Introduction
The brown comber, Serranus hepatus (Linnaeus, 1758), is a small-sized demersal fish that belongs to the family Serranidae. It occurs in the Eastern Atlantic, Mediterranean and Black Seas. The species inhabits sandy and muddy bottoms with seagrass and rocks at depths ranging from 5 to 200 m (Smith, Reference Smith1981; Whitehead et al., Reference Whitehead, Bauchot, Hureau, Nielsen and Tortonese1986; Jardas, Reference Jardas1996). Serranus hepatus is caught mainly by bottom trawl and discarded at sea (Labropoulou et al., Reference Labropoulou, Tserpes and Tsimenides1998; Dulčić et al., Reference Dulčić, Matić-Skoko, Paladin and Kraljević2007; Bilecenoğlu, Reference Bilecenoğlu2009), while two congeneric species, S. cabrilla and S. scriba, are both commercial species albeit of rather low economic value.
Several studies have been conducted on various aspects of the species biology in the Mediterranean Sea. More specifically, age and growth have been studied by Wagué and Papaconstantinou (Reference Wagué and Papaconstantinou1997), Labropoulou et al. (Reference Labropoulou, Tserpes and Tsimenides1998), Dulčić et al. (Reference Dulčić, Matić-Skoko, Paladin and Kraljević2007), Bilecenoğlu (Reference Bilecenoğlu2009), Yapici et al. (Reference Yapici, Filiz and Ozkan2012), Soykan et al. (Reference Soykan, Ilkyaz, Metin and Kinacigil2013) and Erdoğan and Torcu-Koc (Reference Erdoğan and Torcu-Koc2016); weight–length relationship (WLR) by Merella et al. (Reference Merella, Quetglas, Alemany and Carbonell1997), Wagué (Reference Wagué1997), Abdallah (Reference Abdallah2002), Lamprakis et al. (Reference Lamprakis, Kallianiotis, Moutopoulos and Stergiou2003), Valle et al. (Reference Valle, Bayle and Ramos2003), Çiçek et al. (Reference Çiçek, Avsar, Yeldan and Ozutok2006), Dulčić and Glamuzina (Reference Dulčić and Glamuzina2006), Sangün et al. (Reference Sangün, Akamca and Akar2007), Bilecenoğlu (Reference Bilecenoğlu2009) and Başusta et al. (Reference Başusta, Başusta and Sangun2017); feeding habits by Bilecenoğlu (Reference Bilecenoğlu2009) and Yapici et al. (Reference Yapici, Filiz and Ozkan2012); reproduction by Bruslé (Reference Bruslé1983), Soykan et al. (Reference Soykan, Ilkyaz, Metin and Kinacigil2013) and Erdoğan and Torcu-Koc (Reference Erdoğan and Torcu-Koc2016). Finally, Altin and Ayyildiz (Reference Altin and Ayyildiz2017) and Bilge et al. (Reference Bilge, Yapıcı and Filiz2018) studied the relation between the body size and four otolith parameters of this species.
It is well recognized that information on age, growth and WLR is necessary in stock assessment and population dynamics. Moreover, otolith morphology is used to distinguish fish species at taxonomic, phylogenetic, paleontological, geographical and dietary level (Lombarte and Lleonart, Reference Lombarte and Lleonart1993; Tuset et al., Reference Tuset, Rosin and Lombarte2006; Škeljo and Ferri, Reference Škeljo and Ferri2011; Disspain et al., Reference Disspain, Ulm and Gillanders2016; Jawad et al., Reference Jawad, Hoedemakers, Ibáñez, Ahmed, Abu El-Regal and Mehanna2017), providing useful data in species biology, and stock identification studies. Such knowledge is essential if different approaches are required to be implemented for distinct stocks in fisheries management. Information on these topics is also necessary for discarded species, for which the existing excessive lack of adequate data may lead to inaccurate conclusions in stock assessment and management (FAO, 2020). No published work on the above-mentioned biological features of S. hepatus is known in the Eastern Ionian and southwestern Aegean Seas so far, where considerable abundance for this discarded species is known (Labropoulou, Reference Labropoulou, Papaconstantinou, Zenetos, Vassilopoulou and Tserpes2007; Mytilineou et al., Reference Mytilineou, Herrmann, Smith, Mantopoulou-Palouka, Anastasopoulou, Siapatis, Sala, Megalofonou, Papadopoulou, Vassilopoulou, Stamouli, Kavadas, Lefkaditou and Nicolaidou2022).
The present study aims to contribute to the knowledge of the life history of S. hepatus by providing updated information on age, growth and otolith morphometrics, information presented for the first time for this species in the E. Ionian and SW Aegean Seas. An additional objective encompasses the comparison of the above-mentioned life-history characteristics to define potential differences between the two study areas, which may be indicative of stock-related differences, information useful in fisheries management and stock identification studies.
Materials and methods
Study areas and data collection
Samples of S. hepatus were collected during experimental bottom trawl surveys conducted in the E. Ionian (October 2014) and SW Aegean Seas (September 2014 and May 2015) (Figure 1). Samples were collected at depths ranging between 43 and 71 m in the former area and 71 and 99 m in the latter one.
