Introduction
Diatoms are a significant but still largely unknown component of the photosynthetic microbiota of tropical coral reefs. Much of the early taxonomic work on tropical diatoms was based on dredge samples (e.g. Mann, Reference Mann1925) or hand collections with little or no habitat information, as noted by Lobban et al. (Reference Lobban, Schefter, Jordan, Arai, Sasaki, Theriot, Ashworth, Ruck and Pennesi2012). More recently, coral reef diatoms have been studied in diverse regions of tropical oceans: Tahiti, Polynesia (Ricard, Reference Ricard1974, Reference Ricard1975, Reference Ricard1977); Mahé, Seychelles (Giffen, Reference Giffen1980); Puerto Rico (Navarro, Reference Navarro1981a, Reference Navarro1981b, Reference Navarro1982b, Reference Navarro1982c, Reference Navarro1982a, Reference Navarro1983a, Reference Navarro1983b; Navarro et al., Reference Navarro, Pérez, Arce and Arroyo1989); Fiji (Foged, Reference Foged1987); Palawan, Philippines (Podzorski & Håkansson, Reference Podzorski and Håkansson1987); Nassau, The Bahamas (Hein et al., Reference Hein, Winsborough, Sullivan and Lange-Bertalot2008); Rodrigues, Réunion (Al-Handal et al., Reference Al-Handal, Compère and Riaux-Gobin2016); off Basrah coast of Iraq (Al-Handal et al., Reference Al-Handal, Thomas and Pennesi2018). Several of these studies (especially Ricard's and Navarro's) were based on net sampling, which included phytoplankton and tychoplankton, the latter supposedly passively swept up from the benthos (but see Sabir et al., Reference Sabir, Theriot, Manning, Al-Malki, Khiyami, Al-Ghamdi, Sabir, Romanovicz, Hajrah, El Omri, Jansen and Ashworth2018b).
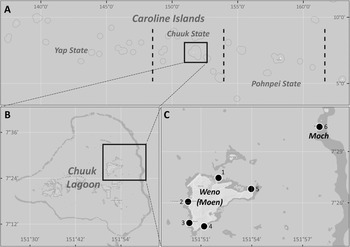
The coral reef diatoms of the Micronesian Islands in the Western Pacific Ocean have been intensely studied in Guam (Lobban et al., Reference Lobban, Schefter, Jordan, Arai, Sasaki, Theriot, Ashworth, Ruck and Pennesi2012; Lobban, Reference Lobban2015a, inter alia). The number of new species reported from Guam (Lobban et al., Reference Lobban, Ashworth, Pennesi, Park, Jordan and Theriot2009–2019) is an indication of the biodiversity still to be discovered in the region, an area larger than the contiguous USA; it stretches 3700 km from Palau in the west to the Marshall Islands in the east and encompasses high islands and many atolls. Chuuk State is located at about 7°N within the Federated States of Micronesia in the western Pacific (Figure 1A). Chuuk lagoon consists of 12 major volcanic islands and 24 coral-reef islands, within a barrier reef (Figure 1B). Of 12 major volcanic islands, Weno (formerly Moen) is located in the eastern part of the lagoon. Park et al. (Reference Park, Lobban and Lee2018) recently began diatom floristic studies there, based on diatoms associated with seaweeds; 143 species were observed. The present collection of new records is based on net sampling of plankton samples at five sites at the same locations, to which we have added two samples from another island in Chuuk Lagoon from the GUAM diatom collection. The new analyses included scanning electron microscopy (SEM), which enabled us to identify to species some taxa previously included at the genus level.

Fig. 1. Sampling sites in Weno (Moen) Island in Chuuk Lagoon. (A) Location of Chuuk State (box) in Caroline Islands. (B) Sampling location (box) in Chuuk Lagoon. (C) Six sampling sites in Weno and Moch Islands.
Materials and methods
Net samples were collected from surface water (2–5 m water depth) at five sites near the coast of Weno Island on 22 May 2017 (Figure 1C, Park et al., Reference Park, Lobban and Lee2018) by horizontally towing a 20 μm mesh net. The collected samples were put in 250 ml polyethylene bottles and fixed immediately with Lugol's solution to a final concentration of about 2%. Preparation of samples followed Park et al. (Reference Park, Lobban and Lee2018). In addition, we analysed two samples from Moch Island (Figure 1C, 7°30′50.7″N 151°57′59.8″E) at the Eastern Passage of the barrier reef, collected by CSL on 30 May 1991 by hand. These had been preserved in 4% formalin and are curated in the GUAM diatom collection.
Organic matter was removed with HCl-KMnO4, the remaining material was mounted in Pleurax and observed with a light microscope (LM; BX51, Olympus, Tokyo) (Park et al., Reference Park, Lobban and Lee2018) or cleaned with HNO3 and mounted in Hyrax, and observed with a Nikon i80 microscope (Lobban, Reference Lobban2015b). Scanning electron microscope (SEM) observations were made with a Phenom G2 Pro desktop instrument (Lobban, Reference Lobban2015b).
Results
A total of 109 diatom taxa were observed (Figures 2–168). Of these, 70 taxa were observed from net samples and 39 taxa were added from seaweed-associated habitats from SEM observations. Of 39 taxa, four uncertainly identified species in Park et al. (Reference Park, Lobban and Lee2018) were also further identified with SEM and re-listed in this study: Thalassiosira cedarkeyensis (as Thalassiosira cf. cedarkeyensis), Pleurosigma cf. elongatum (as Pleurosigma sp. 2), Amphora lunulata (as Amphora sp. 2), Nitzschia cf. amabilis (as Nitzschia sp. 11). Brief diagnostics are included only for 31 species that were unrecorded from Micronesian waters (Table 1), and the figures and dimensions for all diatoms are presented because the evidence of species occurrences may be re-evaluated with further identifications in future. Taxa are listed systematically according to Cox (Reference Cox and Frey2015).
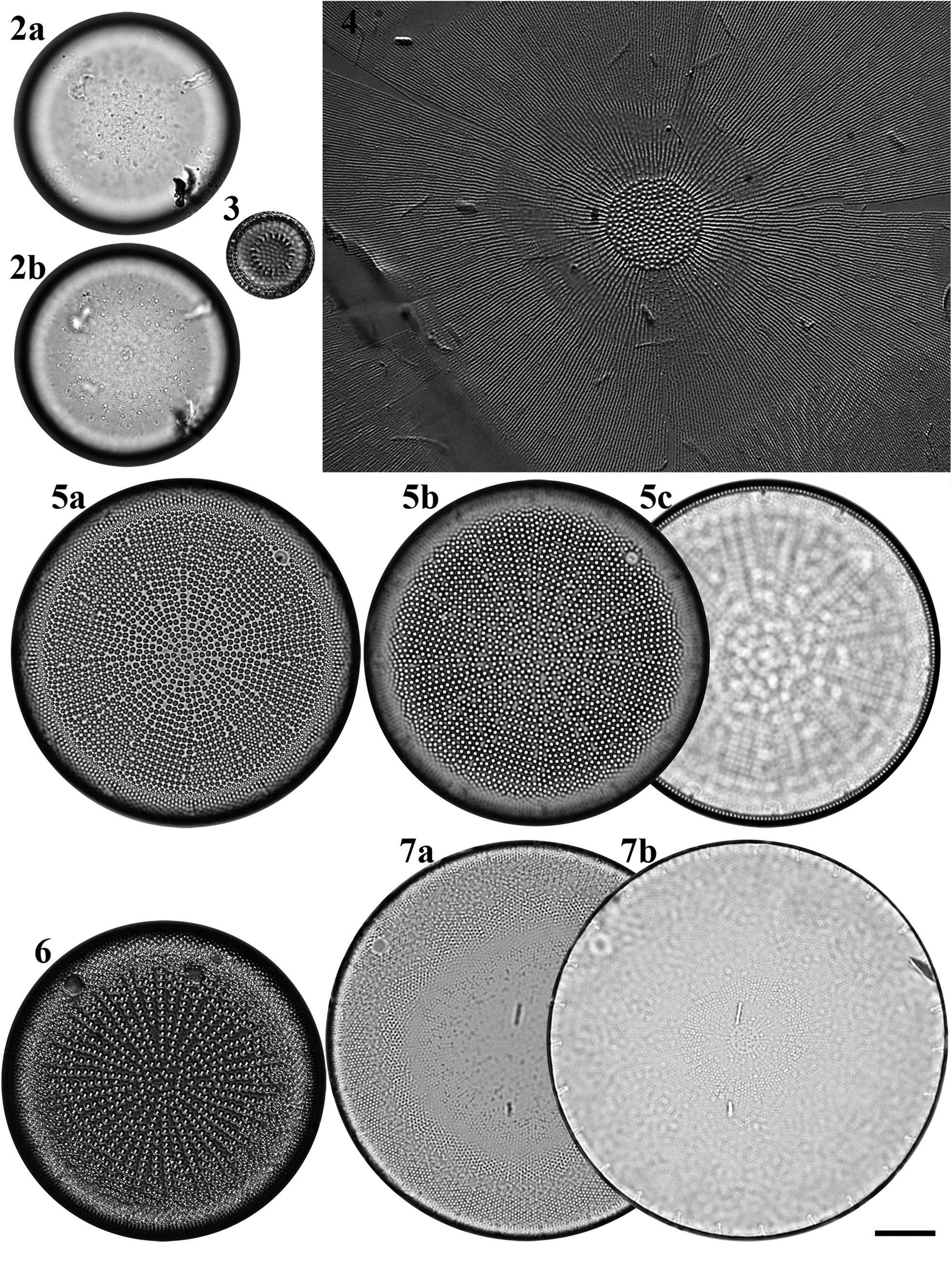
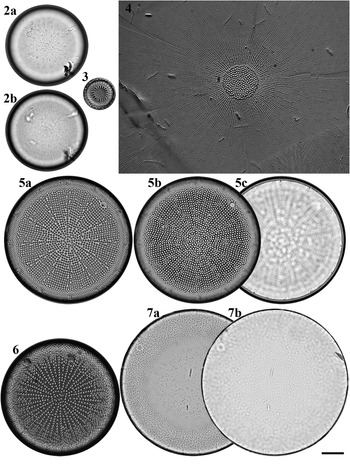
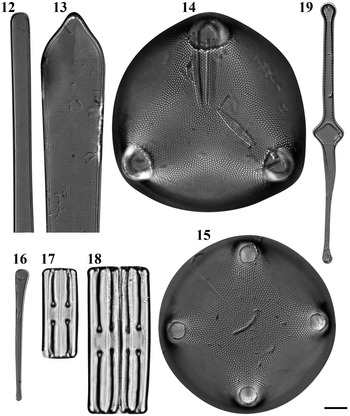
Figs 2–7. Light microscopy images of six taxa in Chuuk, Micronesia. Fig. 2a, b. Podosira hormoides from Weno. Fig. 3. Paralia cf. longispina from Moch. Fig. 4. Chrysanthemodiscus floriatus from Moch. Fig. 5a–c. Actinocyclus octonarius var. tenellus from Moch. Fig. 6. Actinocyclus ocotonarius var. octonarius from Weno. Fig. 7a, b. Actinocyclus subtilis from Weno. Scale bar = 10 μm.
Table 1. List of 31 newly recorded diatoms in Micronesia and their distribution and habitats

Class COSCINODISCOPHYCEAE Round & R.M. Crawford, 1990
Subclass MELOSIROPHYCIDAE Cox, 2015
Order MELOSIRALES R.M. Crawford, 1990
Family HYALODISCACEAE R.M. Crawford, 1990
Podosira hormoides (Montagne) Kützing Figures 2a–b, 72
References: Kützing (Reference Kützing1844): 52, pl. 28, figure 5, pl. 29, figure 84; Giffen (Reference Giffen1970): 95; Lobban et al. (Reference Lobban, Schefter, Jordan, Arai, Sasaki, Theriot, Ashworth, Ruck and Pennesi2012): 248, pl. 4, figures 1 & 2.
Sample: Moen_03 #003.
Dimensions: 35.3 μm in diameter; 30 areolae in 10 μm.
Family PARALIACEAE R.M. Crawford, 1988
Paralia cf. longispina Konno & Jordan Figure 3
References: Konno & Jordan (Reference Konno, Jordan and Likhoshway2008): 56, figures 2–53; Lobban et al. (Reference Lobban, Schefter, Jordan, Arai, Sasaki, Theriot, Ashworth, Ruck and Pennesi2012): 249, pl. 4, figures 6–8.
Sample: TK28.
Dimensions: 14 μm in diameter.
Comments: It is difficult to distinguish Konno & Jordan's (Reference Konno, Jordan and Likhoshway2008) species from three very similar taxa in the P. longispina complex (MacGillivary & Kaczmarska, Reference MacGillivary and Kaczmarska2012), even with SEM, and impossible from LM. None of these other species has yet been identified in samples from Micronesia, making it likely that this specimen is P. longispina, but we will need additional specimens in SEM to confirm this.
Subclass COSCINODISCOPHYCIDAE Round & R.M. Crawford, 1990
Order CHRYSANTHEMODISCALES Round, 1990
Family CHRYSANTHEMODISCACEAE Round, 1990
Chrysanthemodiscus floriatus A. Mann Figure 4
References: Takano (Reference Takano1965): 7, pl. I, figures 12–14; Round (Reference Round1978): 157, figures 1–15; Gibson & Navarro (Reference Gibson and Navarro1981): 338, figures 1–9; Navarro & Lobban (Reference Navarro and Lobban2009): 127, figures 2–4.
Samples: TK4, TK28.
Dimensions: 65–100 μm in diameter (flattened valve).
Diagnostics: Large cells often forming large nebulous colonies and easily crumpled when mounted. Central area with coarser pore and radiating fine rows of pores from central area. Numerous copulae and the domed valves give the cells a bacillus profile in girdle view.
Order COSCINODISCALES Round & R.M. Crawford
Family HEMIDISCACEAE Hendey ex Hasle
Actinocyclus octonarius var. octonarius Ehrenberg Figure 6
Reference: Hasle & Syvertsen (Reference Hasle, Syvertsen and Tomas1996): 117.
Samples: TK4, TK28.
Dimensions: 53.2 μm in diameter, 9 areolae in 10 μm, 3–5 striae in fascicle.
Diagnostics: Areolae arranged in fascicles separated by compete radial rows of areolae extending from the valve centre to the mantle. Fascicles denser than A. octonarius var. tenellus.
Comments: This species is new to Micronesian waters including Guam.
Actinocyclus octonarius var. tenellus (Brébisson) Hendey Figure 5a–c
References: Villareal & Fryxell (Reference Villareal and Fryxell1983): 453, figures 1–14; Lee et al. (Reference Lee, Byun and Lee1992): 194, figures 38–41.
Sample: Moen_05 #002.
Dimensions: 57.3 μm in diameter, 9–11 areolae in 10 μm, 10–11 striae in fascicle.
Diagnostics: Areolae arranged in fascicles separated by compete radial rows of areolae extending from the valve centre to the mantle. Segments distinct. Striae arranged in crisscrossing pattern.
Comments: This species is new to Micronesian waters including Guam.
Actinocyclus subtilis (W. Gregory) Ralfs Figures 7a, b, 73
References: Hustedt (Reference Hustedt1927–1930); Andersen et al. (Reference Andersen, Medlin and Crawford1986): 467, figures 1–35; Witkowski et al. (Reference Witkowski, Lange-Bertalot and Metzeltin2000): 22, pl. 4, figure 1; Al-Handal et al. (Reference Al-Handal, Compère and Riaux-Gobin2016): 5, pl. 1, figure 4 (as Actinocyclus cf. subtilis).
Sample: Moen_04 #001.
Dimensions: 65.9–71.6 μm in diameter; 17–18 areolae in 10 μm in the valve centre.
