing dorsomedial semi-triangular knob (Figures 1A & 2A). All pereomeres bearing conspicuous crenate coxal plates (Figure 2A). Pereomeres 1–4 with semi-rectangular dorsolateral bosses (Figure 2A). Seven pairs of pereopods, increasing in size posteriorly but of similar form. Basis elongate with cuticular extensions on upper margin, ischium elongate with lower margin extended, merus short and rectangular in shape, carpus triangular with tiny scales at distal margin, propodus oblong with minute setae on dactylus insertion, dactylus curved, stout and acute (Figure 2F, G). Pereopod 1 with two long and slender branches on upper margin of basis (Figure 2F), ischium lower margin extended as thin whole plate bounded by slender projections (Figure 2F). Pereopods 1 and 2 with a single tapering projection near distal part of lower margin of basis (Figure 2B, F). Pereopods 2–7 with elongate cuticular extensions on upper margin of basis increasing in number and thickness posteriorly (Figure 2B, G) and lower margin of ischium extending as long and intricate cuticular extensions (Figure 2G). Marsupium tightly closed by five pairs of oostegites (Figures 1B & 2B). First pair of oostegite surfaces smooth, divided by deep groove, anterior section large, ovoid, bearing small setae along margin, with 14 long, slender cuticular extensions on posterior margin; posterior section triangular, shorter but wider than anterior one, lacking posterolateral point (Figure 2H); inner ridge with curved, smooth, protruding lobule on proximal portion, distal portion bearing eight thick projections, first four bifurcated distally (Figure 2I). Oostegites 2–5 increasing in size posteriorly, imbricated from back to front, rectangular, with margins lined by conspicuous setae, dorsally with setae of variable sizes clearly more numerous on pairs 4 and 5 (Figure 2B).
Pleon of six pleomeres, dorsally distinct. Pleomeres 1–5 curved backwards, fused laterally to each other and to triangular lateral plate with tuberculated margins, edges barely indicated by slight intersegmental lines (Figures 1A & 2A), pleomere 6 smaller, triangular, almost enclosed by pleomere 5 (Figure 2A, J). Ventrally, almost entirely covered by five pairs of biramous pleopods decreasing in size posteriorly, directed inwards, slightly overlapping in medial region of pleon (Figure 2B), both rami of pinnate shape with tuberculated projections (Figure 2K, L). Pleopods 1–3 with endopods longer than exopods (Figure 2K, L), 4 and 5 with both rami of similar sizes. Uropods uniramous, short, rectangular (Figure 2J).
Male, allotype (Figures 1C, D & 3A–G): length 3.08 mm, maximal width 1.53 mm (at pereomere 7), head length 0.35 mm, head width 1.18 mm, pereon length 1.76 mm, pleon length 0.91 mm, pleon width 1.35 mm.
Head semicircular, wider than long, surface smooth, posterior margin slightly curved, distinct from first pereomere, eyes on posterolateral margins (Figures 1C & 3A). Antennule short, three-segmented, first segment larger, third segment tiny, tipped with small setae (Figure 3C). Antennae long, seven-segmented, slightly exceeding lateral margin of head, first four segments large and similar in size, latter three ones decreasing in size progressively, last three segments bearing fine setae (Figure 3C).

Figure 3. Male of Ionella fimbriata sp. nov., allotype (CNCR-37084), parasite of Neocallichirus grandimana in Veracruz, Mexico. (A) habitus, flattened under a cover slip, dorsal view; (B) same, not flattened, ventral view, left pereopod 5 damaged; (C) antennule and antenna; (D) right pereopod 1; (E) left pereopod 7; (F) pleopod 1 pair; (G) detail of pleomere 5, pleotelson and uropods. Scale bars: A–B 0.5 mm, C–G 0.1 mm.
Pereon of seven distinct pereomeres, slightly enlarged posteriorly, posterior margin overlapping adjoining anterior margin, rounded lateral margins directed downwards (Figures 1C & 3A). Midventral tubercles absent. Seven pairs of pereopods of similar shape, increasing in size posteriorly (Figures 1D & 3B). Basis elongate, ischium approximately half size of basis, meri and carpi distinct, small, rounded, propodi ovoid, dactyli acute (Figure 3D, E).
Pleon of five pleomeres plus small semicircular pleotelson. First pleomere narrower than final pereomere, others progressively recurved, narrower posteriorly. All segments distinctly separated dorsally and laterally, lateral margins rounded (Figures 1C & 3A). Five pairs of globose biramous pleopods, slightly decreasing in size posteriorly (Figures 1D & 3B). Endopods longer than exopods, directed medially (Figures 1D & 3B, F). One pair of globose uniramous uropods, longer than last pair of pleopods (Figure 3B, G).