Figure 1. Map of the sampling stations in the Ionian (blue) and Aegean Seas (red).
From each specimen, total length (TL) was recorded to the nearest mm and total weight (TW) to the nearest g. Sex was determined based on the macroscopic inspection of the gonads. Sagittal otoliths were removed from the cranial cavity, cleaned in water to remove the organic material and stored dry. Each right otolith was placed, with its proximal side down on a glass Petri dish filled with water and photographed under transmitted light against a black background with the Image-Pro Plus software (Version 4.5.0.29) under the magnification ×12.5.
Age estimation was based on counting the annual growth rings, considered as alternating opaque and translucent zones, along the right sagittal otolith axis, from the core to the post-rostrum edge. Otoliths were read by three readers. To compare the age readings between the different readers, the formulas of percent agreement (PA), coefficient of variation (CV) and average percent error (APE) were calculated (see relevant formulas in Supplementary materials). Broken or damaged otoliths were excluded from the ageing procedure and the analysis of the morphometric parameters. Regardless of the actual spawning date of the species, date of birth was set at 1st January as commonly established by the majority of fish age determination laboratories around the world (ICES, 2018; NOAA, 2020).
The otolith morphometric variables recorded, based on the right otolith observations, were the following: radius (RA, mm); otolith length (OL, mm); otolith width (OW, mm); otolith area (OA, mm2); perimeter (PE, mm) (Figure 2); roundness (RD) which is the ratio between the actual area and the area of a circle of the same diameter, factor larger if and when the shape of otolith is more circular (Ponton, Reference Ponton2006) taking a minimum value of 1 (Pothin et al., Reference Pothin, Gonzalez-Salas, Chabanet and Lecomte-Finiger2006) and circularity (CI) which provides information on the complexity of the otolith contour (Tuset et al., Reference Tuset, Lozano, Gonzĺez, Pertusa and García-Díaz2003) taking a minimum value of 4π (Pothin et al., Reference Pothin, Gonzalez-Salas, Chabanet and Lecomte-Finiger2006). Additionally, the following shape factors were calculated:
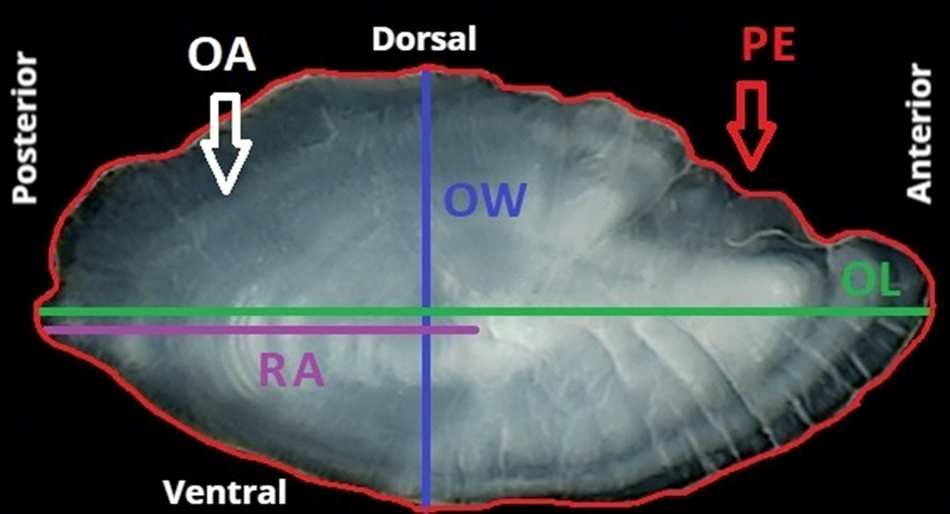
Figure 2. Right otolith of Serranus hepatus, illustrating the measurements analysed. RA, radius (mm); OL, otolith length (mm); OW, otolith width (mm); OA, otolith area (mm2); PE, perimeter (mm).
Form factor (FF) = ([4 × OA/PE 2]), which is a dimensionless value that indicates the similarity of the otolith contour to a circle, taking values from 0 to 1, with a value of 1 corresponding to a perfect circle;
Rectangularity (RC) = (OA/[OL × OW]), which gives information about the approximation to a rectangular or square shape, indicating a perfect rectangle or square if it has a value of 1;
Ellipticity (EL) = (OL – OW/OL + OW), which reflects the similarity to an ellipse, with values close to 0 indicating a tendency towards circularity (Tuset et al., Reference Tuset, Lozano, Gonzĺez, Pertusa and García-Díaz2003).
Data analysis
Growth
The total length (TL) frequency distribution of the samples in each study area was based on classes of 1 cm interval. The WLR relationship was estimated by applying the equation TW = αTLb, where TW is the total weight in g, TL the total length in cm, α the intercept and b the slope of the regression. The null hypothesis for isometric growth (H 0: b = 3) was tested by using Student's t-test in each study area. Analysis of covariance (ANCOVA) was used to compare the intercepts and slopes between the two areas. Differences were considered at the significant level a = 0.05.