Diagnostics: Areolae fine, and arranged bifurcately. Central annulus circular and filled with irregular areolae. Pseudonodule distinct.
Comments: The distinction between Actinocyclus subtilis and A. tenuissimus is subtle. Hustedt's (Reference Hustedt1927–1930) key distinction between A. subtilis and A. tenuissimus is the areola density, but his drawings (figs 304, 305 respectively), show A. subtilis with dense areolae in the centre, separated by a distinct hyaline ring, and the segments not clearly separated, whereas A. tenuissimus has more scattered central areolae and distinct segments with lines of areolae stretching from the rimoportulae to the centre filled in by straight lines of areolae that end at different levels as they meet the segmental lines. The central area of A. tenuissimus is not more than 4 μm (Hustedt, Reference Hustedt1927–1930). Al-Handal et al. (Reference Al-Handal, Compère and Riaux-Gobin2016) mentioned Hustedt's (Reference Hustedt1927–1930) criterion to distinguish both species, namely, the areolae size in the valve: A. subtilis (18–20 in 10 μm) vs A. tenuissimus (12–15 in 10 μm). However, the areolae density of A. subtilis is more variable (12–18 in 10 μm) in Andersen et al. (Reference Andersen, Medlin and Crawford1986) and Witkowski et al. (Reference Witkowski, Lange-Bertalot and Metzeltin2000). We assigned the Chuuk species to A. subtilis based on the areola density and the appearance of the segments and the central area. The taxonomy of both species should be re-considered with the taxonomic history and distribution. This species is a first record from Micronesian waters including Guam.
Class MEDIOPHYCEAE (Jousé & Proshkina-Lavrenko) Medlin & Kaczmarska, 2004
Subclass BIDDULPHIOPHYCIDAE Round & R.M. Crawford, 1990
Order BIDDULPHIALES Krieger, 1954
Family BIDDULPHIACEAE Kützing, 1844
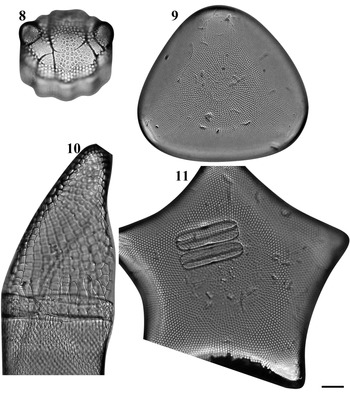
Biddulphia biddulphiana (J.E. Smith) Boyer Figure 8

Figs 8–11. Light microscopy images of four taxa in Chuuk, Micronesia. Fig. 8. Biddulphia biddulphiana from Weno. Fig. 9. Biddulphiopsis titiana from Moch. Fig. 10. Isthmia minima from Moch. Fig. 11. Trigonium formosum f. pentagonale from Moch. Scale bar = 10 μm.
Synonym: Biddulphia pulchella S.F. Gray 1821
References: Hoban (Reference Hoban1983): 273, figures 1–27; Navarro & Lobban (Reference Navarro and Lobban2009): 133, figures 28–31; Witkowski et al. (Reference Witkowski, Lange-Bertalot and Metzeltin2000), pl. 8, figures 8 & 9 (as B. pulchella); Al-Handal et al. (Reference Al-Handal, Compère and Riaux-Gobin2016): 7, pl. 2, figure 4, pl. 3, figure 4 (as B. pulchella).
Sample: Moen_05 #002.
Dimensions: 41.8 μm long.
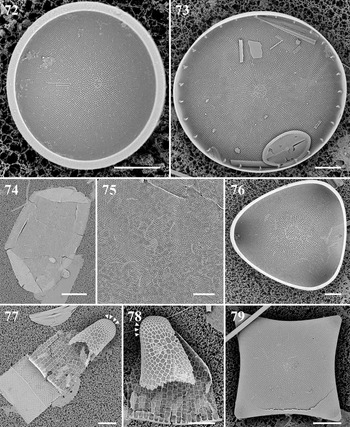
Biddulphopsis membranacea (Cleve) von Stosch & Simonsen Figures 74 & 75
References: von Stosch & Simonsen (Reference von Stosch and Simonsen1984): 15, figures 36–84; Navarro & Lobban (Reference Navarro and Lobban2009): 133, figures 6 & 7.
Sample: TK28.
Dimensions: Valve 217 μm long, 140 μm wide in flattened isolated valves; 11 areolae in 10 μm.
Diagnostics: Huge frustules elliptical in valve view, rectangular in girdle view with many copulae, numerous rimoportulae around each broad apex of the valve. Large oval central patch of randomly arranged areolae differentiated from the striae radiating from it.
Biddulphopsis titiana (Grunow) von Stosch & Simonsen Figures 9 & 76
References: von Stosch & Simonsen (Reference von Stosch and Simonsen1984): 12, figures 1–35; Lobban et al. (Reference Lobban, Schefter, Jordan, Arai, Sasaki, Theriot, Ashworth, Ruck and Pennesi2012): 251, pl. 7, figure 3.
Sample: TK28.
Dimensions: 67 μm diameter, 11–12 areolae in 10 μm.
Diagnostics: Circular to triangular valve with small central area slightly differentiated from the striae radiating from it, numerous small rimoportulae around the margin of the valve.
Isthmia minima Harvey & Bailey Figures 10, 77, 78
Reference: Lobban et al. (Reference Lobban, Schefter, Jordan, Arai, Sasaki, Theriot, Ashworth, Ruck and Pennesi2012): 251, pl. 7, figures 4–6.
Samples: TK4, TK28.
Dimensions: Depth of valve 58–77 μm; width of flattened cell in girdle view 50 μm, length of frustule 200 μm.
Diagnostics: This species is distinguished from congeners by the invaginated cribra on the valve.
Trigonium formosum f. pentagonale Hustedt Figure 11
Reference: Navarro & Lobban (Reference Navarro and Lobban2009): 133, figures 32–35.
Samples: TK28.
Dimensions: 109 μm in diameter, 7 areolae in 10 μm.
Trigonium formosum f. quadrangularis (Greville) Desikachary & Sreelatha Figure 79
References: Navarro (Reference Navarro1981b): 617, figure 25; Lobban et al. (Reference Lobban, Schefter, Jordan, Arai, Sasaki, Theriot, Ashworth, Ruck and Pennesi2012): 252, pl. 8, figure 3.
Sample: TK28.
Dimensions: 71–76 μm wide, 8 areolae in 10 μm.
Order TOXARIALES Round, 1990
Family ARDISSONEACEAE Round, 1990
Ardissonea fulgens (Greville) Grunow Figure 12

Figs 12–19. Light microscopy images of six taxa in Chuuk, Micronesia. Fig. 12. Ardissonea fulgens from Moch, portion of valve. Fig. 13. Synedrosphenia gomphonema from Moch, apical portion of valve. Figs 14, 15. Lampriscus shadboltianus from Moch, specimens with three and four ocelli. Fig. 16. Koernerella recticostata from Moch. Figs 17, 18. Grammatophora macilenta from Weno. Fig. 19. Hanicella moenia from Weno, copula. Scale bar = 10 μm.
Synonym: Synedra fulgens (Greville) W. Smith 1853, p. 74, pl. 12, figure 103.
References: Witkowski et al. (Reference Witkowski, Lange-Bertalot and Metzeltin2000): 44, pl. 31, figures 9–11; Lobban et al. (Reference Lobban, Schefter, Jordan, Arai, Sasaki, Theriot, Ashworth, Ruck and Pennesi2012): 259, pl. 16, figures 3–5; Poulin et al. (Reference Poulin, Bérard-Therriault and Cardinal1986), figures 31 & 32 (as S. fulgens).
Samples: TK4, TK28.
Dimensions: 324–427 μm long, 13.2 μm wide, 15 transapical striae in 10 μm, 22 areolae in 10 μm.
Family CLIMACOSPHENIACEAE Round, 1990
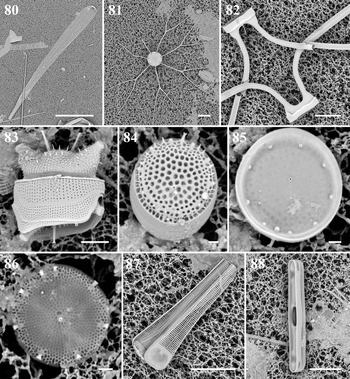
Climacosphenia moniligera Ehrenberg Figure 80
References: Round (Reference Round1982), figures 1–3, 7, 8, 9; Witkowski et al. (Reference Witkowski, Lange-Bertalot and Metzeltin2000): 44, pl. 18, figure 1.
Samples: TK4, TK28.
Dimensions: 338–367 μm long, 36 μm wide at the apex, 9 μm at the base.
Comments: Valve margins gradually sloping (in contrast to C. elongata, which is strongly spathulate), often curved.
Synedrosphenia gomphonema (Janisch & Rabenhorst) Hustedt Figure 13
References: Janisch & Rabenhorst (Reference Janisch, Rabenhorst and Rabenhorst1863): 13, pl. 2, figure 6; Hustedt 1914 in Schmidt et al. (Reference Schmidt, Schmidt, Frike, Heiden, Müller and Hustedt1874–1959), pl. 305, figures 32–34; Hustedt (Reference Hustedt1931–1959): 231, figure 723.
Samples: TK4, TK28.
Dimensions: 250 μm long, 27.8 μm wide near apex, 19 transapical striae in 10 μm, 24 areolae in 10 μm.
Diagnostics: Valves long, broadly clavate with subrostrate head pole.
Comments: This species is new to Micronesian waters including Guam.
Subclass CHAETOCEROPHYCIDAE Round & R.M. Crawford, 1990
Order CHAETOCEROTALES Round & R.M. Crawford, 1990
Family CHAETOCEROTACEAE Ralfs in Pritchard, 1861
Bacteriastrum furcatum Shadbolt Figure 81
References: Hustedt (Reference Hustedt1927–1930): 612, figure 353 (as B. delicatulum Cleve); Sarno et al. (Reference Sarno, Zingone and Marino1997): 262, figures 19–34; Bosak et al. (Reference Bosak, Šupraha, Nanjappa, Kooistra and Sarno2015): 132, figures 1–17.
Samples: TK4, TK28.
Dimensions: 12 μm in diameter, length of setae 50 μm. 6–10 inner setae per valve.
Diagnostics: Inner setae bifurcated in valve plane, and its fused part long.
Comments: Hustedt (Reference Hustedt1927–1930: 612, figure 353) shows the very similar B. delicatulum Cleve, implying synonymy, but these species are still both considered valid. There appear to be no SEM data for B. delicatulum, and it was described from temperate waters, whereas B. furcatum is a warm-water species (Bosak et al., Reference Bosak, Šupraha, Nanjappa, Kooistra and Sarno2015). This species is new to Micronesian waters including Guam.
Chaetoceros cf. atlanticus var. skeleton (F. Schütt) Hustedt Figure 82
References: Cupp (Reference Cupp1943): 104, figures 59–B; Lobban et al. (Reference Lobban, Schefter, Jordan, Arai, Sasaki, Theriot, Ashworth, Ruck and Pennesi2012): 253, pl. 9, figure 3.
Sample: TK28.
Dimensions: 13.5 μm in diameter.
Comments: Although the nominate variety of this species is cold/temperate Atlantic, var. skeleton, with extremely shallow valves and slender chaetae, ‘reaches its peak in warmer seas’ (Hustedt Reference Hustedt1927–1930: 645).
Subclass THALASSIOSIROPHYCIDAE Round & R.M. Crawford, 1990
Order EUPODISCALES V.A. Nikolaev & D.M. Harwood, 2000
Family EUPODISCACEAE Ralfs, 1861
Lampriscus shadboltianus (Greville) Peragallo & Peragallo Figures 14 & 15
References: Hustedt (Reference Hustedt1927–1930): 807–810, figures 470, 471; Navarro (Reference Navarro1981b): 618, figures 33a–36.
Samples: TK4, TK28.
Dimensions: 50–83 μm in diameter.
Comments: We did not observe crenulate copulae (var. crenulatus Navarro), as are most specimens collected in Guam (Navarro & Lobban, Reference Navarro and Lobban2009), and have thus left the name at the species level. This is a first record from Micronesian waters.
Odontella aurita (Lyngbye) C. Agardh Figure 83
Reference: Lobban et al. (Reference Lobban, Schefter, Jordan, Arai, Sasaki, Theriot, Ashworth, Ruck and Pennesi2012) 250, pl. 6, figures 1 & 2.
Sample: CHP3-2 st1265.
Dimensions: 16.4–42.3 μm long; 5.2–22.3 μm wide; 11–20 areolae in 10 μm.
Order THALASSIOSIRALES Glezer & I.V. Makarova, 1986
Family THALASSIOSIRACEAE Lebour, 1930
Thalassiosira cedarkeyensis Prasad Figures 84 & 85
Reference: Park et al. (Reference Park, Lobban and Lee2018): 105, figure 4 (as Thalasssiosira cf. cedarkeyensis).
Samples: Moen_01 #001, Moen_02 #001, Moen_03 #001, CHP-2 st1285, CHP-3 st1286.
Diagnosis: Valves tangentially undulate; single fultoportula in the depressed part of valve; external tube of marginal rimoportula prominent.
Comments: With SEM examination, the morphological details of this small taxon confirm its identity as T. cedarkeyensis. This species was abundant with over 5.4 × 105 cells l−1 in the seaweed-associated sample at sites 1 and 2. Thalassiosira cedarkeyensis was first described from Florida benthos (Prasad et al., Reference Prasad, Fryxell and Livingston1993, Reference Prasad, Nienow and Hargraves2011), and also reported from the Guangdong coast (Li et al., Reference Li, Zhao and Lu2013). In addition, Risjani et al. (Reference Risjani, Witkowski, Kryk, Yunianta, Krzywda, Safitri, Sapar, Dąbek, Arsad, Gusev, Rudiyansyah and Wróbel2021) also identified this species from Indonesian coral reef habitats. Despite the few reports of T. cedarkeyensis, the occurrences of tropical benthic diatoms in different ocean basins (Atlantic and Pacific) might be widespread along the coral reefs in the western Pacific Ocean based on its occurrence from coral reef regions.
Thalassiosira cf. catharinensis M. Garcia Figure 86
Reference: García & Dutra (Reference Garcia and Dutra2016): 62, figures 1–14.
Samples: CHB1-1 st1263, CHP-2 st1285.
Dimensions: 6.9 μm in diameter; 45 areolae in 10 μm, arranged fasciculate.
Diagnostics: Valve very small. Areolation fasciculate. Rimoportula adjacent to a marginal fultoportula.
Comments: Although the small valve size, areolae density and areolation of the Chuuk specimen match the T. catharinensis from Brazilian waters (García & Dutra, Reference Garcia and Dutra2016), the internal structures such as number of satellite pores and slit direction of the rimoportula are needed to identify positively.
Class FRAGILARIOPHYCEAE Round, 1990
Subclass FRAGILARIOPHYCIDAE Round, 1990
Order FRAGILARIALES P.C. Silva, 1962
Family FRAGILARIACEAE Kützing, 1844
Koernerella recticostata (Körner) Ashworth, Lobban & Theriot Figures 16 & 87
Reference: Lobban et al. (Reference Lobban, Ashworth, Arai, Jordan and Theriot2011a): 181, figures 1, 3, 8, 9, 21–25.
Samples: TK28.
Dimensions: 58 μm long, 5 μm wide at base.
Order RHABDONEMATALES Round & Crawford, 1990
Family GRAMMATOPHORACEAE Lobban & Ashworth, 2014
Grammatophora macilenta W. Smith Figures 17 & 18
References: Witkowski et al. (Reference Witkowski, Lange-Bertalot and Metzeltin2000): 58, pl. 15, figures 16–18; Lobban et al. (Reference Lobban, Schefter, Jordan, Arai, Sasaki, Theriot, Ashworth, Ruck and Pennesi2012): 262, pl. 19, figures 3–5.