Distribution
Currently known only from southern Veracruz, Mexico, in the southwest Gulf of Mexico.
Remarks
Several characteristics of the pair of bopyrid isopods examined, such as female with body broadly oval, barely distorted, suboval maxilliped lacking palp and densely fringed by setae, first pair of oostegites without posterolateral point, biramous pleopods and uniramous uropods; male with extended head, all body regions distinct, lacking midventral tubercles, biramous pleopods and uniramous uropods, show their affinity with Ionella (Bonnier, Reference Bonnier1900; Markham, Reference Markham and Crosnier1994). Currently, this genus is comprised of four species (Boyko et al., Reference Boyko, Bruce, Hadfield, Merrin, Ota, Poore and Taiti2023): Ionella agassizii Bonnier, Reference Bonnier1900, I. compressa (Shiino, Reference Shiino1964a, Reference Shiino1964b), I. maculata Markham, Reference Markham and Crosnier1994 and I. murchisoni Danforth, Reference Danforth1970.
The female examined differs from the other species of the genus in several characters (Table 1). In I. agassizii, both segments of the maxilliped are fused, the barbula has one large oblique and pointed projection on each side and bears a series of numerous very small secondary lamellae on the medial margin, the digitations on the inner margin of the first pair of oostegites are small and not distally bifurcated, the margins expanded at bases and ischia extend as a single thin sharp-edged blade and the five pairs of biramous pleopods are foliaceous in shape (Bonnier, Reference Bonnier1900; Shiino, Reference Shiino1964a). In I. compressa, the coxal plates are distinct on the first four segments but only on the longer side of the body, all pereopods are relatively long, the two last pleomeres and pleotelson are short, narrow and crowded together and the uropods are very small and tuberculate (Shiino, Reference Shiino1964b). In I. maculata, the antennae are minute, the barbula bears two short broad projections on each side, pereomeres 6 and 7 are produced laterally into slender falcate posterolateral projections and the biramous pleopods are foliate (Markham, Reference Markham and Crosnier1994). In I. murchisoni, pereomeres 2–7 are fused medially into a slightly elevated margin, the dorsolateral bosses on pereomeres 1–4 are triangular in outline, the swelled margins on the bases and ischia of pereopods 2–7 are a single rounded blade, based on the illustration and description (Danforth, Reference Danforth1970, Figure 1B), and the pleon is flat and triangular in shape with no dorsal evidence of segmentation (Danforth, Reference Danforth1970).
Table 1. Comparison of some morphological characteristics of bopyrid isopod females belonging to the genus Ionella Bonnier, Reference Bonnier1900

a Based on the illustrations and descriptions; DLB, dorsolateral bosses on pereomeres; Cox Pls, coxal plates; Ls, long side of body; Ss, short side of body; Ant 1, antennule; Ant 2, antenna; Max, maxilliped; Lp, lateral plate.
(1) Bonnier, Reference Bonnier1900; (2) Danforth, Reference Danforth1970; (3) Markham, Reference Markham and Crosnier1994; (4) Shiino, Reference Shiino1964a; (5) Shiino, Reference Shiino1964b; (6) this study.
Differences between males of the new species and all others of Ionella species are also recognized (Table 2). In I. agassizii, the pleopods are rod-like with the endopod longer than the exopod and the oval uropods bear small rigid setae. Males of I. compressa have pereomeres with truncated margins and uniramous pleopods. In I. murchisoni, the pleopods are biramous with the endopod longer than the exopod, both finger-shaped and arising from a common peduncle, and the uropods are ‘extremely tiny’ (Danforth, Reference Danforth1970). Males of I. maculata can be recognized by their minute antennae and pleopods varying from biramous in pairs 1 and 2 to uniramous in pairs 3–5 (Markham, Reference Markham and Crosnier1994).
Table 2. Comparison of some morphological characteristics of bopyrid isopod males belonging to the genus Ionella Bonnier, Reference Bonnier1900
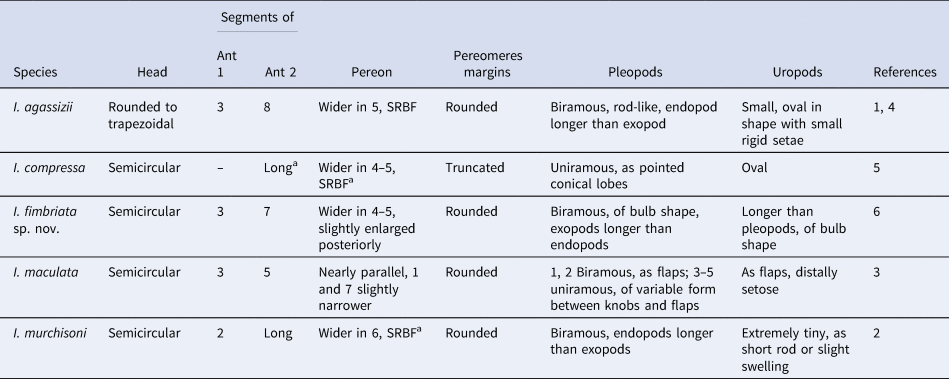
a Based on the illustrations and descriptions; Ant 1 = antennule; Ant 2 = antenna; SRBF = slightly reduced anteriorly and posteriorly.