For the age study, age–length keys were constructed by area. The growth parameters were calculated through the Von Bertalanffy equation: Lt = L∞ (1 – e−k(t−t0)) where, Lt is the predicted length at age t in cm, L∞ is the mean theoretical asymptotic length in cm, k is a growth rate parameter in year−1 and t 0 the theoretical age at zero length in years (von Bertalanffy, Reference Von Bertalanffy1938). Differences in the growth parameters between the two study areas were tested using Student's t-test. In addition, the growth performance index Φ΄ (Φ΄ = logk + 2logL ∞; Pauly and Munro, Reference Pauly and Munro1984) was applied to discuss the growth rate in the two study areas and that of the existing published literature.
Otolith morphometrics
The values of the mean ± standard error, and minimum and maximum of each otolith morphometric variable were presented by area. Since the examination of the length frequency distribution between the two study areas revealed statistically significant difference in the sizes of the samples (Kolmogorov–Smirnov test, P < 0.01), to minimize the effect of the size differences on the otolith variables between the two study areas, only individuals included in the length classes 70–109 mm were used in the following analyses (E. Ionian: N = 98 and SW Aegean: N = 52). These length classes were selected because no difference was identified in the length frequency of this size range (Kolmogorov–Smirnov test, P = 0.07). Differences were considered at the significant level a = 0.05.
The relationship of each otolith morphometric variable with TL was also described for each area by the exponential regression: y = axb, where y is the otolith morphometric variable in mm, x the total length in mm, a is the intercept and b the slope of the regression. The significance of these relationships was based on the P-value. ANCOVA was used to test the between-area differences by comparing the slopes of the regressions, which indicated significant correlation between otolith variable and TL. Differences were considered at the significant level a = 0.05.
The identification of the effect of the study area on the otolith variables was examined using the following approach:
As proposed by Agüera and Brophy (Reference Agüera and Brophy2011), the otolith variables which do not present a consistent b in the two study areas should be excluded from further analyses. Otolith variables that were significantly correlated with TL were used for the comparison of the two study areas, based on their standardized values calculated using a common within-group slope (b). The following equation, reported by Elliott et al. (Reference Elliott, Haskard and Koslow1995), was used for these standardizations to remove the size effect: Ms = Mo(TLs/TLo)b where, Ms = standardized otolith variable, Mo = measured otolith variable, TLs = overall (arithmetic) mean length for all fish from all samples in each analysis, TLo = length of the specimen and b the common within-group slope for each measured otolith variable, estimated from the combined data of both study areas by the equation Mo = aTLob. The estimated common within-group slope (b) is presented in Supplementary Table S1. The relationship of TL with each standardized otolith morphometric variable was re-examined to cross-validate that the size effect has been removed successfully from the data.