Sample: Moen_04 #001.
Dimensions: 39.2–65.0 μm long; 15.6–27.6 μm in pervalvar axis.
Diagnostics: Septa almost straight in girdle view.
Comments: Lobban et al. (Reference Lobban, Schefter, Jordan, Arai, Sasaki, Theriot, Ashworth, Ruck and Pennesi2012) showed G. macilenta from Guam with the valve view under SEM observation. The valve in Guam specimens were heteropolar and two spines were present in the narrow pole (cf. Lobban et al., Reference Lobban, Schefter, Jordan, Arai, Sasaki, Theriot, Ashworth, Ruck and Pennesi2012, figures 3–5). In the Chuuk specimens, the valve outline was not observed, the marginal spine seems to absent from the pole, and we did not observe the apical pseudosepta. Al-Handal et al. (Reference Al-Handal, Compère and Riaux-Gobin2016) described Grammatophora cf. macilenta from the coral reefs of Réunion and Rodrigues Islands and it differed from G. macilenta by the slightly bent frustule and wavy septa in the upper part of the frustule.
Grammatophora marina (Lyngbye) Kützing Figure 88
Reference: Witkowski et al. (Reference Witkowski, Lange-Bertalot and Metzeltin2000): 58, pl. 16, figures 4, 6, 7; Sato et al. (2003): 186, figures 5a, b, 15, 16, 25, 26.
Sample: TK28.
Dimensions: 39 μm long, striae 18 in 10 μm.
Diagnostics: Valve undulate, septum with a distinct wave at the apices.
Comments: We reported G. marina in our previous work (Park et al., Reference Park, Lobban and Lee2018), which actually matches G. oceanica in both the septum shape and the stria density according to Sato et al. (Reference Sato, Nagumo and Tanaka2003). Therefore, we correctly report G. marina again.
Hanicella moenia Lobban & Ashworth Figure 19
Reference: Lobban & Ashworth (Reference Lobban and Ashworth2014): 881, figures 1a, b, 4–11.
Sample: TK28.
Dimensions: 119 μm long, 3.4 μm wide except 6.6 μm at apical inflations and 13.7 μm at central inflation.
Diagnosis: Valves with capitate apices and a diamond-shaped central inflation; silica flaps attached to the valve margin that resemble ramparts on a castle wall. Distinctive open copulae with half septa (illustrated).
Comments: This species has been only observed from Guam (Lobban & Ashworth, Reference Lobban and Ashworth2014) and Chuuk in the North-western Pacific.
Microtabella interrupta (Ehrenberg) F.E. Round Figure 89
References: Round et al. (Reference Round, Crawford and Mann1990): 673; Lobban & Ashworth (Reference Lobban and Ashworth2014): 873, figures 3E–J, 12, 13.
Sample: CHB1-1 st1293.
Dimensions: 20.2 μm long; 2.1 μm wide; 28 transapical striae in 10 μm; 34 areolae in 10 μm.
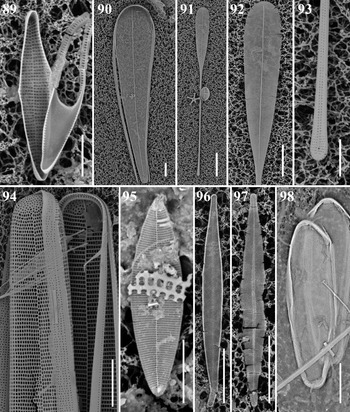
Rhabdonema cf. adriaticum Kützing Figure 20
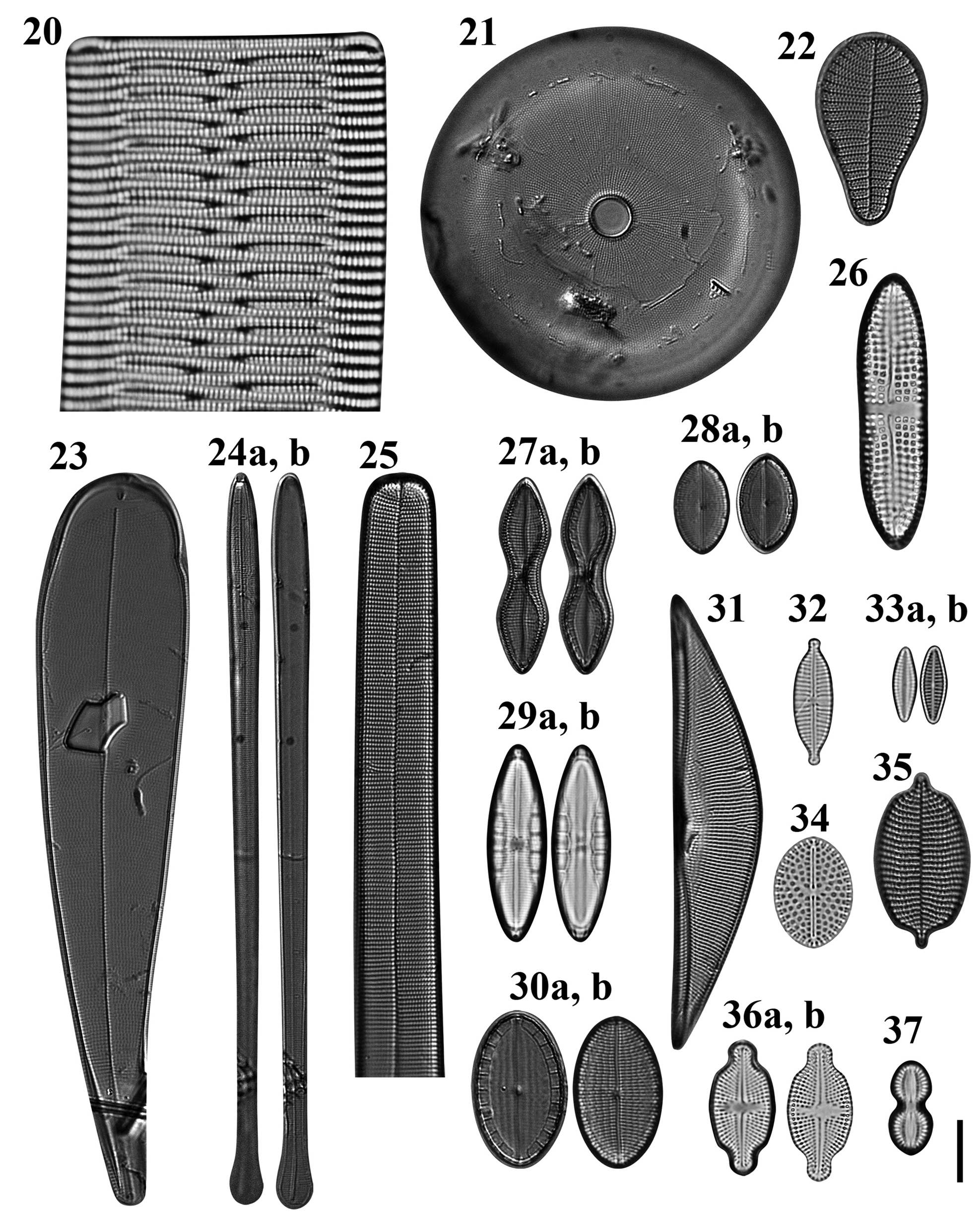
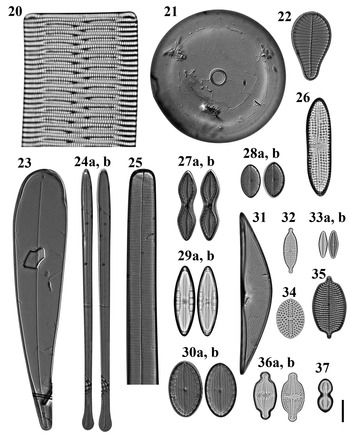
Figs 20–37. Light microscopy images of 18 taxa in Chuuk, Micronesia. Fig. 20. Rhabdonema cf. adriaticum from Weno. Fig. 21. Astrosyne radiata from Moch. Fig. 22. Podocystis adriatica from Moch. Fig. 23. Licmophora curvata from Moch. Fig. 24a, b. Licmophora flabellata from Moch. Fig. 25. Synedra bacillaris from Moch, portion of valve. Fig. 26. Achnanthes brevipes from Weno. Fig. 27a, b. Mastogloia constricta from Moch. Fig. 28. (A, B) Mastogloia emarginata from Moch. Fig. 29. (A, B) Mastogloia exigua from Weno. Fig. 30a, b. Mastogloia ovata from Moch. Fig. 31. Tetramphora intermedia from Moch. Fig. 32. Gomphonema lagenula from Weno. Fig. 33a, b. Planothidium campechianum from Weno. Fig. 34. Cocconeiopsis wrightii from Weno. Fig. 35. Schizostauron cf. trachyderma from Moch. Fig. 36. (A, B) Luticola inserata from Weno. Fig. 37. Diploneis gravelleana from Weno. Scale bar = 10 μm.
Reference: Lobban & Ashworth (Reference Lobban and Ashworth2014): 873, figures 3D, 15–17.
Sample: Moen_05 #001.
Dimensions: 51.0 μm long; 13 striae in 10 μm.
Comments: Lobban & Ashworth (2014: 882) referred Guam specimens to ‘Rhabdonema cf. adriaticum’ because of a large divergence in the molecular tree between samples from Mexico and Guam, raising the possibility that the Guam flora (and by implication Micronesian flora) might have a different species not yet distinguished morphologically, instead of or in addition to authentic R. adriaticum.
Order CYCLOPHORALES Round & R.M. Crawford, 1990
Family CYCLOPHORACEAE Round & R.M. Crawford, 1990
Astrosyne radiata Ashworth & Lobban Figure 21
Reference: Ashworth et al. (Reference Ashworth, Ruck, Lobban, Romanovicz and Theriot2012): 688, figures 10–13, 37–41.
Samples: TK4, TK28.
Dimensions: 33–61 μm in diameter.
Diagnosis: The distinctive circular valve with a cuplike pseudoseptum in the centre is actually a pennate diatom, related to Cyclophora.
Comment: This species has been only reported from Guam, Chuuk and Marshall along the tropical coral reefs in the North-western Pacific (Ashworth et al., Reference Ashworth, Ruck, Lobban, Romanovicz and Theriot2012).
Order LICMOPHORALES Round, 1990
Family LICMOPHORACEAE Kützing, 1844
Podocystis adriatica Kützing Figure 22
Reference: Lobban et al. (Reference Lobban, Schefter, Jordan, Arai, Sasaki, Theriot, Ashworth, Ruck and Pennesi2012): 256, pl. 12, figures 2 & 3.
Sample: TK4.
Dimensions: 30–35 μm long, 17–18 μm wide; striae 17 in 10 μm.
Licmophora curvata Lobban, Tharngan & Ashworth Figures 23 & 90
Reference: Lobban et al. (Reference Lobban, Tharngan and Ashworth2018): 95, figures 76–117.
Samples: TK4, TK28.
Dimensions: 120–153 μm long, 19–28 μm wide; striae 19 in 10 μm.
Diagnostics: Broad, slightly curved, clavate Licmophora; its curved congener L. normaniana (Greville) Wahrer is narrow and arcuate.
Comment: The occurrence of L. curvata from Chuuk is the second record after Guam by Lobban et al. (Reference Lobban, Tharngan and Ashworth2018). Beside these studies, there was no report from outside of Micronesia until now.
Licmophora flabellata (Carmichael ex Greville) C.A. Agardh Figures 24a, b
References: Sar & Ferrario (Reference Sar and Ferrario1990): 404, figures 1–13; Honeywill (Reference Honeywill1998): 230, figures 1a–i; Lobban et al. (Reference Lobban, Schefter and Ruck2011b): 20, figures 29–33.
Samples: CHB1-1 st1262, CHB1-1 st1263, TK4.
Dimensions: 94.4–149.4 μm long; 6.2–6.8 μm; 36 transapical striae in 10 μm.
Diagnostics: Valves with numerous intercalary rimoportulae along the sternum; differing from L. comnavmaria Lobban & Schefter in being areolate throughout.
Licmophora remulus (Grunow) Grunow Figures 91–93
References: Navarro & Lobban (Reference Navarro and Lobban2009): 136, figures 54–57; Lobban et al. (Reference Lobban, Schefter and Ruck2011b): 19, figures 26–28.
Samples: TK28.
Dimensions: 144 μm long, 12 μm wide; striae 29 in 10 μm.
Diagnostics: Slender, strongly spathulate valves, the apical portion areolate throughout; striae on the ‘stems’ comprising a single areola on each side of the sternum.
Comments: This species has been found in Guam and Yap but is also present in the Honduras material that Grunow used to describe L. remulus, and probably is also pantropical epiphyte on coral reef seaweeds (Lobban, Reference Lobban2021a), sometimes abundant, but the shape is not unique. There is a similar species, L. romuli Lobban, that may have been mistaken for L. romulus. It has decreasing vimines toward the apex.
Family ULNARIACEAE Cox, 2015
Hyalosynedra al-shareefii Sabir & Theriot Figure 95
Reference: Sabir et al. (Reference Sabir, Theriot, Lobban, Alhebshi, Al-Malki, Hajrah, Khiyami, Obaid, Jansen and Ashworth2018a): 16, figures 33–36.
Sample: CHP3-2 st1265.
Dimensions: 15.3 μm long; 4.1 μm wide; 61 transapical striae in 10 μm.
Diagnostics: Small, wide cells with narrow linear sternum, apex rounded.
Comments: This is a first record from Micronesian waters.
Hyalosynedra cf. sublaevigata Álvarez-Blanco & S. Blanco Figures 96 & 97
References: Álvarez-Blanco & Blanco (Reference Álvarez-Blanco and Blanco2014): 105, pl. 54, figures 1–6; Sabir et al. (Reference Sabir, Theriot, Lobban, Alhebshi, Al-Malki, Hajrah, Khiyami, Obaid, Jansen and Ashworth2018a): 16, figures 20–26.
Sample: CHB1-1 st1263.
Dimensions: 28.5–38.3 μm long; 3.3–3.7 μm wide; 58–66 transapical striae in 10 μm.
Diagnostics: Medium cells with lanceolate sternum, apex subcapitate.
Comments: Although Sabir et al. (Reference Sabir, Theriot, Lobban, Alhebshi, Al-Malki, Hajrah, Khiyami, Obaid, Jansen and Ashworth2018a) described the sternum as lanceolate, and these specimens key out to this species in their key, Álvarez-Blanco & Blanco (Reference Álvarez-Blanco and Blanco2014) describe the sternum as ‘narrow’, implying it is linear rather than lanceolate. Moreover, the sterna in our specimens are perhaps more broadly lanceolate than specimens shown by Sabir et al. (Reference Sabir, Theriot, Lobban, Alhebshi, Al-Malki, Hajrah, Khiyami, Obaid, Jansen and Ashworth2018a).
Synedra bacillaris (Grunow) Hustedt Figures 25 & 94
References: Sullivan & Wear (Reference Sullivan and Wear1995): 182, figures 9–12; Lobban et al. (Reference Lobban, Schefter, Jordan, Arai, Sasaki, Theriot, Ashworth, Ruck and Pennesi2012): 256, pl. 13, figures 1 & 2.
Samples: TK4, TK28.
Dimensions: 410–432 μm long, 16–19 μm wide; striae 13–14 in 10 μm.
Comments: Dimensions given by Lobban et al. (Reference Lobban, Schefter, Jordan, Arai, Sasaki, Theriot, Ashworth, Ruck and Pennesi2012) may confound two different species, the smaller one undescribed and also present in Chuuk samples. The size range given by Hustedt (Reference Hustedt1931–1959) matches the specimens shown here, but his stria density does not.