(1) Bonnier, Reference Bonnier1900; (2) Danforth, Reference Danforth1970; (3) Markham, Reference Markham and Crosnier1994; (4) Shiino, Reference Shiino1964a; (5) Shiino, Reference Shiino1964b; (6) this study.
The females of I. fimbriata sp. nov. can be distinguished by a barbula with one stout, acute and falcate projection on each side, a medial margin bearing triangular rounded projections of variable size; seven pairs of pereopods that increase in size posteriorly and bear semicylindrical elongate cuticular extensions on the upper margins of bases and flatter and thinner cuticular extensions on the lower margins of ischia; five pleomeres curved backwards, fused laterally to each other and to a triangular lateral plate of tuberculated margins, as well as five pairs of biramous pleopods of pinnate shape with tuberculated projections. Likewise, males of this species can be recognized by seven pairs of pereopods that increase in size posteriorly but are of similar shape; five pairs of globose biramous pleopods, in which the endopods are longer than the exopods, and uniramous uropods of similar shape to the pleopods but larger in size than last pair of pleopods.
Reproductive remarks
The ovigerous female examined had 2194 embryos in the brood chamber, which were classified as stage II because they were oblong in shape and with no traces of developing appendages or ocular line pigments (Beck, Reference Beck1980). The embryos' length and width varied from 0.309 to 0.364 mm and from 0.182 to 0.236 mm, respectively; the mean length (0.336 ± 0.018 mm) and width (0.215 ± 0.017 mm) were also estimated. The mean volume calculated was 0.008 ± 0.001 mm3, with a range that varied between 0.006 and 0.011 mm3.
Discussion
Currently, most species included in Ionella do not fulfil the three characters used to define the genus by Bonnier (Reference Bonnier1900): females with pleomeres lacking lateral plates, uropods uniramous and biramous pleopods in both females and males. Ionella maculata is the only species reported as not having lateral plates on the pleomeres (Markham, Reference Markham and Crosnier1994) while the type-species of the genus, I. agassizii, has lateral plates on the pleomeres (Shiino, Reference Shiino1964a). Likewise, the pleopods of I. compressa males are uniramous and those of I. maculata males are not entirely biramous (Table 2). Based on a wider list of generic characters provided by Markham (Reference Markham and Crosnier1994), we suggest that the following could improve the characterization of this genus: females with oval body, barely distorted; maxilliped rounded or suboval in shape, lacking palp and anterior segment densely fringed by setae; first pair of oostegites with subcircular anterior segment, inner ridge ornamented only laterally, posterior segment shorter without posterolateral point; bases and ischia of pereopods with wide margins, and biramous pleopods. Males with rounded extended head, midventral tubercles absent, at least the first two pairs of pleopods biramous, and uniramous uropods. However, some of these characters are not described for I. compressa and I. murchisoni (Table 1) which remain poorly known (Markham, Reference Markham and Crosnier1994). Examination of specimens is therefore needed to confirm the morphological details of these species in order to complete the characterization of Ionella. Based on the current taxonomic information available, keys are provided herein for both females and males of species in this genus.
All hosts of Ionella species are axiidean ghost shrimps belonging to Callianassidae and Callichiridae (Table 3). To our knowledge, N. grandimana had not been recognized as a host of Ionella species, or any other bopyrid species. The record of I. fimbriata sp. nov. in the northwest Atlantic represents a significant distribution range extension for Ionella, because this genus was previously restricted to the Pacific Ocean (Table 3). Similarly, the record of I. fimbriata sp. nov. increases to 14 the number of bopyrid species in Veracruz, Mexico, where until 2005 only three bopyrid had been recorded. Recent studies reported nine more bopyrid species in the region, three of them new species (Romero-Rodríguez and Álvarez, Reference Romero-Rodríguez and Álvarez2023; this study), which makes to Veracruz one of the regions with the greatest diversity of bopyrid parasites in Mexico.