For a more comprehensive picture, multivariate general linear models (multivariate GLM) were used to identify the effect of the factor area on the otolith variables. The standardized values of the otolith variables were used as dependent variables and the study area was used as independent variable. When needed, square root transformation was performed as more appropriate on the parameters to achieve normality. The partial eta squared was applied to evaluate the relative importance of each variable in differentiating the two study areas.
The standardized values of the otolith variables were also used to explore the relationships between them using principal component analysis (PCA).
Results
Growth
Length distribution
A total of 239 individuals were examined for the present study: 150 from the E. Ionian Sea and 89 from the SW Aegean Sea. The total length TL of the samples from the E. Ionian Sea ranged from 6.6 to 10.0 cm, while those from the SW Aegean Sea ranged from 5.7 to 10.0 cm (Figure 3). The TL distribution showed that the length class of 8.0 cm was predominant in both areas.
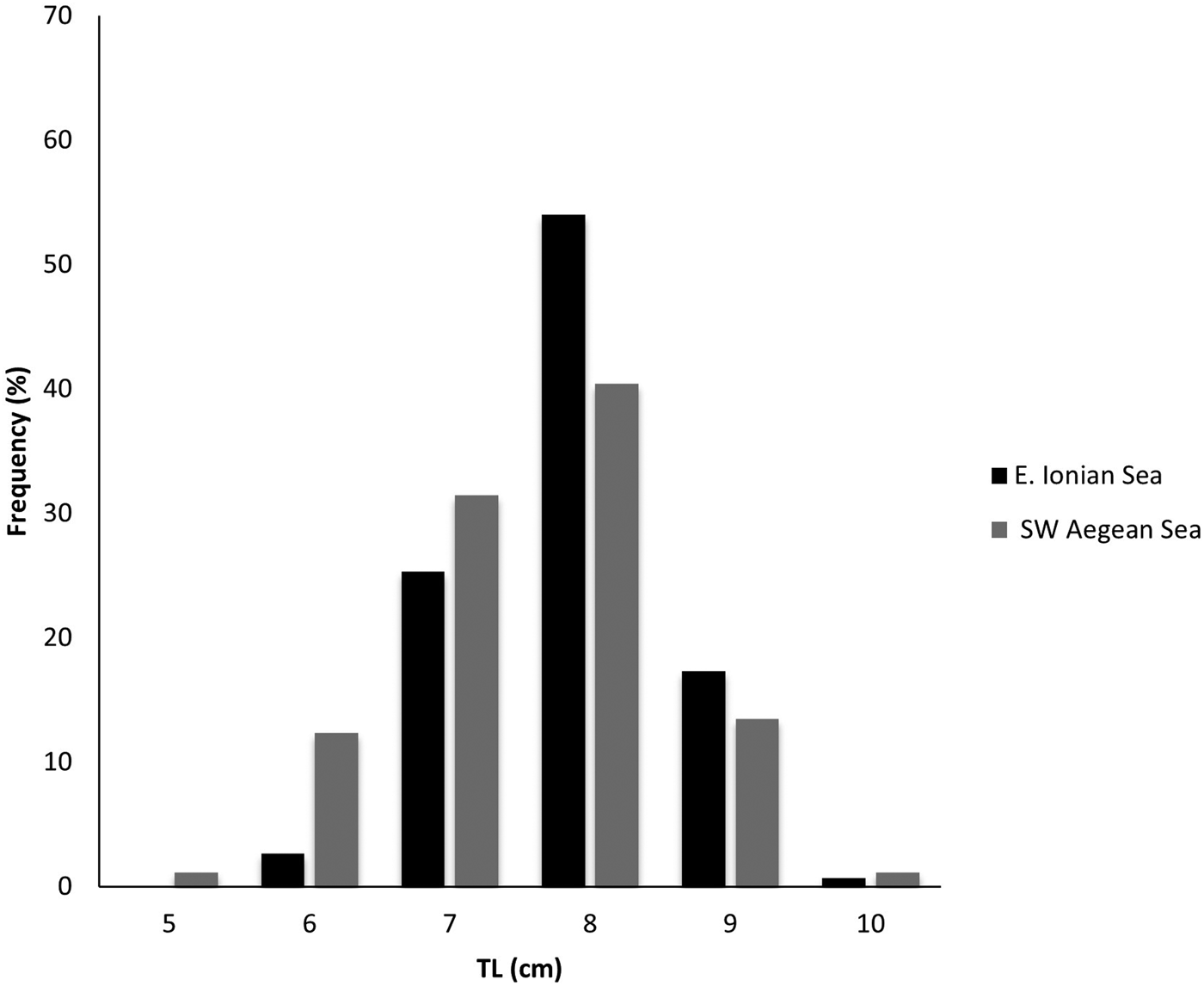
Figure 3. Length frequency distribution (TL, cm) of Serranus hepatus in the E. Ionian and SW Aegean Seas.
Total weight–total length relationship
The values of the total weight TW ranged between 3.67–13.66 g and 3.06–14.50 g in the E. Ionian and the SW Aegean, respectively. For each study area, the WLR was as follows:
TW = 0.016382526 × TL 2.93331 (R 2 = 91%) for the E. Ionian Sea;
TW = 0.009369 × TL 3.18154 (R 2 = 89%) for the SW Aegean.
The WLR revealed that the value of b did not differ significantly than 3 in both areas, which indicated isometric growth (E. Ionian Sea: t-test = −0.83, P = 0.41; SW Aegean Sea: t-test = 1.45, P = 0.15). The comparison of b in the WLR of the two study areas did not show statistically significant differences (ANCOVA, for b: P = 0.08).
Age and growth
Age readings of the three readers were quite similar (PA = 95.99%, CV = 4.70% and APE = 3.60%). For those otoliths with age disagreement, re-reading or exclusion of them resulted in a common data set with PA = 100%. Three age groups (from 1 to 3) in the E. Ionian and four age groups (from 0 to 3) in the SW Aegean were identified from the otolith readings of S. hepatus. One false ring was always observed before the first annual growth ring. The first annulus was identified at a distance from the core (posterior area) ranging from 1.5 to 2.0 mm (Figure 4). The relevant age–length keys of S. hepatus for the two study areas are presented in Table 1. Most of the sampled individuals belonged to the age group one, followed by the age group two.
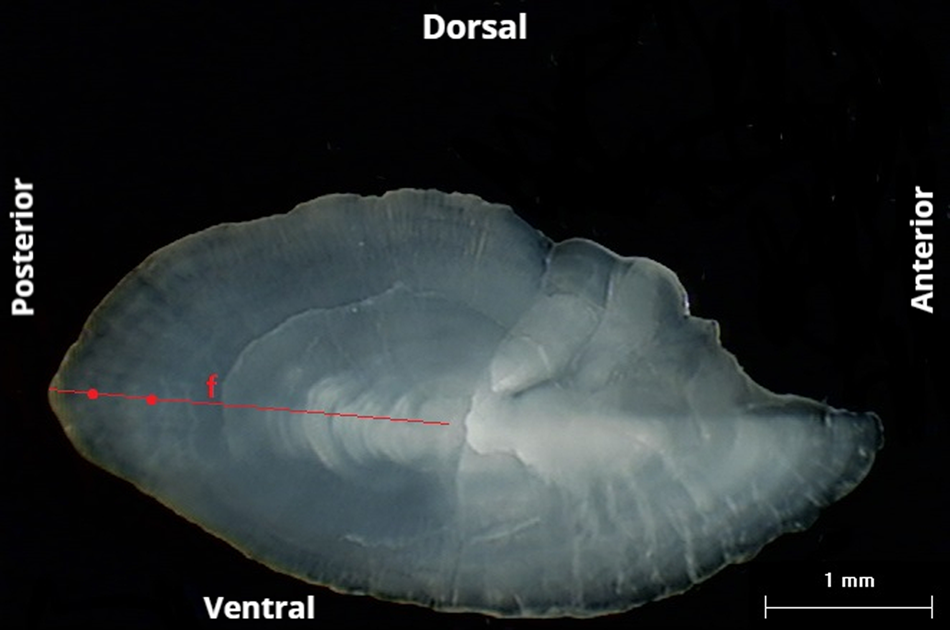
Figure 4. Otolith of Serranus hepatus with two annual rings (red dots) and the false ring (f); date of capture October.