Incertae Sedis
Gato hyalinus Lobban & Navarro Figure 98
Reference: Lobban & Navarro (Reference Lobban and Navarro2013): 23, figures 1–21.
Samples: TK4, TK28.
Dimensions: 33 μm long, 12 μm wide.
Diagnostics: Asymmetrical, hyaline, oval cells in mucilage tubes, striae extremely fine, visible only in SEM.
Comments: This is a first record from Micronesian waters.
Subclass BACILLARIOPHYCIDAE D.G. Mann, 1990
Order MASTOGLOIALES D.G. Mann, 1990
Family ACHNANTHACEAE Kützing, 1844
Achnanthes brevipes C. Agardh Figure 26
Reference: Lobban et al. (Reference Lobban, Schefter, Jordan, Arai, Sasaki, Theriot, Ashworth, Ruck and Pennesi2012): 284, pl. 38, figures 1–4.
Sample: Moen_03 #002.
Dimensions: 44.0 μm long, 11.2 μm wide; 9 transapical striae in 10 μm; 10 areolae in 10 μm.
Diagnosis: Valve linear with somewhat acute apices. Transapical fascia distinct in valve centre and reaching valve margin.
Comments: Toyoda & Williams (Reference Toyoda and Williams2004) clearly summarized three differences between A. brevipes var. brevipes and A. brevipes var. intermedia. According to criteria to distinguish between both varieties by Toyoda & Williams (Reference Toyoda and Williams2004), the linear valve and almost flat valve surface of Chuuk specimen match A. brevipes var. brevipes. Although Park et al. (Reference Park, Lobban and Lee2018) reported A. brevipes from seaweed-associated diatoms, its quadrangular areolae and narrow fascia reaching the valve margin are closer to A. cuneata than A. brevipes. Therefore, we correctly report A. brevipes again.
Family MASTOGLOIACEAE Mereschkowsky, 1903
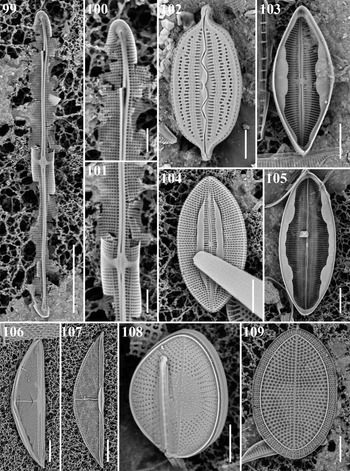
Craspedostauros cf. neoconstrictus E.J. Cox Figures 99–101
Reference: Cox (Reference Cox1999): 140, figures 1–5, 11–14, 24, 31, 35, 40, 46, 50.
Sample: CHB1-2 st1263.
Dimensions: 45 μm long, 4 μm wide, striae 23 in 10 μm.
Diagnostics: The length, number of striae and form of the internal central nodule serve to distinguish C. neoconstrictus, but it has hyaline extensions of the mantle beyond the stauros, which are not visible in this perpendicular image.
Comments: The stria density distinguishes the Chuck specimen from C. paradoxa Ashworth & Lobban, recently described from Guam, which has much finer striae and a very reduced stauros (Ashworth et al., Reference Ashworth, Lobban, Witkowski, Theriot, Sabir, Baeshen, Hajarah, Baeshen, Sabir and Jansen2017).
Mastogloia constricta Cleve Figures 27a, b
Reference: Hustedt (Reference Hustedt1931–1959): 506, figure 931B.
Sample: TK28.
Dimensions: 32 μm long, 8.5 μm wide, constricted to 5.5 μm; striae 22 in 10 μm.
Comments: This species, characterized by Hustedt (Reference Hustedt1931–1959) as rare, has been reported from Java and Borneo. This is a first record from Micronesian waters.
Mastogloia corsicana Grunow Figure 102
References: Yohn & Gibson (Reference Yohn and Gibson1981): 643, figures 17a, b, c, 18–21; Lobban et al. (Reference Lobban, Schefter, Jordan, Arai, Sasaki, Theriot, Ashworth, Ruck and Pennesi2012): 269, pl. 26, figures 1–3.
Sample: CHP3-2 st1265.
Dimensions: 24.3–26.3 μm long, 11.1 μm wide; 19–20 striae in 10 μm.
Diagnostics: Valves elliptical with protracted apices. Raphe sinuous. Two longitudinal ribs interrupting the transapical striae.
Comments: This is a first record for Micronesian waters.
Mastogloia emarginata Hustedt Figures 28a, b
References: Witkowski et al. (Reference Witkowski, Lange-Bertalot and Metzeltin2000): 245, pl. 77, figures 9–12; Lobban (Reference Lobban2015a): 7, figures 51–55; Pennesi et al., Reference Pennesi, Poulin, Hinz, Rmoagnoli, de Stefano and Totti2013, figures 65, 66.
Sample: TK28.
Dimensions: 16 μm long, 9 μm wide; striae 27 in 10 μm.
Diagnostics: Characterized by the prominent pores on the outer side of the elongate chambers. The stria density is higher than the range in Hustedt (Reference Hustedt1931–1959) or Lobban (Reference Lobban2015a).
Mastogloia exigua F.W. Lewis Figures 29a, b
References: Schmidt et al. (Reference Schmidt, Schmidt, Frike, Heiden, Müller and Hustedt1874–1959), pl. 185, figures 33, 35; Kemp & Paddock (Reference Kemp and Paddock1990), figures 43–45.
Sample: Moen_02 #001.
Dimensions: 31.7 μm long, 9.6 μm wide; 23 striae in 10 μm.
Diagnostics: Valves elliptic-lanceolate with rounded apices. Transapical striae slightly radial in valve centre and convergence near the valve ends. Five partecta positioned in the middle, their size decreasing towards the apices.
Comments: This is a first record from Micronesian waters.
Mastogloia exilis Hustedt Figure 103
Reference: Lobban et al. (Reference Lobban, Schefter, Jordan, Arai, Sasaki, Theriot, Ashworth, Ruck and Pennesi2012): 273, pl. 28, figures 6 & 7.
Sample: CHB1-2 st1295.
Dimensions: 21.3 μm long, 8.6 μm wide; 31 striae in 10 μm.
Diagnostics: Small slightly rostrate valves with half-lanceolate hyaline areas extending from the central area in the form of an H, chambered only in the middle.
Mastogloia hustedtii Meister Figure 104
Reference: Lobban et al. (Reference Lobban, Schefter, Jordan, Arai, Sasaki, Theriot, Ashworth, Ruck and Pennesi2012): 274, pl. 29, figures 3–5.
Sample: CHB1-2 st1263.
Dimensions: 20.5 μm long, 10.5 μm wide; 32 striae in 10 μm.
Diagnostics: Recognized in external SEM by the sinuous raphe (whereas M. mediterranea has a straight raphe) and the conopea.
Mastogloia mauritiana Brun Figure 105
Reference: Lobban et al. (Reference Lobban, Schefter, Jordan, Arai, Sasaki, Theriot, Ashworth, Ruck and Pennesi2012): 278, pl. 32, figures 4–10.
Sample: CHP3-2 st1265.
Dimensions: 57.5 μm long, 22.8 μm wide; 14 striae in 10 μm.
Diagnostics: Valves lanceolate with broadly rounded apices. Raphe slightly sinuous. Partectal ring with inflated cells in each quadrant.
Mastogloia ovata Grunow Figure 30a, b
References: Lobban et al. (Reference Lobban, Schefter, Jordan, Arai, Sasaki, Theriot, Ashworth, Ruck and Pennesi2012): 279, pl. 33, figures 7, 8; Pennesi et al. (Reference Pennesi, Poulin and Totti2016): 153, figures 4A–H.
Sample: TK28.
Dimensions: 24.3 μm long, 14 μm wide; striae 19 in 10 μm.
Diagnostics: Distinguished from M. emarginata by the shorter, wider partecta without prominent pore.
Tetramphora decussata (Grunow) Stepanek & Kociolek Figures 106 & 107
Basionym: Amphora decussata Grunow 1877: 178, pl. 195, figure 9.
References: Stepanek & Kociolek (Reference Stepanek and Kociolek2016): 139; Lobban et al. (Reference Lobban, Schefter, Jordan, Arai, Sasaki, Theriot, Ashworth, Ruck and Pennesi2012): 298, pl. 1, figures 7–9, pl. 54, figure 5, pl. 55 figures 1–3 (as A. decussata).
Sample: TK28.
Dimensions: 61 μm long, 14 μm wide; striae 19 in 10 μm.
Comments: The taxonomy was revised by Stepanek & Kociolek (Reference Stepanek and Kociolek2016).
Tetramphora intermedia (Cleve) Stepanek & Kociolek Figure 31
Basionym: Amphora rhombica var. intermedia Cleve 1895: 127.
References: Stepanek & Kociolek (Reference Stepanek and Kociolek2016): 131, figures 35–44; Lobban (Reference Lobban2015a): 2, figures 7–9 (as A. rhombica var. intermedia).
Sample: TK28.
Dimensions: 60 μm long, 13 μm wide; striae 18 in 10 μm.
Order CYMBELLALES D.G. Mann, 1990
Family GOMPHONEMATACEAE Kützing, 1844
Gomphonema lagenula Kützing Figure 32
Synonym: Gomphonema parvulum var. lagenulum (Kützing) O.Müller 1905
References: Sala & Ramírez (Reference Sala and Ramírez2008): 1171, figures 25–27; Abarca et al. (Reference Abarca, Jahn, Zimmermann and Enke2014): figures 2.26–28, 3.4, 5.6; Medeiros et al. (Reference Medeiros, Amaral, Ferreira, Ludwig and Bueno2018): 15, figures 44–46, 116–119; Foged (Reference Foged1978): 71, pl. XL, figures 10, 11 (as G. parvulum var. lagenulum).
Sample: Moen_01 #001.
Dimensions: 20.2 μm long, 6.2 μm wide; 16 striae in 10 μm.
Diagnostics: Valves heteropolar, elliptical with protruded ends. Striae slightly radial in the centre and parallel near the ends. Central area delimited by the shortening of one median stria. A stigma near one shortening stria.
Comments: Gomphonema lagenula is distinguished from G. parvulum by strongly protruding valve ends (Abarca et al., Reference Abarca, Jahn, Zimmermann and Enke2014). This is a freshwater species (M.D. Guiry in Guiry & Guiry, Reference Guiry and Guiry2020), and a first report from Micronesian waters.
Order COCCONEIDALES Cox
Family ACHNANTHIDIACEAE D.G. Mann, 1990
Planothidium campechianum (Hustedt) Witkowski & Lange-Bertalot Figure 33a, b
Reference: Lobban et al. (Reference Lobban, Schefter, Jordan, Arai, Sasaki, Theriot, Ashworth, Ruck and Pennesi2012): 285, pl. 39, figure 4.
Samples: Moen_03 #001, CHP-3 st1286.
Dimensions: 8.4–12.2 μm long, 4.1–4.8 μm wide; 16–18 striae in 10 μm in both valves.
Diagnostics: Valves lanceolate with slightly obtuse apices.
Family COCCONEIDACEAE Kützing, 1844
Anorthoneis eurystoma Cleve Figure 108
References: Witkowski et al. (Reference Witkowski, Lange-Bertalot and Metzeltin2000): 97, pl. 54, figures 4–8; Garcia & Talgatti (Reference Garcia and Talgatti2006): 8, figures 1, 2, 5–8; Pennesi et al. (Reference Pennesi, Majewska, Sterrenburg, Totti and de Stefano2018): 214, figures 35–48.
Sample: CHP3-2 st1265.
Dimensions: 16.5 μm long, 12.0 μm wide; 19 striae in 10 μm in rapheless valve.
Diagnostics: Valves broadly elliptical. In rapheless valve, sternum elliptical in the middle and abruptly narrowing towards apices. Transapical striae radial.
Comments: Only two species of Anorthneis have a broad hyaline area on the sternum valve (SV), the other being A. hyalina Hustedt. The SV of A. hyalina can be distinguished from A. eurystoma by the transapically elongated areolae, and the latter has a round areolae (Pennesi et al., Reference Pennesi, Majewska, Sterrenburg, Totti and de Stefano2018). This is a first record from Micronesian waters.
Cocconeiopsis wrightii (O'Meara) Witkowski, Lange-Bertalot & Metzeltin Figure 34
References: Witkowski et al. (Reference Witkowski, Lange-Bertalot and Metzeltin2000): 176, pl. 67, figure 24; Riaux-Gobin & Compère (Reference Riaux-Gobin and Compère2004): 62, Pl. III, figures 16–22, Pl. IV, text figs d, e; Park et al. (Reference Park, Lee, Kang and Lee2014): 233, figures 1b–d.
Sample: Moen_01 #001.
Dimensions: 17.7 μm long, 12.7 μm wide; 12 striae in 10 μm.
Diagnostics: Valve elliptical. Striae radiate in a single row of areolae and two rows near valve margin. Raphe straight. Axial area linear, central area transapically expanded and connected with an H-shaped area.
Comments: This is a first record from Micronesian waters.
Cocconeis coronatoides Riaux-Gobin & Romero Figure 109
Synonym: Cocconeis coronata Riaux-Gobin & Romero in Riaux-Gobin et al., Reference Riaux-Gobin, Romero, Al-Handal and Compère2010: 279, figures 1–17, 30.
References: Lobban et al. (Reference Lobban, Schefter, Jordan, Arai, Sasaki, Theriot, Ashworth, Ruck and Pennesi2012): 286, pl. 39, fig. 6; Al-Handal et al. (Reference Al-Handal, Compère and Riaux-Gobin2016): 21, pl. 7, figure 10, pl. 12, figure 8; Riaux-Gobin et al. (Reference Riaux-Gobin, Romero, Al-Handal and Compère2010): 279, figures 1–17, 30 (as C. coronata).
Sample: Moen_03 #002.
Dimensions: 14.2 μm long, 8.1 μm wide; 13 striae in 10 μm in sternum valve; 16 areolae in 10 μm in a stria.
Diagnostics: Valve elliptical. Striae parallel to slightly radiate. Crista marginalis distinct. Numerous round spine-like expansions around the areolae.
Order NAVICULAES Bessey, 1907
Suborder NEIDIINEAE D.G. Mann, 1990
Family BERKELEYACEAE D.G. Mann, 1990
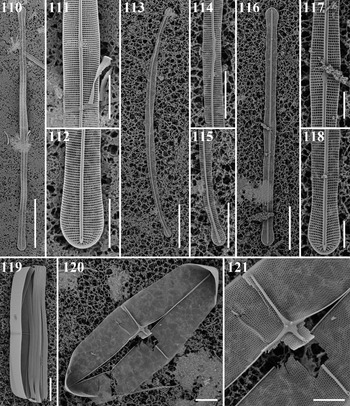
Climaconeis cf. petersonii Lobban, Ashworth & Theriot Figures 110–112
Reference: Lobban et al. (Reference Lobban, Ashworth and Theriot2010): 300, figures 9, 10, 26–32.
Sample: CHB1-2 st1263.
Dimensions: 254 μm long, 7 μm wide except 9 μm at apex and centre; striae 21 in 10 μm.
Diagnostics: Long straight valve with square to apically elongate areolae.
Comments: This specimen is considerably shorter than the range given in Lobban et al. (Reference Lobban, Ashworth and Theriot2010), 350–380 μm, but the stria density and the shapes of the areolae are similar, so there is little reason to think this is a new species.
Climaconeis riddleae A.K.S.K. Prasad Figures 113–115
References: Prasad (Reference Prasad2003): 14, figures 26–41; Lobban et al. (Reference Lobban, Ashworth and Theriot2010): 302, figures 16, 17, 39–43.
Sample: CHB1-1 st1262.
Dimensions: 107 μm long, 4 μm wide; striae 25 in 10 μm.
Diagnostics: Curved valves with ± square areolae, the stria density distinguishing it from C. inflexa (Brébisson) Cox and C. tarangensis (Lobban ms submitted).