Table 3. Hosts and geographic distribution of Ionella Bonnier, Reference Bonnier1900 species
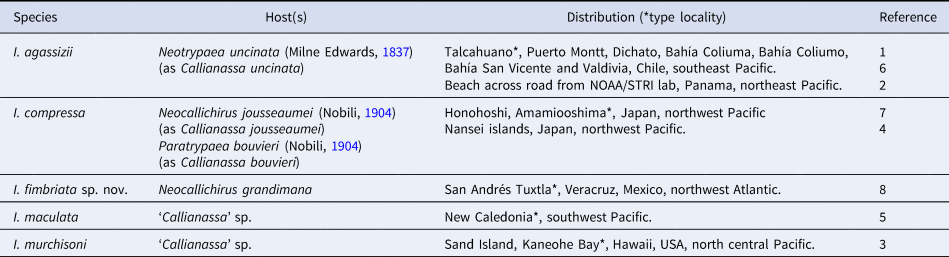
(1) Bonnier, Reference Bonnier1900; (2) Boyko et al., Reference Boyko, Williams and Shields2017; (3) Danforth, Reference Danforth1970; (4) Itani, Reference Itani2004; (5) Markham, Reference Markham and Crosnier1994; (6) Shiino, Reference Shiino1964a; (7) Shiino, Reference Shiino1964b; (8) this study.
The fecundity of I. fimbriata sp. nov. is similar to the ~2000 embryos reported from females of I. agassizii of 6–8 mm TL (Muñoz and George-Nascimento, Reference Muñoz and George-Nascimento1999, Figure 3), but is lower than that reported for other bopyrid females of similar size (Cericola and Williams, Reference Cericola and Williams2015). The latter could be related to the mean embryo size and volume calculated for I. fimbriata sp. nov. which is higher than those calculated for other bopyrid species (Cericola and Williams, Reference Cericola and Williams2015; Romero-Rodríguez and Álvarez, Reference Romero-Rodríguez and Álvarez2023) because in other decapod crustacean groups it has been observed that fecundity is related to egg size, i.e. species with reduced fecundity produce eggs large in size while those species with small eggs have a high fecundity (Ramírez-Llodra, Reference Ramírez-Llodra2002), as was observed in Pseudostegias atlantica Lemos de Castro, Reference Lemos de Castro1965 (Romero-Rodríguez and Álvarez, Reference Romero-Rodríguez and Álvarez2023). However, it is important to highlight that all the reproductive information of I. fimbriata sp. nov. comes from a single individual. This shows the need to enhance the knowledge on the reproductive biology of this species and other bopyrid isopods.
Key to females of species of Ionella Bonnier.
1a Pereon of seven pereomeres clearly distinct dorsally……………………………………………2
1b Pereon of seven pereomeres weakly indicated by demarcations, only first pereomere distinct side to side, all others fused dorsomedially…………………………….…….…Ionella murchisoni
2a Coxal plates on both sides of the body, at least on pereomeres 1 and 2…………..……..……..3
2b Coxal plates only on pereomeres 1–4 of long side of body…………………..Ionella compressa
3a Short antennule and long antenna; barbula with one acute projection on each side………………..4
3b Antennae minute, barbula with two broad projections on each side………..…Ionella maculata
4a Pereopods with upper margin of bases and lower margin of ischia expanded as thin plates; pleopods of foliate shape with irregular margins………………..……….………..Ionella agassizii
4b Pereopods with upper margin of bases and lower margin of ischia with elongate cuticular extensions; pleopods of pinnate shape with tuberculated projections……Ionella fimbriata sp. nov.
Key to males of species of Ionella Bonnier.
1a Pereomeres of rounded margins, at least first two pairs of pleopods biramous……………….2
1b Pereomeres with truncated margins, five pairs of uniramous pleopods…….Ionella compressa
2a Five pairs of biramous pleopods ……………………………………………………….………3
2b Pleopods 1 and 2 biramous, 3–5 uniramous……………………….…………..Ionella maculata
3a Uropods uniramous, resembling pleopods in appearance…..………………………….……….4
3b Uropods uniramous, extremely tiny, appearing as short rods or swelling……..Ionella murchisoni
4a Uropods small, oval-shaped bearing small rigid setae………………..………..Ionella agassizii
4b Uropods longer than last pleopods, globose in shape….…….………..Ionella fimbriata sp. nov.
Acknowledgments
We thank S. Guzmán-Gómez (LANABIO/IB/UNAM) for her assistance in taking the photographs for Figure 1, and J. L. Villalobos-Hiriart (Colección Nacional de Crustáceos/IB/UNAM) for providing the specimens examined and for his support with the laboratory work. We also thank the reviewers for their helpful comments on the manuscript.
Author contributions
J. R.-R.: conceptualization, specimen identification, writing original draft preparation and editing. F. A.: conceptualization, original draft reviewing and editing. All authors reviewed and accepted the latest version of the manuscript.
Financial support
This work was supported by a scholarship granted to the first author by the Consejo Nacional de Humanidades, Ciencia y Tecnología (CONAHCyT) through the program ‘Becas Posdoctorales por México’.
Competing interest
None.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.