Table 1. Age–length key of Serranus hepatus from the E. Ionian and SW Aegean Sea
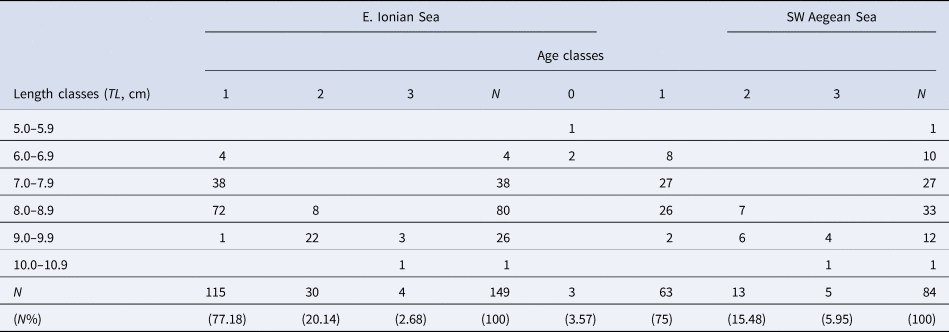
TL, total length (cm); N, total number of individuals; N%, percentage to the total number of individuals.
Table 2 and Supplementary Figure S1 present the von Bertalanffy parameters and curves, respectively, per area. The von Bertalanffy growth parameters did not present statistically significant differences between the two areas (L∞: P = 0.99; k: P = 0.94; t 0: P = 0.80). The growth performance index Φ΄ is also shown in Table 2.
Table 2. Von Bertalanffy growth parameters of Serranus hepatus in the E. Ionian and the SW Aegean Sea

N, total number of individuals; L∞, the mean theoretical asymptotic length in cm; k, a growth rate parameter in year−1; t 0, the theoretical age at zero length in years; SE, standard error; Φ΄, the growth performance index.
Otolith morphometrics
The mean (± standard error) and the minimum and maximum values of RA (radius); OL (otolith length); OW (otolith width); OA (otolith area); PE (perimeter); RD (roundness); CI (circularity); FF (form factor); RC (rectangularity) and EL (ellipticity) of the right otolith are presented in Table 3 per area. Their standardized values for the length classes 70–109 mm are also shown in Table 3.
Table 3. Mean ± standard error (minimum–maximum) of the otolith morphometric variables
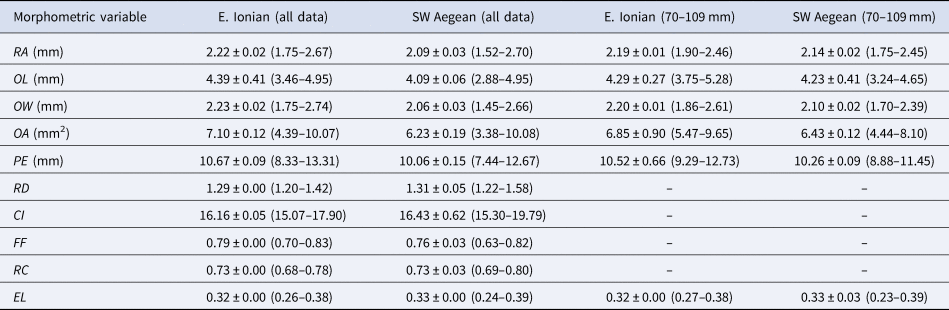
RA, radius (mm); OL, otolith length (mm); OW, otolith width (mm); OA, otolith area (mm2); PE, perimeter (mm); RD, roundness; CI, circularity; FF, form factor; RC, rectangularity; EL, ellipticity of Serranus hepatus for the E. Ionian and SW Aegean Seas.
The standardized otolith morphometric variables for the length classes 70–109 mm are also shown.
TL was found to be statistically significantly correlated (P < 0.05) with all examined otolith variables in the E. Ionian Sea (Table 4, Supplementary Figure S2). In the SW Aegean Sea, all morphometric variables were statistically significantly correlated with TL (P < 0.05); however, the otolith shape variables (RD, CI, FF, RC, except EL) showed a low no significant correlation with size (Table 4, Supplementary Figure S3). For the otolith variables that were significantly correlated with TL in both the E. Ionian and SW Aegean Seas (RA, OL, OW, OA, PE and EL), no significant differences (ANCOVA, P > 0.05) were detected for the slope b between these areas (Table 4). As a result, only these otolith variables were used for the estimation of the common within-group slope (see Supplementary Table S1) for the estimation of their standardized values and for the identification of differences between the two study areas.