Climaconeis scalaris (Brébisson) Cox Figures 116–118
Reference: Lobban (Reference Lobban2018): 358, figures 4, 34–38.
Sample: CHB1-1 st1292.
Dimensions: 110 μm long, 5.7 μm wide; striae 21 in 10 μm.
Diagnostics: Straight valves with apically elongate areolae, the stria density and width distinguishing it from C. undulata (Meister) Lobban, Ashworth & Theriot.
Comments: This is a first record from Micronesian waters.
Parlibellus biblos (Cleve) Cox Figures 119–121
Synonym: Stauroneis biblos (Cleve) Hustedt, p. 145.
References: Cox (Reference Cox1988): 23; Lobban et al. (Reference Lobban, Schefter, Jordan, Arai, Sasaki, Theriot, Ashworth, Ruck and Pennesi2012): 296, pl. 52, figures 4 & 5; Hustedt (Reference Hustedt1931–1959): 703, figure 1178 (as S. biblos).
Sample: Moen_05#001.
Dimensions: 70.1 μm long, 16.0 μm wide; 17 striae in 10 μm; 20 areolae in 10 μm.
Comments: Lobban et al. (Reference Lobban, Schefter, Jordan, Arai, Sasaki, Theriot, Ashworth, Ruck and Pennesi2012) identified this species as Stauroneis retrostauron (Mann) Meister, noting its similarity to Stauroneis biblos. On re-examining Mann (Reference Mann1925) and Meister (Reference Meister1937), in comparison with Hustedt (Reference Hustedt1931–1959) and Cox (Reference Cox1988), we conclude that we have P. biblos, not S. retrostauron both here and in the Guam flora. The dimensions given by Mann (Reference Mann1925: 118, pl. 25, figure 5, pl. 26, figures 1 & 2) are: length 90–141 μm, width 29–35 μm, rather large and the stria density (11 in 10 μm) much too coarse. Most tellingly, Mann's girdle view (pl. 26, figure 1) seems to show only one wide girdle band, not a large number of narrow ones. It is also different from the planktonic species Meunieria membranacea (Cleve) P.C. Silva, especially in the plastids (see Al-Yamani & Subarova, Reference Al-Yamani and Subarova2009).
Family DIADESMIDACEAE D.G. Mann, 1990
Diadesmis confervacea Kützing Figure 122
Synonym: Navicula confervacea (Kützing) Grunow 1880, pl. 14, figure 36.
References: Navarro & Lobban (Reference Navarro and Lobban2009), Novelo et al. (Reference Novelo, Tavera and Ibarra2007): 143, figures 80, 81; Schmidt (Reference Schmidt, Schmidt, Frike, Heiden, Müller and Hustedt1874–1959), pl. 297, figures 77, 78 (as N. confervacea).
Sample: CHP-2 st1285.
Dimensions: 20.8–2.7 μm long, 8.2–8.5 μm wide; 21 striae in 10 μm; 34–40 areolae in 10 μm.
Diagnostics: Frustule rectangular in girdle view, formed band-like colonies. Valve elliptical with slightly protracted apices. Marginal spines present along the junction of valve margin and mantle.
Comments: This is a terrestrial species (M.D. Guiry in Guiry & Guiry, Reference Guiry and Guiry2020). Lobban (unpubl.) has observed blooms of this species in nutrient-enriched water in a terraced secondary water treatment plant in Guam and it is also common in natural streams.
Luticola inserata (Hustedt) D.G. Mann Figures 36a, b
Basionym: Navicula inserata Hustedt 1955, p. 125, figure 18.
References: Hustedt (Reference Hustedt1961–1966): 627, figure 1624a (as N. inserata); Foged (Reference Foged1978): 91, pl. 28, figure 8; Simonsen (Reference Simonsen1987c), pl. 638, figures 7 & 8 (as N. inserata).
Samples: Moen_01 #002, Moen_03 #001.
Dimensions: 21.7–21.0 μm long, 10.5 μm wide; 19–20 striae in 10 μm; 16–17 areolae in 10 μm in a stria.
Diagnostics: Valve elliptical with capitate ends. Striae radiate. Sternum tapered towards apices, central area transapical expanded.
Comments: This is a brackish-water species, common in the western Pacific (Rybak et al., Reference Rybak, Witkowski, Peszek, Kociolek, Risjani, Nguyen, Zhang, Yunianta, Gastineau, Duong, Rosa and Meleder2021). However, it is distinguished only by some ultrastructural details from L. seposita (Hustedt) D.G. Mann, a freshwater species that Rybak et al. (Reference Rybak, Witkowski, Peszek, Kociolek, Risjani, Nguyen, Zhang, Yunianta, Gastineau, Duong, Rosa and Meleder2021) also reported from a marine habitat. Although we lack the details of this species by SEM, we identified this species as L. inserta on the basis of the habitat. This is the first record of the nominate variety from Micronesia, although L. inserata var. undulata (Hustedt) Navarro & Lobban was reported from Guam (Navarro & Lobban, Reference Navarro and Lobban2009).
Suborder SELLAPHORINEAE D.G. Mann, 1990
Family PINNULARIACEAE D.G. Mann, 1990
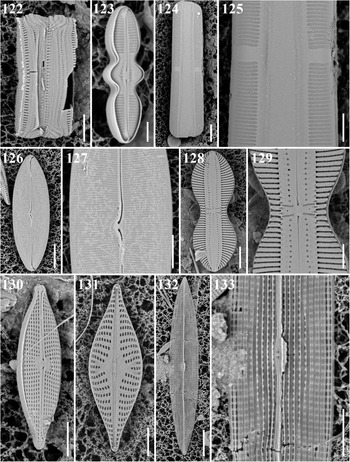
Caloneis egena (A. Schmidt) Cleve Figures 38 & 123
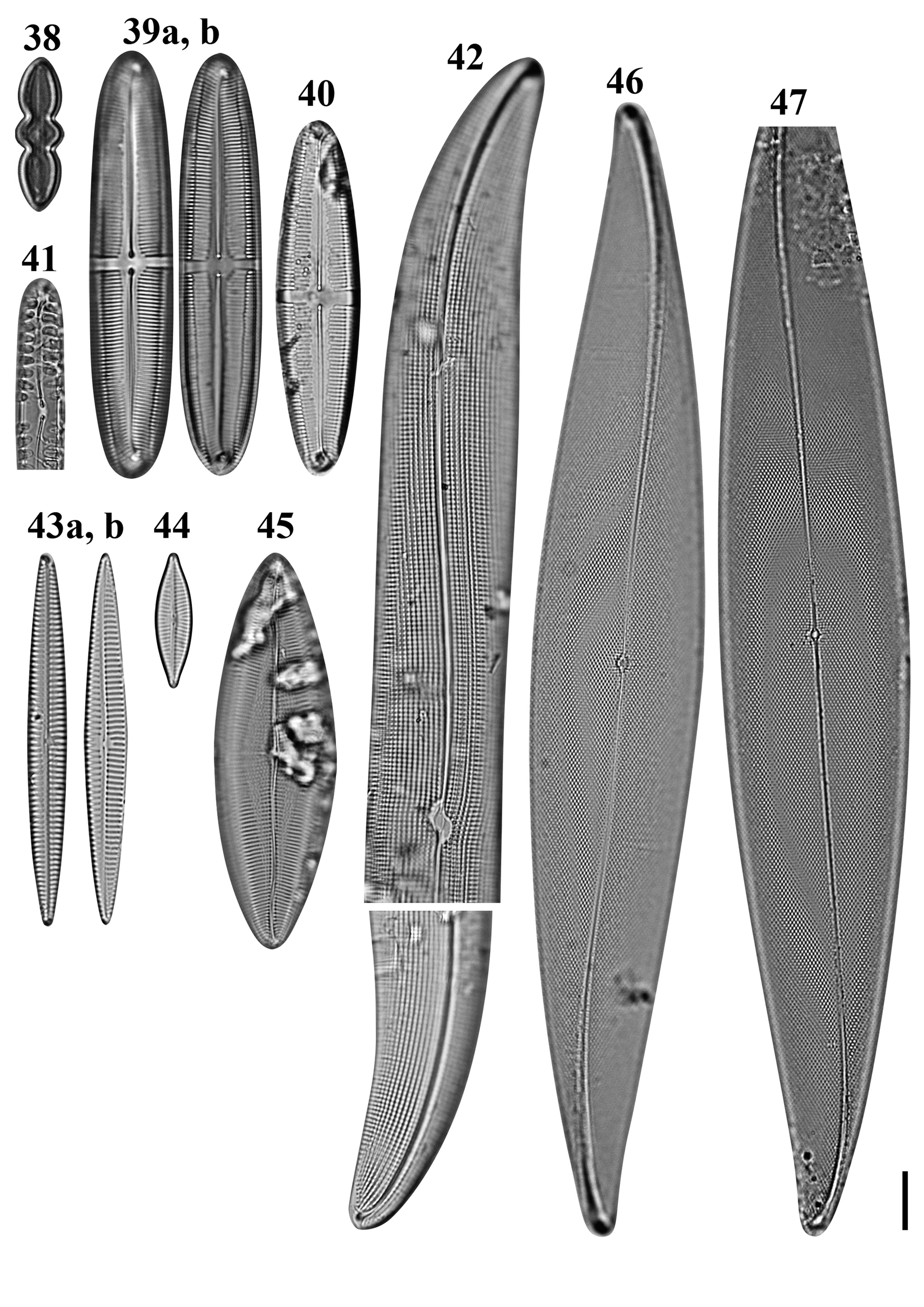
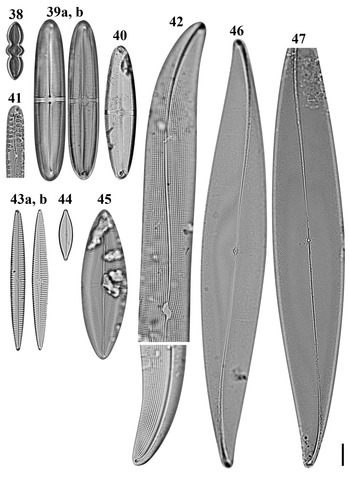
Figs 38–47. Light microscopy images of eight taxa in Chuuk, Micronesia. Fig. 38. Caloneis egena from Moch. Figs 39a, b, 40. Caloneis macquariensis from Weno. Fig. 41. Pinnularia cf. borealis from Weno. Fig. 42a, b. Gyrosigma cf. balticum from Weno. Fig. 43a, b. Navicula directa from Weno. Fig. 44. Navicula gregaria from Weno. Fig. 45. Navicula plicatula from Weno. Figs 46, 47. Pleurosigma marinum from Weno. Scale bar = 10 μm.
References: Schmidt et al. (Reference Schmidt, Schmidt, Frike, Heiden, Müller and Hustedt1874–1959), pl. 160, figures 42 & 43, pl. 212, figure 1; Lobban et al. (Reference Lobban, Schefter, Jordan, Arai, Sasaki, Theriot, Ashworth, Ruck and Pennesi2012): 294, pl. 49, figures 3 & 4.
Samples: TK4, TK28.
Dimensions: 13 μm long, 2.5–3.0 μm wide; striae 37 in 10 μm.
Caloneis macquariensis Foged Figures 39a, b, 40, 124, 125
Reference: Foged (Reference Foged1978): 38, pl. 24, figure 1.
Samples: Moen_03 #002, CHP3-2 st1265.
Dimensions: 60.2–80.1 μm long, 14.1–14.2 μm wide; 15–16 striae in 10 μm.
Diagnostics: Valve linear-lanceolate with broadly rounded apices. Transapical striae regularly parallel throughout. Axial area almost linear, narrow near ends. Central fascia present.
Comment: This is a freshwater species described from eastern Australia; new record for Micronesian waters.
Oestrupia sp. 4 sensu Hein et al. Figures 126 & 127
References: Hein et al. (Reference Hein, Winsborough, Sullivan and Lange-Bertalot2008): 101, pl. 67, figure 15; Lobban et al. (Reference Lobban, Schefter, Jordan, Arai, Sasaki, Theriot, Ashworth, Ruck and Pennesi2012): 294, pl. 50, figure 4.
Sample: CHP3-2 st1265.
Dimensions: 48.2 μm long, 15.5 μm wide; 30 transapical striae in 10 μm.
Diagnostics: Valve linear-lanceolate with broadly rounded apices. Raphe linear-sigmoid.
Pinnularia cf. borealis Ehrenberg Figure 41
Synonym: Navicula borealis (Ehrenberg) Kützing Reference Kützing1844: 96. pl. 28, figures 68, 72c.
References: Foged (Reference Foged1978): 113, pl. 33, figure 15, pl. 34, figure 9 (as N. borealis); Novelo et al. (Reference Novelo, Tavera and Ibarra2007): 49, pl. 9, figure 4 (as N. borealis).
Samples: Moen_03 #002, CHP3-2 st1265
Dimensions: 45.2 μm long, 8.4 μm wide; 5 striae in 10 μm.
Diagnostics: Valve linear with broadly rounded apices. Raphe curved in axial area. Central fascia present. Striae coarse.
Suborder DIPLONEIDINEAE D.G. Mann, 1990
Family DIPLONEIDACEAE D.G. Mann, 1990
Diploneis cerebrum Pennesi, Caputo & Lobban Figures 128 & 129
Reference: Pennesi et al. (Reference Pennesi, Caputo, Lobban, Poulin and Totti2017): 213, figures 66–75.
Sample: CHP3-2 st1265.
Dimensions: 55.8 μm long, 19.8 μm wide; 10 striae in 10 μm.
Diagnostics: Valve panduriform with broadly rounded apices. Striae consisted of transapically elongated slit-like areolae. Longitudinal canals straight, the areolae having a characteristic brain-like pattern in the volae.
Comments: This is a first record for Micronesian waters.
Diploneis gravelleana R. Hagelstein Fig. 37
References: Hagelstein (Reference Hagelstein1939): 352, pl. 5, figure 2; Foged (Reference Foged1978): 52, pl. 24, figure 11.
Sample: Moen_03 #001; Siqueiros Beltrones & López Fuerte (Reference Siqueiros Beltrones and López Fuerte2006), figure 3.11.
Dimensions: 14.7 μm long, 7.1 μm wide; 20 striae in 10 μm.
Diagnostics: Valve small, strongly constricted. Striae radiate.
Comments: This species has been observed with SEM in samples from Yap (http://www.protistcentral.org/Taxa/get/taxa_id/586012) and Palau, but there is no comparable SEM of the Puerto Rico species, which in LM resembles D. caffra (Giffen) Witkowski, Lange Bertalot & Metzeltin. The longitudinal canals follow a biarcuate path close to the alveolate striae rather than paralleling the raphe sternum. This is a first record from Micronesian waters.
Suborder NAVICULINEAE Hendey, 1937
Family NAVICULACEAE Kützing, 1844
Gyrosigma cf. balticum (Ehrenberg) Rabenhorst Figure 42a, b
References: Navarro (Reference Navarro1982a): 324, figures 66–68; Cardinal et al. (Reference Cardinal, Poulin and Bérard-Therriault1989), figures 3 & 46; Sterrenburg (Reference Sterrenburg1995): 402, figures 1–4, 13–15.
Samples: Moen_02 # 001, CHP-2 st1285.
Dimensions: 261.7 μm long, 22.9 μm wide; 13 striae in 10 μm transapically, 12 areolae in 10 μm apically.
Diagnostics: Valves sigmoid with rather obtusely rounded ends. Raphe sternum with double curvature, gradually curved throughout.
Comments: Gyrosigma sterrenburgii Stidolph (Stidolph, Reference Stidolph1992; Sterrenburg, Reference Sterrenburg1995) is a simulacrum species, differing only in some ultrastructural details and we cannot distinguish them on the basis of LM.
