Article contents
Does size matter? The effects of body size and declining oxygen tension on oxygen uptake in gastropods
Published online by Cambridge University Press: 03 November 2011
Abstract
Metabolic rate is one of the most frequently measured physiological variables and the relationship between oxygen uptake and body mass is one of the most controversial issues in biology. The present study used closed chamber respirometry to compare the oxygen uptake of 32 species of benthic British gastropod molluscs of a wide size-range (from less than 0.001 g to greater than 10 g dry tissue weight). We investigated the effects of body size on the respiratory rate at 10°C to explore the evolutionary and phylogenetically determined patterns of metabolic scaling both among different gastropods groups, and within siphonate and asiphonate caenogastropods. Resting oxygen uptake (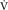 O2) increased with body mass (W) with a slope value of 0.6 using both ordinary least squares (OLS) and standard major axis (SMA) where N = 488, over a 6 fold range of body mass. The slopes b of the regression lines relating oxygen uptake to body mass were similar for all heterobranch molluscs and most caenogastropods. Highest mass-specific rates for oxygen consumption were found for the smallest littorinid species. Trophic mode significantly affected the amount of oxygen consumed with higher oxygen uptake in herbivores than other groups, including detritivores and predators. All of the gastropods reduced their oxygen consumption when exposed to declining oxygen conditions; however, about a third of the species exhibited partial regulation at higher oxygen partial pressures. When exposed to 20% normal saturation levels, smaller gastropods respired at approximately 25% of their rates in fully saturated seawater whereas larger species (above 0.1 g dry tissue weight) respired at approximately 35% of the values recorded at full saturation. Our study suggests that a scaling exponent relating O2 to body mass of 0.6 is typical and may be ‘universal’ for gastropods. It is below the 0.75 scaling exponent which has been proposed for ectothermic invertebrates. It is concluded that size does matter in determining the metabolic patterns of gastropods and that the quantity of oxygen consumed and the energy balance of gastropods is affected by activity, food type and exposure to declining oxygen conditions.
O2) increased with body mass (W) with a slope value of 0.6 using both ordinary least squares (OLS) and standard major axis (SMA) where N = 488, over a 6 fold range of body mass. The slopes b of the regression lines relating oxygen uptake to body mass were similar for all heterobranch molluscs and most caenogastropods. Highest mass-specific rates for oxygen consumption were found for the smallest littorinid species. Trophic mode significantly affected the amount of oxygen consumed with higher oxygen uptake in herbivores than other groups, including detritivores and predators. All of the gastropods reduced their oxygen consumption when exposed to declining oxygen conditions; however, about a third of the species exhibited partial regulation at higher oxygen partial pressures. When exposed to 20% normal saturation levels, smaller gastropods respired at approximately 25% of their rates in fully saturated seawater whereas larger species (above 0.1 g dry tissue weight) respired at approximately 35% of the values recorded at full saturation. Our study suggests that a scaling exponent relating O2 to body mass of 0.6 is typical and may be ‘universal’ for gastropods. It is below the 0.75 scaling exponent which has been proposed for ectothermic invertebrates. It is concluded that size does matter in determining the metabolic patterns of gastropods and that the quantity of oxygen consumed and the energy balance of gastropods is affected by activity, food type and exposure to declining oxygen conditions.
- Type
- Research Article
- Information
- Journal of the Marine Biological Association of the United Kingdom , Volume 92 , Issue 7 , November 2012 , pp. 1603 - 1617
- Copyright
- Copyright © Marine Biological Association of the United Kingdom 2011
References
REFERENCES
- 29
- Cited by