Table 4. Parameters of the exponential regression of the total length (TL) of Serranus hepatus with the otolith morphometric variables
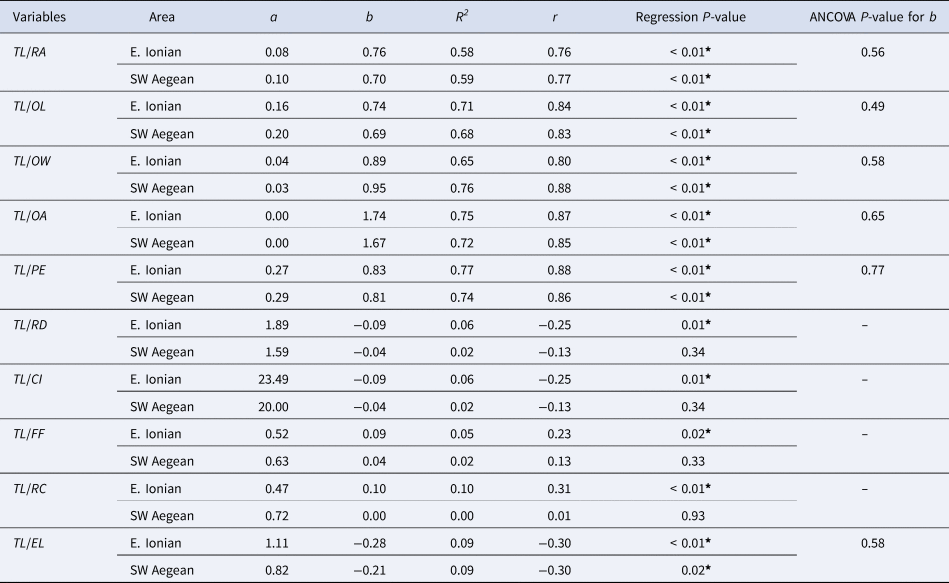
RA, radius (mm); OL, otolith length (mm); OW, otolith width (mm); OA, otolith area (mm2); PE, perimeter (mm); RD, roundness; CI, circularity; FF, form factor; RC, rectangularity; EL, ellipticity in the E. Ionian and SW Aegean Seas.
R 2, coefficient of determination; r, correlation coefficient. The P-value of the regressions and the P-value of the comparison of the slope b of the regression lines between the two areas (ANCOVA) are also shown.
*Significance level a = 0.05
Τhe standardized values of RA, OL, OW, OA, PE and EL (square root of OL and RA) of the length classes 70–109 mm were analysed by multivariate GLM to identify the effect of the area factor on these variables and the relative importance of each one in differentiating the two areas. Since the variable EL was not found to be statistically significant, the model was rerun excluding this variable. The final model was found to be statistically significant (P = 0.012, Supplementary Table S2). The variables contributing to the separation of the two study areas ordered by their relative importance, based on the partial eta squared, were OL, OW, OA, PE and RA (Table 5).
Table 5. Results of the multivariate GLM analysis showing the relative importance of each otolith variable (based on the partial eta squared η2) in differentiating the two study areas
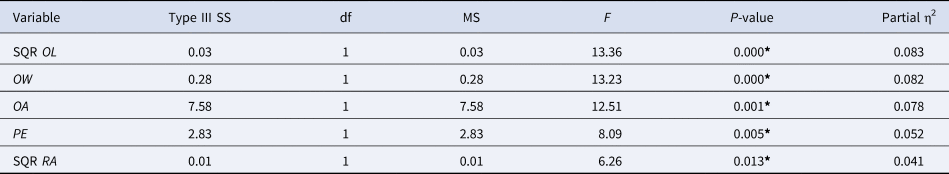
SQR OL, square root of otolith length; OW, otolith width; OA, otolith area; PE, perimeter; SQR RA, square root of radius; SS, sum of squares; df, degrees of freedom; MS, mean square.
*Significance level a = 0.05.
The PCA showed two principal components explaining the 93.7% of the variability of the otolith variables. The most important variables of the first principal component were (OA, OL and OW) expressing 72.9% while the second one included only EL which expressed 20.8% (Table 6).
Table 6. Component matrix of PCA results of the total variance explained and the weight of each initial variable at each principal component
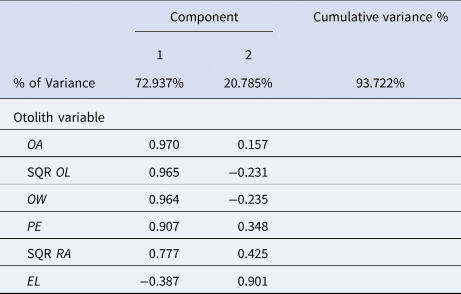
OA, otolith area; SQR OL, square root of otolith length; OW, otolith width; PE, perimeter; SQR RA, square root of radius; EL, ellipticity.