Navicula directa (W. Smith) Ralfs Figure 43a, b
References: Witkowski et al. (Reference Witkowski, Lange-Bertalot and Metzeltin2000): 275, pl. 129, figure 1, pl. 133, figures 10–12; Al-Handal et al. (Reference Al-Handal, Thomas and Pennesi2018): 127, figure 50.
Samples: Moen_03 #001, Moen_04 #001.
Dimensions: 63.2–73.7 μm long, 6.7–8.0 μm wide; 10–11 striae in 10 μm.
Diagnostics: Valves narrowly long lanceolate with acute apices. Transapical striae parallel throughout but convergent near apices.
Comments: Hendey (Reference Hendey1964) mentioned ‘A wide range of variation exists in this species and several subspecific taxa have been described. In all of them the striae are strongly lineate.’ This is a first report from Micronesian waters.
Navicula gregaria Donkin Figures 44 & 130
References: Cox (Reference Cox1995): 109, figures 37–42, 68–72; Witkowski et al. (Reference Witkowski, Lange-Bertalot and Metzeltin2000): 280, pl. 124, figures 8–25, pl. 129, figure 9, pl. 142, figures 4 & 5; Bruder & Medlin (Reference Bruder and Medlin2008), figures 5a–d; Van de Vijver et al. (Reference Van de Vijver, Zidarova, Sterken, Verleyen, de Haan, Vyverman, Hinz and Sabbe2011): 293, figures 97–106.
Samples: Moen_01 #001, Moen_02 #002, Moen_03 #001, CHP-3 st1286.
Dimensions: 20.0–35.5 μm long, 5.7–7.1 μm wide; 14–18 transapical striae in 10 μm.
Diagnostics: Valves lanceolate with protracted apices. Transapical striae radiate in the middle and convergent at apices. Areolae visible in LM. Central area variable in size, markedly asymmetric.
Comments: The small size of Chuuk specimen is considered as a freshwater form. This is a first record from Micronesian waters.
Navicula mannii Hagelstein Figure 131
Reference: Lobban et al. (Reference Lobban, Schefter, Jordan, Arai, Sasaki, Theriot, Ashworth, Ruck and Pennesi2012): 293, pl. 47, figure 9.
Sample: CHB1-1 st1262, CHB1-2 st1295
Dimensions: 29 μm long, 9.4 μm wide; 9 striae in 10 μm.
Comments: The shape and low stria density distinguish this from other small, ± rhomboidal Navicula spp. This species has been reported from Guam but elsewhere known only from Puerto Rico (Lobban et al., Reference Lobban, Schefter, Jordan, Arai, Sasaki, Theriot, Ashworth, Ruck and Pennesi2012).
Navicula plicatula Grunow Figure 45
Reference: Lobban et al. (Reference Lobban, Schefter, Jordan, Arai, Sasaki, Theriot, Ashworth, Ruck and Pennesi2012): 293, pl. 48, figures 1–3.
Sample: Moen_05 #001.
Dimensions: 66.9 μm long, 20.2 μm wide; 15 striae in 10 μm; 19 areolae in 10 μm in a stria.
Navicula tsukamotoi (Sterrenburg & Hinz) Yuhang Li & Kuidong Xu Figures 132 & 133
Synonym: Haslea tsukamotoi Sterrenburg & Hinz 2015, p 151, figures 14, 15, 33–38.
References: Sterrenburg et al. (Reference Sterrenburg, Tiffany, Hinz, Herwig and Hargraves2015): 151, figures 14, 15, 33–38 (as H. tsukamotoi); Li et al. (Reference Li, Chen, Sun and Xu2017): 454, figures 5–21 (as H. tsukamotoi).
Sample: CHP3-2 st1265.
Dimensions: 26.1 μm long, 5.3 μm wide; 16 transapical striae in 10 μm.
Diagnostics: Valve linear-lanceolate with acute apices. Transapical striae slightly radial.
Comments: Lobban et al. (Reference Lobban, Perez and Ashworth2020) concluded that Guam specimens identified as H. howeana are correctly identified with the new species N. tsukamotoi on the basis of genetic and structural evidence in Li et al. (Reference Li, Chen, Sun and Xu2017) and Sterrenburg et al. (Reference Sterrenburg, Tiffany, Hinz, Herwig and Hargraves2015), but could not say whether H. howeana, originally described from the Caribbean, is conspecific with N. tsukamotoi.
Family PLEUROSIGMATACEAE Mereschkowsky, 1903
Pleurosigma marinum Donkin Figures 46 & 47
Reference: Foged (Reference Foged1978): 120, pl. XXIII, figure 2.
Samples: Moen_04 #001, CHP3-2 st1286.
Dimensions: 89.0–204.2 μm long, 10.1–32.0 μm wide; 20–23 transverse striae in 10 μm; 17–20 diagonal striae in 10 μm.
Diagnostics: Valve lanceolate with slightly sigmoid ends. Raphe straight from axial area to middle of valve and curved near valve ends.
Comments: This is a first record from Micronesian waters.
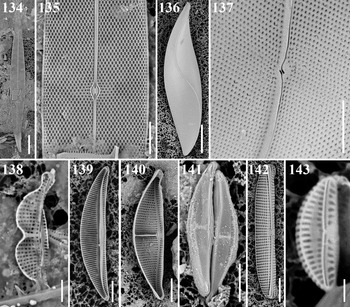
Pleurosigma cf. elongatum W. Smith Figures 134 & 135
References: Sar et al. (Reference Sar, Sterrenburg and Sunesen2014): 160, figures 48–55; Al-Handal et al. (Reference Al-Handal, Abdulla, Wulff and Abdulwahab2014), figure 55.
Samples: Moen_04 #003, CHP3-2 st1265.
Dimensions: 153.0–162.9 μm long, 16.2–16.6 μm wide; 23 transverse striae in 10 μm; 20 diagonal striae in 10 μm.
Diagnostics: Valves long, slender. Central area small and symmetric. Transverse and oblique striae angle 61°.
Comments: Park et al. (Reference Park, Lobban and Lee2018) reported this taxon in an unidentified status as Pleurosigma sp. 2. Valve shape and internal bisected areolae by bar are similar to P. elongatum (Sar et al. Reference Sar, Sterrenburg and Sunesen2014), but the absence of calcar at the terminal ends in the Chuuk specimen is different from P. elongatum. We did not observe the external details such as central raphe fissure and areolae slits in the Chuuk sample. Further details are needed to identify this taxon positively. Nevertheless, there was no record on this P. elongatum-like species from Micronesian waters until now.
Rhoicosigma compactum (Greville) Grunow Figures 136 & 137
Reference: Lobban (Reference Lobban2015a): 14, figures 139–143.
Sample: CHB2-1.
Dimensions: 106 μm long; striae 25 in 10 μm.
Comments: The stria density is at the high end of the several different ranges given in the literature, but appears to match specimens from Guam identified as this species by Lobban (Reference Lobban2015a).
Family STAURONEIDACEAE D.G.Mann, 1990
Schizostauron cf. trachyderma (F.Meister) Górecka, Riaux-Gobin & Witkowski Figure 35
Synonyms: Cocconeis citronella A. Mann; Achnanthes citronella (A. Mann) Hustedt
Reference: Davidovich et al. (Reference Davidovich, Davidovich, Witkowski, Li, Dabek, Mann, Zgłobicka, Kurzydłowski, Gusev, Górecka and Krzywda2017): 80, figures 1–24 (as Schizostauron sp. 1); Górecka et al. (Reference Górecka, Ashworth, Davidovich, Davidovich, Dąbek, Sabir and Witkowski2021): 1475, figure 2.
Sample: TK4.
Dimensions: 28 μm long, 15 μm wide; striae 10 in 10 μm (sternum valve).
Comments: This species, which has been reported as A. citronella from Guam (Lobban et al., Reference Lobban, Schefter, Jordan, Arai, Sasaki, Theriot, Ashworth, Ruck and Pennesi2012) and Tahiti (Ricard, Reference Ricard1977), now falls into Schizostauron (Davidovich et al., Reference Davidovich, Davidovich, Witkowski, Li, Dabek, Mann, Zgłobicka, Kurzydłowski, Gusev, Górecka and Krzywda2017; Górecka et al., Reference Górecka, Ashworth, Davidovich, Davidovich, Dąbek, Sabir and Witkowski2021), but we cannot tell from the LM if the Chuuk specimen belongs to S. trachyderma as proposed or to S. kajotkei Dabek, Górecka & Witkowski. The distinction is hard to pin down because while Davidovich et al. (Reference Davidovich, Davidovich, Witkowski, Li, Dabek, Mann, Zgłobicka, Kurzydłowski, Gusev, Górecka and Krzywda2017) state that their Schizostauron sp.1 is very similar to S. trachyderma except for the latter having only 9.8–12.1 striae in 10 μm on the SV, in transferring trachyderma from Achnanthes to Schizostauron, Górecka et al. (Reference Górecka, Ashworth, Davidovich, Davidovich, Dąbek, Sabir and Witkowski2021) give the stria density as 10–17 (vs 10–14). Even without that confusion, our cell, with 10 striae in 10 μm fits both. Schizostauron trachyderma has been reported from several Western Pacific sites as well as the Indian Ocean, S. kajotkei so far only from Madagascar. It is possible that our material fits neither but we will need good SEM to tell. Schizostauron is not related to Achnanthes, but in gene trees is sister to Astartiella and was transferred to Naviculales: Stauroneidaceae (Górecka et al., Reference Górecka, Ashworth, Davidovich, Davidovich, Dąbek, Sabir and Witkowski2021).
Order THALASSIOPHYSALES D.G. Mann, 1990
Family CATENULACEAE Mereschkowsky, 1902
Amphora biggiba Grunow Figure 138
Reference: Navarro & Lobban (Reference Navarro and Lobban2009): 150, figures 135 & 136.
Sample: CHP3-2 st1265.
Dimensions: 11.1 μm long, 2.5 μm wide; 34 striae in 10 μm in dorsal part, 37 striae in 10 μm in ventral part.
Amphora lunulata Wachnicka & Gaiser Figure 139
Reference: Wachnicka & Gaiser (Reference Wachnicka and Gaiser2007): 426, figures 141–143.
Sample: CHB1-1 st1262 0018.
Dimensions: 30.0–31.9 μm long, 5.3–6.2 μm wide; 23 striae in 10 μm in dorsal part, 23 striae in 10 μm in ventral part.
Diagnosis: Valves semi-elliptical with slightly acute apices. Raphe dorsally arched. Hyaline longitudinal line crossing dorsal striae.
Comments: Park et al. (Reference Park, Lobban and Lee2018) described this taxon in an unidentified status as Amphora sp. 2. The striae in the Chuuk specimen are denser than original description (17–20 in 10 μm). The most striking feature of this specimen is the internal rib alongside the raphe. As Wachnicka & Gaiser (Reference Wachnicka and Gaiser2007) did not show SEM for this species, the identification is not strongly supported by our single SEM image.
Amphora ostrearia var. vitrea Cleve Figure 140
References: Wachnicka & Gaiser (Reference Wachnicka and Gaiser2007): 411, figures 82 & 83; Lobban et al. (Reference Lobban, Schefter, Jordan, Arai, Sasaki, Theriot, Ashworth, Ruck and Pennesi2012): 299, pl. 56, figures 5 & 6.
Sample: Moen_03 #002.
Dimensions: 55.1 μm long, 13.3 μm wide; 11 striae in 10 μm in dorsal part, 11 striae in 10 μm in ventral part.
Amphora cf. abuldens Simonsen Figure 141
References: Simonsen (Reference Simonsen1960): 129, pl. 2, figures 10–12; Witkowski et al. (Reference Witkowski, Lange-Bertalot and Metzeltin2000): 128, pl. 163, figures 18 & 19.
Sample: CHB1-1 st1262.
Dimensions: 17.5 μm long, 3.5 μm wide; 50 striae in 10 μm in dorsal part.
Diagnostics: Valve semi-lanceolate, almost straight ventral margin with acute apices. Transapical striae only distinct in the valve margin of dorsal part. Raphe slightly arched. Axial area on the dorsal part broad. Transverse fascia distinct.
Comments: Simonsen (Reference Simonsen1960) and Witkowski et al. (Reference Witkowski, Lange-Bertalot and Metzeltin2000) described the stria density of A. abludens as above 38 in 10 μm, but the Chuuk specimen density is even denser.
Amphora cf. egregia Ehrenberg Figure 142
References: Schmidt et al. (Reference Schmidt, Schmidt, Frike, Heiden, Müller and Hustedt1874–1959), pl. 28, figures 13–15, 18, pl. 39, figures 26, 27, 31; Levkov (Reference Levkov2009): 271, pl. 113, figure 2, pl. 269, figures 1–6, pl. 283, figures 4 & 5.
Sample: CHB1-1 st1263.
Dimensions: 56.5 μm long, 8.3 μm wide; 9 striae in 10 μm in dorsal part, 9 striae in 10 μm in ventral part.
Diagnostics: Valve linear semi-elliptic with broadly round apices. Dorsal and ventral part parallel in the middle part and smoothly curved toward ventral part. Areolae coarse and loculate. Striae slightly radiate in middle part and convergent in ends. Raphe sternum flap toward dorsal part covering adjacent areolae.
Comments: Although this species has the general characters of A. egregia, our specimen has striae all along the ventral side rather than just at the apices and along the margin, and appears to lack a longitudinal rib across the dorsal striae near the dorsal margin (Levkov, Reference Levkov2009).
Amphora cf. helenesis Giffen Figure 143
References: Giffen (Reference Giffen1973): 5, figures 7–9; Witkowski et al. (Reference Witkowski, Lange-Bertalot and Metzeltin2000): 139, pl. 163, figures 31–33; Al-Handal et al. (Reference Al-Handal, Compère and Riaux-Gobin2016): 32, pl. 13, figures 5 & 6; Kim et al. (Reference Kim, Kim, Park and Witkowski2017): 607, figures 2A–L.
Sample: CHP3-2 st1265.
Dimensions: 5.0–10.3 μm long; 1.8–2.6 μm wide; 40–46 striae in 10 μm in dorsal part, 31–37 striae in 10 μm in ventral part.
Diagnosis: Valve lunate with rounded apices. Raphe slightly bi-arcuate.
Comments: Chuuk specimen is similar to A. helenensis, but the stria density is substantially higher than in the original description (17–20 in 10 μm).
Order BACILLARIALES Hendey, 1937
Family BACILLARIACEAE Ehrenberg, 1831
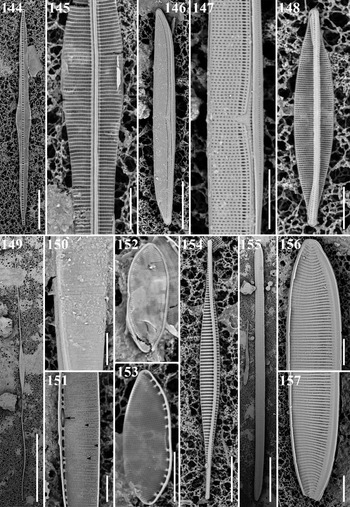
Bacillaria Group B sensu Schmid var. tumidula Figures 144 & 145
Synonym: Bacillaria paxillifer var. tumidula (Grunow) Witkowski et al., Reference Witkowski, Lange-Bertalot and Metzeltin2000: 357, pl. 196, figure 8.
References: Witkowski et al. (Reference Witkowski, Lange-Bertalot and Metzeltin2000): 357, pl. 196, figures 5–7, pl. 207, figure 9; Schmid (Reference Schmid2007): 304, figure 9.
Sample: CHB1-1 st1263.
Dimensions: 110 μm long, 8 μm wide; striae 21 in 10 μm, 10 fibulae in 10 μm.
Diagnostics: The variety tumidula is distinguished by an inflated centre with silica flaps at the edge of the valve face.
Comments: This group has still not been given a species name, which it lacks since Schmid (Reference Schmid2007) restricted B. paxillifer to estuarine forms. This form is also abundant in mangrove samples from Vietnamese coasts (A. Witkowski, personal communication). This is a first record from Micronesian waters.
Giffenia cocconeiformis (Grunow) Round & Basson Figures 48 & 49
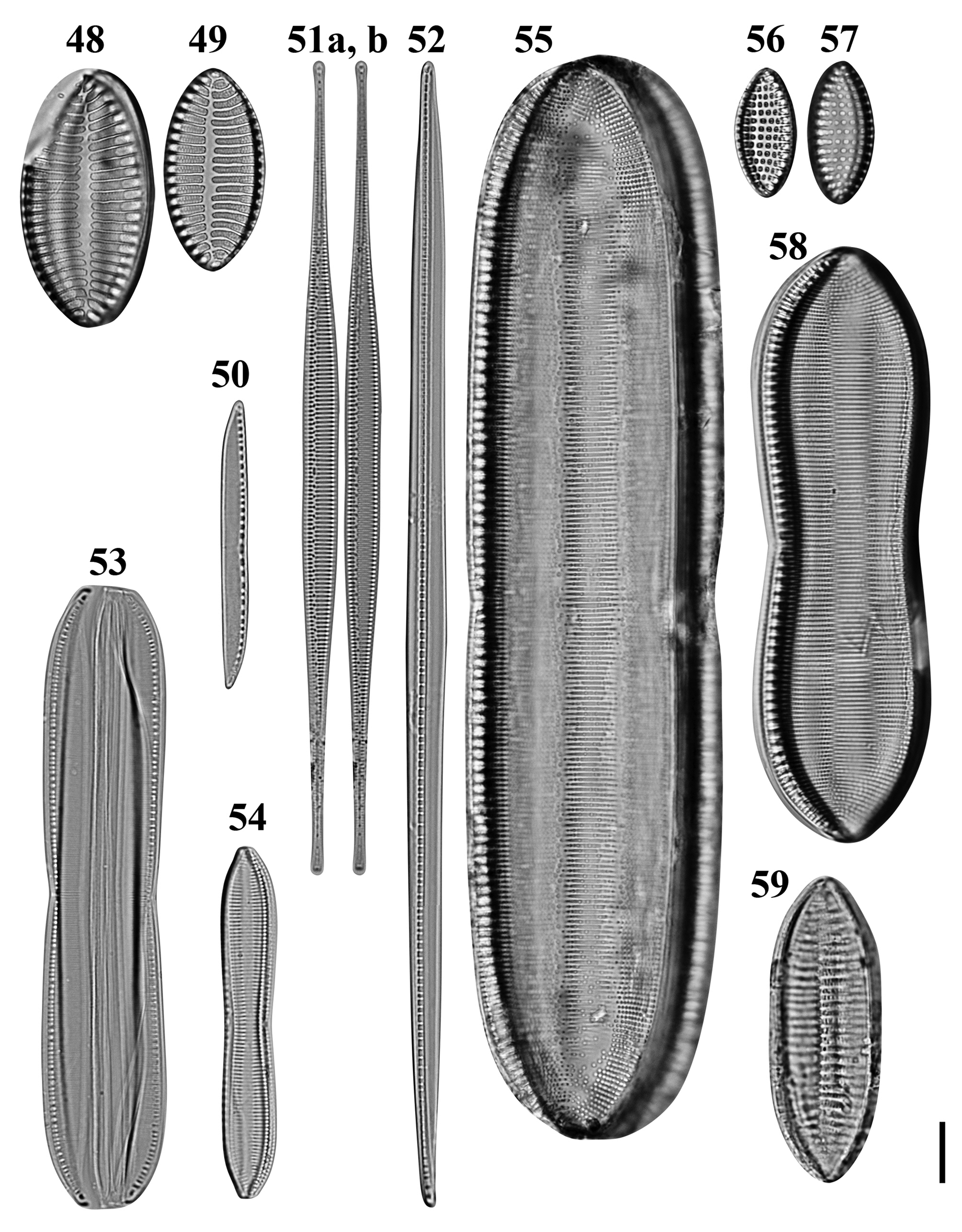
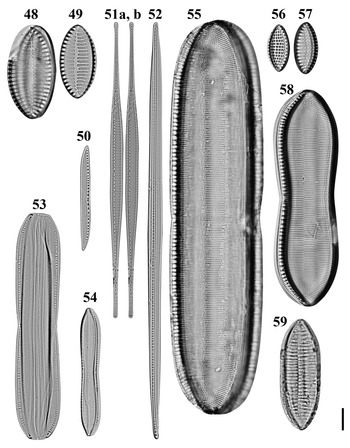
Figs 48–59. Light microscopy images of 10 taxa in Chuuk, Micronesia. Figs 48, 49. Giffenia cocconeiformis from Weno. Fig. 50. Nitzschia cf. clausii from Weno. Fig. 51a, b. Nitzschia lorenziana from Weno. Fig. 52. Nitzschia cf. electrae from Weno. Fig. 53. Nitzschia cf. hybrida from Weno. Fig. 54. Tryblionella apiculata from Weno. Fig. 55. Tryblionella graeffii from Weno. Figs 56, 57. Tryblionella granulata from Weno. Fig. 58. Tryblionella cf. jelineckii from Weno. Fig. 59. Tryblionella cf. levidensis from Weno. Scale bar = 10 μm.
References: Giffen (Reference Giffen1963): 244; Foged (Reference Foged1975): 45, pl. 29, figure 6; Witkowski et al. (Reference Witkowski, Lange-Bertalot and Metzeltin2000): 374, figures pl. 188, figures 8 & 9.
Sample: Moen_03 #001.
Dimensions: 33.1–42.1 μm long, 16.5–21.5 μm wide; 7 striae in 10 μm; 21–22 areolae in a stria.
Diagnostics: Valves elliptical with cuneate apices. Transapical striae biseriate, composed of rows of circular areolae, parallel, at apices radiate and interrupted by hyaline fold.
Comments: This is a first record from Micronesian waters.
Nitzschia lorenziana Grunow Figures 51a, b, 154
Reference: Navarro (Reference Navarro1982a, Reference Navarro1982b): 54, pl. 35, figure 4; Poulin et al. (Reference Poulin, Bérard-Therriault, Cardinal and Hamilton1990): 87, figures 63, 64, 67, 68, 71, 72; Witkowski et al. (Reference Witkowski, Lange-Bertalot and Metzeltin2000): 392, pl. 211, figure 3.
Sample: Moen_04 #001, CHB1-1 st1263.
Dimensions: 55.4–133.0 μm long; 4.4–5.7 μm wide; 15 striae in 10 μm.
Diagnostics: Valves linear, abruptly tapered into long rostrate apices. Raphe eccentric. Striae coarse and separated by externally solid equidistant costae.
Comments: This is a brackish-water species and has been observed from the Atlantic Ocean for example the Indian River (Florida, Navarro, Reference Navarro1982a, Reference Navarro1982b), Quebec (Canada, Poulin et al., Reference Poulin, Bérard-Therriault, Cardinal and Hamilton1990). This is a first report from Micronesian waters, North-western Pacific.
Nitzschia maiae Lobban, Ashworth, Calaor & E.C. Theriot Figure 148
References: Park et al. (Reference Park, Lobban and Lee2018): 133, figure 155 (as Nitzschia sp. 5); Lobban et al. (Reference Lobban, Ashworth, Calaor and Theriot2019): 208, figures 13, 26–34.
Samples: CHB1-1 st1263; CHP3-2 st1265.
Dimensions: 24.7–38.72 μm long, 3.8–5.0 μm wide; 41–42 transapical striae in 10 μm.
Diagnosis: Valves lanceolate with rostrate apices. Transapical striae parallel. Raphe slightly eccentric. Conopea present only at apices.
Comments: Park et al. (Reference Park, Lobban and Lee2018) reported this species with an unidentified status as Nitzschia sp. 5.
Nitzschia rectilonga Takano Figures 149–151
References: Takano (Reference Takano1983): 18, figures 4, 24–30, 31; Shorenko et al. (Reference Shorenko, Davidovich, Kulikovskiy and Davidovich2016): 23, figures 2a–c, 3a–c, 5, 6.
Samples: Moen_02 #001, CHP-2 st1285, CHP-3 st1286.
Dimensions: 264.4 μm long, 8.3 μm wide; 37 striae in 10 μm, 8 fibulae in 10 μm.
Diagnostics: Valves linear-lanceolate usually straight, abruptly narrowed into very long rostrate ends. Raphe strongly eccentric, central nodule present, transapical striae in LM not resolvable.
Comments: This species is similar to N. longissima, but N. rectilonga differs in the presence of the hyaline field, a labiate cylinder on the margin of fibulae and with respect to the size of the projection of the raphe central nodule on the inside (Shorenko et al., Reference Shorenko, Davidovich, Kulikovskiy and Davidovich2016). This is a first record from Micronesian waters.
Nitzschia cf. amabilis Suzuki Figures 152 & 153
Synonym: Nitzschia laevis Hustedt 1939: 662, figures 116–118.
References: Suzuki et al. (Reference Suzuki, Hanai, Nagumo and Tanaka2009): 274, figures 1–23; Rivera & Cruces (Reference Rivera and Cruces2011): 96, figure 1A–K; Simonsen (Reference Simonsen1987b), pl. 385, figures 10–18 (as N. laevis); Witkowski et al. (Reference Witkowski, Lange-Bertalot and Metzeltin2000): 387, pl. 188, figures 13–15, pl. 190, figures 1–6 (as N. laevis).
Samples: CHP3-2 st1265.
Dimensions: 12.6–13.5 μm long; 4.8 μm wide; 51–53 striae in 10 μm.
Diagnostics: Cells small, delicate. Valves broadly linear-elliptical with blunt, rounded and slightly protruding ends. Sometimes middle part constricted.
Comments: Park et al. (Reference Park, Lobban and Lee2018) reported this taxon with an unidentified status as Nitzschia sp. 11. The valve outline of our material is close to N. amabilis, but a special structure on the central nodule of N. amabilis and stria density is finer than previous reports: ~40 by Witkowski et al. (Reference Witkowski, Lange-Bertalot and Metzeltin2000); 32–34 in 10 μm by Rivera & Cruces (Reference Rivera and Cruces2011).
Nitzschia cf. clausii Hantzsch Figures 50, 146, 147
Reference: Witkowski et al. (Reference Witkowski, Lange-Bertalot and Metzeltin2000): 373, pl. 199, figures 8–10, pl. 200, figures 1 & 2.
Sample: Moen_01 #001.
Dimensions: 47.1 μm long, 4.3 μm wide; striae 38 in 10 μm, 8 fibulae in 10 μm.
Diagnostics: Valves linear, slightly concave in the middle with sigmoid short rostrate apices. Striae fine, invisible in LM.
Nitzschia cf. electrae Lobban, Ashworth, Calaor & E.C.Theriot Figure 52
Reference: Lobban et al. (Reference Lobban, Ashworth, Calaor and Theriot2019): 228, figures 22, 130–140.
Sample: Moen_04 #001.
Dimensions: 187.4 μm long, 6.4 μm wide; 8 fibulae in 10 μm.
Diagnostics: Valve narrowly lanceolate with acute apices. Transapical striae invisible. Identity uncertain without SEM.
Nitzschia cf. hybrida Grunow Figure 53
References: Poulin & Cardinal (Reference Poulin and Cardinal1983): 113, figure 12; Witkowski et al. (Reference Witkowski, Lange-Bertalot and Metzeltin2000): 385, pl. 191, figures 12–14; Al-Handal et al. (Reference Al-Handal, Abdulla, Wulff and Abdulwahab2014), figure 92; Al-Handal et al. (Reference Al-Handal, Compère and Riaux-Gobin2016): 35, pl. 10, figure 10.
Sample: Moen_01 #001.
Dimensions: 101.4 μm long, 19.8 μm wide; 27 striae in 10 μm; 13 fibulae in 10 μm.
Diagnostics: Frustule rectangular, slightly constricted in the middle with acutely rounded apices.
Tryblionella apiculata W. Gregory Figure 54
References: Schmidt et al. (Reference Schmidt, Schmidt, Frike, Heiden, Müller and Hustedt1874–1959), pl. 331, figures 14 & 15, Giffen (Reference Giffen1963): 243; Hendey (Reference Hendey1964): 279; Lange-Bertalot & Krammer (Reference Lange-Bertalot and Krammer1987): 8, 13.
Sample: Moen_01 #001.
Dimensions: 57.4 μm long, 9.1 μm wide; 16 striae in 10 μm; 10 fibulae in 10 μm.
Diagnostics: Valve constricted in the middle part with subcapitate apices. Striae parallel.
Comments: The apparent hyaline area present is a focus artefact of the longitudinal undulation, in contrast to T. acuminata W. Smith, which has a longitudinal hyaline stripe (Witkowski et al., Reference Witkowski, Lange-Bertalot and Metzeltin2000: 366, pl. 188, figures 1–3). This is a first record from Micronesian waters.
Tryblionella graeffii (Grunow ex Cleve) D.G. Mann Figure 55
Reference: Witkowski et al. (Reference Witkowski, Lange-Bertalot and Metzeltin2000): 383, pl. 192, figure 1.
Sample: Moen_03 #001.
Dimensions: 176.6 μm long, 37.6 μm wide; 12 striae in 10 μm.
Diagnostics: Valve linear, robust. Striae packed throughout the valve and loose in the valve ends.
Comments: This is a first record from Micronesian waters.
Tryblionella granulata (Grunow) D.G. Mann Figures 56 & 57
Reference: Navarro & Lobban (Reference Navarro and Lobban2009): 145.
Sample: Moen_01 #001, Moen_02 #001.
Dimensions: 21.1–23.3 μm long, 10.0–10.8 μm wide; 7 striae in 10 μm; 9 areolae in 10 μm; 8 fibulae in 10 μm.
Tryblionella jelineckii (Grunow) D.G. Mann Figure 58
Reference: Peragallo & Peragallo (Reference Peragallo and Peragallo1897–1908): 268, pl. 69, figure 19.
Sample: Moen_04 #001.
Dimensions: 97.5 μm long, 28.5 μm wide; 13 striae in 10 μm; 8–9 fibulae in 10 μm.
Diagnostics: Valve elliptical in the constricted middle with obtusely rounded apices.
Comments: The fibula density here is slightly higher than given by Peragallo and Peragallo (Reference Peragallo and Peragallo1897–1908).
Comments: This is a first record from Micronesian waters.
Tryblionella scalaris (Ehrenberg) Siver & P.B. Hamilton Figures 155–157
References: Witkowski et al. (Reference Witkowski, Lange-Bertalot and Metzeltin2000): 404, pl. 205, figures 1–4; Ruck & Kociolek (Reference Ruck and Kociolek2004): 55, pl. 62, figure 1–5, pl. 63–65, figures 6–24; Siver & Hamilton (Reference Siver and Hamilton2005): 374, figures 67–80; Al-Handal et al. (Reference Al-Handal, Abdulla, Wulff and Abdulwahab2014), figure 94.
Samples: CHB1-1 st1292, CHB1-1 st1262, CHP-2 st1285.
Dimensions: 581.2 μm long; 21.6 μm wide; 10 transapical striae in 10 μm.
Diagnostics: Very long, robust valve, linear with obtusely rounded apices.
Comments: This is a first record from Micronesian waters.
Tryblionella cf. levidensis W. Smith Figure 59
References: Giffen (Reference Giffen1963): 246; Hendey (Reference Hendey1964): 277; Lange-Bertalot & Krammer (Reference Lange-Bertalot and Krammer1987): 32; Krammer & Lange-Bertalot (Reference Krammer, Lange-Bertalot, Ettl, Gerloff, Heynig and Mollenhauer1997): 37; Al-Handal et al. (Reference Al-Handal, Abdulla, Wulff and Abdulwahab2014), figure 106.