Discussion
Serranus hepatus is a species of widespread occurrence; however, information on its biology and ecology is rather scarce, particularly in the Ε. Ionian and SW Aegean Seas. Although some published information on age, growth and WLR from other localities of the Greek seas exists from the past (Wagué, Reference Wagué1997; Wagué and Papaconstantinou, Reference Wagué and Papaconstantinou1997; Labropoulou et al., Reference Labropoulou, Tserpes and Tsimenides1998; Lamprakis et al., Reference Lamprakis, Kallianiotis, Moutopoulos and Stergiou2003), the results of the present study provided updated information for the Aegean Sea and new information for the Ionian Sea, which is needed for fisheries stock assessment and management.
The study of the WLR in this work revealed an isometric growth for the species in both areas. No significant differences were detected between the two study areas. Isometric somatic growth was also reported by Çiçek et al. (Reference Çiçek, Avsar, Yeldan and Ozutok2006), Sangün et al. (Reference Sangün, Akamca and Akar2007) and Başusta et al. (Reference Başusta, Başusta and Sangun2017). However, positive allometry was mentioned by Merella et al. (Reference Merella, Quetglas, Alemany and Carbonell1997), Lamprakis et al. (Reference Lamprakis, Kallianiotis, Moutopoulos and Stergiou2003), Valle et al. (Reference Valle, Bayle and Ramos2003), Dulčić and Glamuzina (Reference Dulčić and Glamuzina2006), Dulčić et al. (Reference Dulčić, Matić-Skoko, Paladin and Kraljević2007) and Soykan et al. (Reference Soykan, Ilkyaz, Metin and Kinacigil2013). In opposite, negative allometry was reported by Wagué (Reference Wagué1997), Abdallah (Reference Abdallah2002), Bilecenoğlu (Reference Bilecenoğlu2009), Yapici et al. (Reference Yapici, Filiz and Ozkan2012) and Erdoğan and Torcu (Reference Erdoğan and Torcu-Koc2016) (Table 7). The differences in the parameters of WLR are usually attributed to factors such as genotype (Garvey et al., Reference Garvey, Devries, Wright and Miner2003), habitat, differences in length composition, number of specimens examined, preservation techniques, health, stomach conditions, diet, sex, maturity stages (Tesch, Reference Tesch and Ricker1971; Wootton, Reference Wootton1990), sampling season, growth rate and age (Shepherd and Grimes, Reference Shepherd and Grimes1983; Weatherly and Gill, Reference Weatherly and Gill1987). The spawning period of the species extends from spring to autumn in both areas of the present study, which coincides with the sampling period of our samples. Therefore, the body morphometry of the examined individuals of both areas is expected to be similarly affected.
Table 7. Weight–length relationship and growth parameters of Serranus hepatus from different study areas
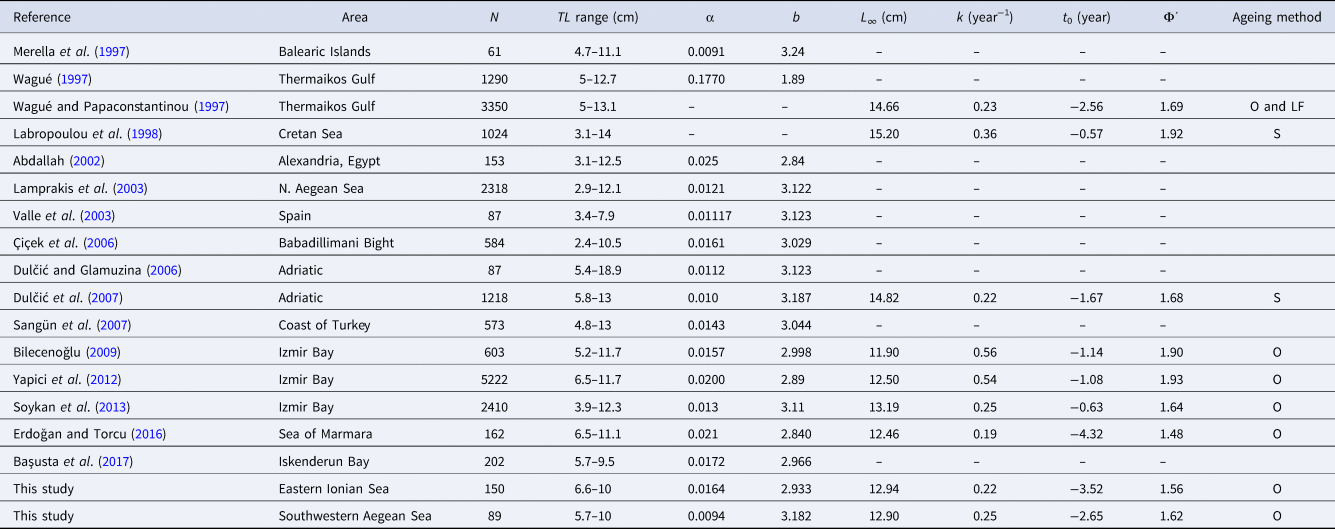
N, total number of individuals; TL range, minimum–maximum total length in cm; α, intercept; b, slope of the weight–length relationship; L∞, mean theoretical asymptotic length in cm; k, growth rate in year−1; t 0, theoretical age at zero length in years; Φ΄, growth performance index; O, otoliths; LF, length-based analysis; S, scales.
In the present work, four age groups (0–3 years old) were identified. More age groups have been reported in the published literature, which however, corresponded to a wider size range than the examined samples (Table 7). The von Bertalanffy parameters did not show statistically significant differences between the E. Ionian and SW Aegean, similarly, the growth performance index Φ΄. The growth parameters and Φ΄ estimated in this work were included within the range of parameters found in the published literature (Table 7) and seem close to the values of some works from the Aegean and Adriatic Seas (but see Labropoulou et al. [Reference Labropoulou, Tserpes and Tsimenides1998] from the Cretan Sea). Differences in the growth parameters may be related to differences in the environmental conditions, life-history traits, methodological approaches and size range of the examined samples.
The study of the relationship of TL with the otolith morphometric variables revealed statistically significant correlation with the ten examined morphometric variables in the E. Ionian, while only with six (RA, OL, OW, OA, PE and EL) in the SW Aegean Sea. The low sample size in the latter area may be the reason of the low correlation of the other variables (RD, CI, FF and RC) with TL and the absence of statistically significant P-value in these cases. Our results are in accordance with the findings of Altin and Ayyildiz (Reference Altin and Ayyildiz2017) and Bilge et al. (Reference Bilge, Yapıcı and Filiz2018), who also indicated a high correlation in the relationships between total length and otolith radius, length and width in S. hepatus. No published information was found for the other otolith variables examined in the present work in S. hepatus (OA, PE, RD, CI, FF, RC and EL). No significant differences (ANCOVA, P > 0.05) were detected for the slope b of the regression lines of RA, OL, OW, OA, PE and EL between the two study areas.
According to the results of the multivariate GLM, the variables RA, OL, OW, OA and PE, which are related more to the otolith morphology, were found to differ statistically significantly between the E. Ionian and SW Aegean Seas. It is noteworthy to mention that the otolith shape variable EL did not contribute to this difference. The OL, OW and OA were the variables with the greatest contribution in differentiating the two study areas. The PCA also showed that these three variables expressed a major part of the variability of the first principal component. However, although OA was the third variable in importance in differentiating the two study areas, it was the first in variability. This was expected since OA includes the variability of both OL and OW. The differences in otolith morphometric variables between the two study areas may indicate differences between the populations of S. hepatus in the broader area. However, similar growth patterns characterized the species in the two study areas. Variation in otolith morphology and shape is known to be related to genetic and/or environmental factors such as temperature, salinity, depth and food availability (Campana and Casselman, Reference Campana and Casselman1993; Lombarte and Lleonart, Reference Lombarte and Lleonart1993; Capoccioni et al., Reference Capoccioni, Costa, Aguzzi, Menesatti, Lombarte and Ciccotti2011; Bostanci et al., Reference Bostanci, Yilmaz, Yedier, Kurucu, Kontas, Darçin and Polat2016; Smolinski et al., Reference Smolinski, Deplanque-Lasserre, Hjörleifsson, Geffen, Godiksen and Campana2020; Nazir and Khan, Reference Nazir and Khan2021). In addition, Bose et al. (Reference Bose, Zimmermann, Winkler, Kaufmann, Strohmeier, Koblmüller and Sefc2020) suggest that the otolith width is highly influenced by selected environmental factors. Moreover, Friedland and Reddin (Reference Friedland and Reddin1994) and Libungan et al. (Reference Libungan, Slotte, Husebø, Godiksen and Pálsson2015) mentioned that subpopulation differences in otolith shape increase with increasing geographical separation. Avigliano et al. (Reference Avigliano, Riaños and Volpedo2014) mentioned that the differences in the water chemistry and salinity among regions may be responsible for variations in the otolith morphometrics of a fish species in two geographical locations. Velaoras et al. (Reference Velaoras, Kassis, Perivoliotis, Pagonis, Hondronassios and Nittis2013) mentioned that the Aegean Sea exhibits greater values for both temperature and salinity than the Ionian Sea. Therefore, the variations in the otolith morphometrics found in the present work could be related to different environmental conditions of the two study areas. This needs to be proved by specific studies relating otolith morphometrics with the environmental conditions of the two areas. In addition, further genetic studies are also necessary to investigate the genotype of the species in the two regions.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0025315423000474
Acknowledgements
The present work was carried out based on samples collected during the surveys conducted in the framework of DeFishGear (IPA Adriatic 2007–2013, STR/00010) and Epilexis (EPAL 2007–2013, 185365) research projects.
Author contributions
Vasiliki Nikiforidou: designing the study, carrying out the study, analysing the data, interpreting the findings and writing of the article. Emmanouil Gkikas: carrying out the study. John Haralabous: analysing the data. Chryssi Mytilineou: designing the study, interpreting the findings and writing the article. Drosos Koutsoubas: writing the article. Aikaterini Anastasopoulou: designing the study, carrying out the study, analysing the data, interpreting the findings and writing the article.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Competing interest
None.