Sample: Moen_03 #001.
Dimensions: 49.7 μm long, 18.1 μm wide; 8 transapical ribs in 10 μm.
Diagnostics: Valve linear-elliptic with cuneate, obtusely rounded apices. Transapical striae in LM barely discernible and obscured by coarse transapical ribs in the middle interrupted by a longitudinal fold.
Order RHOPALOIDALES D.G. Mann, 1990
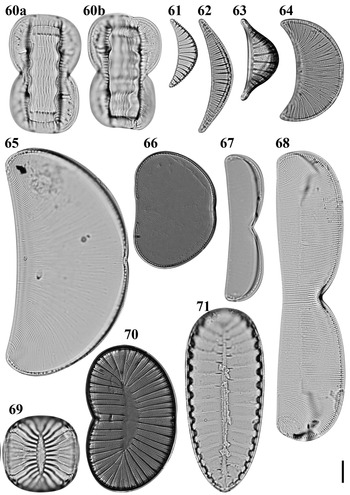
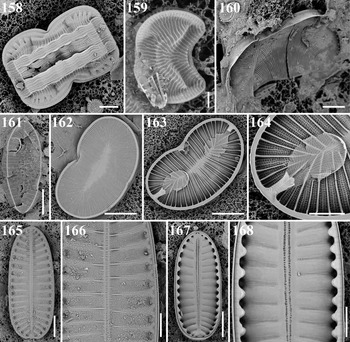
Entomoneis corrugata (Giffen) Witkowski et al., Reference Witkowski, Lange-Bertalot and Metzeltin2000 Figures 60a, b, 158

Figs 60–71. Light microscopy images of six taxa in Chuuk, Micronesia. Fig. 60a, b. Entomoneis corrugata from Weno. Figs 61, 62. Epithemia gibberula var. baltica from Weno. Fig. 63. Epithemia gibberula var. tumida from Weno. Fig. 64. Epithemia globosa from Weno. Fig 65. Auricula complexa from Weno. Fig. 66. Auricula flabelliformis from Weno. Fig. 67. Auricula intermedia from Weno. Fig. 68. Auricula sp. from Weno. Fig. 69. Campylodiscus cf. biangulatus from Weno. Fig. 70. Plagiodiscus martensianus from Moch. Fig. 71. Surirella oblongoelliptica from Weno. Scale bar = 10 μm.
Reference: Navarro & Lobban (Reference Navarro and Lobban2009): 147, figures 108–111.
Samples: Moen_01 #001, CHP-3 st1286.
Dimensions: 50.7–53.5 μm long, 31.1–33.0 μm wide; 16–17 striae in 10 μm.
Diagnostics: Valve with crenulated margin.
Comments: The Chuuk specimen has a smaller valve and finer striae than other observations (Giffen, Reference Giffen1963, Reference Giffen1967; Navarro & Lobban, Reference Navarro and Lobban2009).

Figs 72–79. Scanning electron microscopy images of six taxa in Chuuk, Micronesia. Fig. 72. Podosira hormoides from Weno. Fig. 73. Actinocyclus subtilis from Weno. Figs 74, 75. Biddulphopsis membranacea from Moch, whole valve and detail showing broad central area and radiating striae. Fig. 76. Biddulphopsis titiana from Moch, interior. Figs 77, 78. Isthmia minima from Moch, valve with copulae and detail of valve, both showing the mesh invaginations (arrowheads). Fig. 79. Trigonium formosum f. quadrangularis from Moch, exterior. Scale bars: 50 μm (Fig. 74), 20 μm (77–79), 10 μm (Figs 72, 73, 75, 76).

Figs 80–88. Scanning electron microscopy images of eight taxa in Chuuk, Micronesia. Fig. 80. Climacosphenia moniligera from Moch. Fig. 81. Bacteriastrum furcatum from Moch. Fig. 82. Chaetoceros cf. atlanticus var. skeleton from Moch. Fig. 83. Odontella aurita from Weno. Figs 84, 85. Thalassiosira cedarkeyensis from Weno. Fig. 86. Thalassiosira cf. catharinensis from Weno. Fig. 87. Koernerella recticostata from Moch (apex missing). Fig. 88. Grammatophora marina from Weno, oblique advalvar view of valvocopula with septum and valve. Scale bars: 100 μm (Figure 80), 10 μm (Figs 81, 82, 87, 88), 5 μm (Fig. 83), 1 μm (Figs 84–86).

Figs 89–98. Scanning electron microscopy images of seven taxa in Chuuk, Micronesia. Fig. 89. Microtabella interrupta from Weno, interior valve and copula with septum. Fig. 90. Licmophora curvata from Moch, interior valve and valvocopula. Figs 91–93. Licmophora remulus from Moch, showing valve, details of spathulate apex and basal region with rimoportula and multiscissura. Fig. 94. Synedra bacillaris from Moch. Fig. 95. Hyalosynedra al-shareefii from Weno. Figs 96, 97. Hyalosynedra cf. sublaevigata from Weno. Fig. 98. Gato hyalinus from Moch, two frustules in mucilage tube. Scale bars: 20 μm (Figs 91, 99), 10 μm (Figs 90, 92, 94, 96–98), 5 μm (Figs 89, 93, 95).

Figs 99–109. Scanning electron microscopy images of eight taxa in Chuuk, Micronesia. Figs 99–101. Craspedostauros cf. neoconstrictus from Weno, whole valve interior and details of apical and central portions. Fig. 102. Mastogloia corsicana from Moch. Fig. 103. Mastogloia exilis from Weno. Fig. 104. Mastogloia hustedtii from Weno. Fig. 105. Mastogloia mauritiana from Weno. Figs 106, 107. Tetramphora decussata from Moch, exterior and interior views, respectively. Fig. 108. Anorthoneis eurystoma from Weno, sternum valve. Fig. 109. Cocconeis coronatoides from Weno, sternum valve. Scale bars: 10 μm (Figs 99, 105–107), 5 μm (Figs 102–104, 108, 109), 2 μm (Figures 100, 101).

Figs 110–121. Scanning electron microscopy images of four taxa in Chuuk, Micronesia. Figs 110–112. Climaconeis cf. petersonii from Weno. Figs 113–115. Climaconeis riddleae from Weno. Figs 116–118. Climaconeis scalaris from Weno. Figs 119–121. Parlibellus biblos from Weno. Scale bars: 50 μm (Fig. 110), 20 μm (Figs 113 & 116), 10 μm (Figs 111, 112, 114, 115, 119, 120), 5 μm (Figs 117, 118, 121).

Figs 122–133. Scanning electron microscopy images of eight taxa in Chuuk, Micronesia. Fig. 122. Diadesmis confervacea from Weno. Fig. 123. Caloneis egena from Moch. Figs 124, 125. Caloneis macquariensis from Weno, frustule in girdle view. Figs 126, 127. Oestrupia sp. 4 sensu Hein et al., from Weno. Figs 128, 129. Diploneis cerebrum from Weno. Fig. 130. Navicula gregaria from Weno. Fig. 131. Navicula mannii from Weno. Figs 132, 133. Navicula tsukamotoi from Weno. Scale bars: 10 μm (Figs 124, 126, 128, 132), 5 μm (Figs 122, 125, 127, 129–131, 133), 2 μm (Figure 123).

Figs 134–143. Scanning electron microscopy images of seven taxa in Chuuk, Micronesia. Fig. 135. Pleurosigma cf. elongatum from Weno. Figs 136, 137. Rhoicosigma compactum from Weno. Fig. 138. Amphora biggiba from Weno. Fig. 139. Amphora lunulata from Weno. Fig. 140. Amphora ostrearia var. vitrea from Weno. Fig. 141. Amphora cf. abuldens from Weno. Fig. 142. Amphora cf. egregia from Weno. Fig. 143. Amphora cf. helenesis from Weno. Scale bars: 20 μm (Figs 134, 136, 140, 142), 10 μm (Fig. 141), 5 μm (Figs 135, 137, 139), 2 μm (Fig. 138), 1 μm (Fig. 143).

Figs 144–157. Scanning electron microscopy images of seven taxa in Chuuk, Micronesia. Figs 144, 145. Bacillaria Group B sensu Schmid var. tumidula from Weno, valve internal view and detail of central portion exterior showing silica flap. Figs 146, 147. Nitzschia cf. clausii from Weno. Fig. 148. Nitzschia maiae from Weno. Figs 149–151. Nitzschia rectilonga from Weno, entire valve, external central area, and internal central area showing the raphe central nodule (arrow), and hyaline field in the internal valve (arrowheads). Figs 152, 153. Nitzschia cf. amabilis from Weno. Fig. 154. Nitzschia lorenziana from Weno. Figs 155–157. Tryblionella scalaris from Weno. Scale bars: 100 μm (Figs 149, 155), 20 μm (Fig. 144), 10 μm (Figs 146, 152, 154, 156, 157), 5 μm (Figs 145, 147, 148, 150, 151, 153).

Figs 158–168. Scanning electron microscopy images of six taxa in Chuuk, Micronesia. Fig. 158. Entomoneis corrugata from Weno. Fig. 159. Protokeelia cholnokyi from Weno. Fig. 160. Auricula complexa from Weno. Fig. 161. Petrodictyon patrimonii from Weno. Figs 162–164. Plagiodiscus martensianus from Moch, external valve, internal valve, and detail of the palmulae. Figs 165–168. Surirella oblongoelliptica from Weno. Scale bars: 20 μm (Figs 161–163, 165, 167), 10 μm (Figs 158, 160, 164, 166, 168), 5 μm (Fig. 162), 2 μm (Fig. 159).
Epithemia gibberula var. baltica (O. Müller) J.S. Park & Lobban, comb. nov. Figures 61 & 62
Reference: Schmidt et al. (Reference Schmidt, Schmidt, Frike, Heiden, Müller and Hustedt1874–1959), pl. 253, figures 33–37.
Samples: Moen_01 #001, Moen_02 #001, Moen_03 #001.
Dimensions: 30.6–50.8 μm long, 8.7–9.7 μm wide.
Diagnostics: Valve lunate, strongly convex dorsal margin and moderately concave ventral margin with acutely rounded apices.
Comments: We propose a new combination name for Rhopalodia gibberula var. baltica following Ruck et al. (Reference Ruck, Nakov, Alverson and Theriot2016). The formal nomenclatural changes are made below. This is a first record from Micronesian waters.
Epithemia gibberula var. tumida (Zanon) J.S. Park & Lobban, comb. nov. Figure 63
Reference: Zanon (Reference Zanon1941): 536, pl. 4, figures 10 & 11.
Sample: Moen_03 #001.
Dimensions: 34.5 μm long, 14.1 μm wide.
Diagnostics: Valve strongly convex dorsal margin and straight ventral margin with rounded rostrate apices. Striae multiseriate.
Comments: We propose a new combination name for Rhopalodia gibberula var. tumida following Ruck et al. (Reference Ruck, Nakov, Alverson and Theriot2016). This is a first record from Micronesian waters.
Epithemia globosa (Hustedt) J.S. Park & Lobban, comb. nov. Figure 64
References: Simonsen (Reference Simonsen1987b): 240, pl. 350, figures 6 & 7; Moser et al. (Reference Moser, Lange-Bertalot and Metzeltin1998): 215, pl. 67, figures 1, 2, 6.
Sample: Moen_03 #001.
Dimensions: 46.0 μm long, 21.3 μm wide; 17 striae in 10 μm; 19 areolae in 10 μm in a stria.
Diagnostics: Valve dorsiventral, orange-segment shaped. Striae multiseriate, flabellate with transapical costae. Raphe eccentric, running along the dorsal margin.
Comments: We propose a new combination name for Rhopalodia globosa following Ruck et al. (Reference Ruck, Nakov, Alverson and Theriot2016). This is a first record from Micronesian waters.
Protokeelia cholnokyi (M.H. Giffen) Round & Basson Figure 159
Basionym: Auricula cholnokyi M.H. Giffen
References: Round & Basson (Reference Round and Basson1995): 213, figures 2–9; Lobban (Reference Lobban2015a): 12, figures 114–117; Giffen (Reference Giffen1963): 220, pl. II, figures 27–29 (as A. cholnokyi).
Sample: CHB1-1 st1263.
Dimensions: 11.8 μm long, 7.0 μm wide; 23 striae in 10 μm.
Order SURIRELLALES D.G. Mann, 1990
Auricula complexa (Gregory) Cleve Figures 65 & 160
References: Navarro & Lobban (Reference Navarro and Lobban2009): 147, figures 112–114; Park et al. (Reference Park, Lee, Kang and Lee2014): 238, figure 3a.
Sample: Moen_03 #001.
Dimensions: 68.9–101.7 μm long, 38.6–49.8 μm wide; 15–16 striae in 10 μm.
Diagnostics: Frustule semi-elliptical with a slightly concave ventral and a fairly convex dorsal margin in girdle view. Striae flabellate, radiating from ventral margin to dorsal raphe canal. Costae frequently bifurcated.
Comments: Lobban et al. (Reference Lobban, Schefter, Jordan, Arai, Sasaki, Theriot, Ashworth, Ruck and Pennesi2012) reported A. complexa from Guam, but the valve size and striae density match Auricula densestriata K. Osada (Osada, Reference Osada1997).
Auricula flabelliformis Voigt Figure 66
Reference: Lobban (Reference Lobban2015a): 3, figures 10–12.
Sample: Moen_03 #001.
Dimensions: 58.2 μm long; 18 striae in 10 μm.
Diagnostics: Frustule reniform. Striae flabellate, radiating from centre of the ventral margin to dorsal raphe canal. Costae frequently bifurcated.
Auricula intermedia (F.W. Lewis) Cleve Figure 67
Reference: Lobban et al. (Reference Lobban, Schefter, Jordan, Arai, Sasaki, Theriot, Ashworth, Ruck and Pennesi2012): 304, pl. 63, figures 3 & 4.
Samples: Moen_05 #001.
Dimensions: 65.7 μm long, 16.3 μm wide; 21 striae in 10 μm.
Diagnostics: Frustule semi-elliptical with a slightly concave ventral and a round dorsal margin that constricted in the middle part in girdle view. Striae very fine and parallel in the middle part and radiating from ventral margin to dorsal raphe canal in the valve ends.
Comments: The Chuuk specimen of A. intermedia has finer striae than the other specimens from Guam (16–18 in 10 μm (Lobban et al., Reference Lobban, Schefter, Jordan, Arai, Sasaki, Theriot, Ashworth, Ruck and Pennesi2012) and off Basra, Iraq (16–17 in 10 μm (Al-Handal et al., Reference Al-Handal, Thomas and Pennesi2018)).
Auricula sp. Figure 68
Sample: Moen_03 #001.
Dimensions: 130.5 μm long, 30.3 μm wide; 13 striae in 10 μm.
Diagnostics: Frustule semi-elliptical with a slightly undulate ventral and a round dorsal margin that constricted in the middle part in girdle view. Striae coarse and parallel in the middle part and radiating from ventral margin to dorsal raphe canal in the valve ends. Costae bifurcated in the radiating striae area.
Comments: This species resembles A. intermedia at a first glance, but the valve size is larger and striae density sparser than A. intermedia.
Campylodiscus cf. biangulatus Greville Figure 69
References: Greville (Reference Greville1862): 20, pl. III, figure 2; Schmidt et al. (Reference Schmidt, Schmidt, Frike, Heiden, Müller and Hustedt1874–1959), pl. 14, figures 18–22, pl. 208, figures 8?, 9, 15; Williams (Reference Williams1988): 21, pl. 26, figures 3 & 4.
Sample: Moen_03 #002.
Dimensions: 264.4 μm long, 8.3 μm wide; 8 fibulae in 10 μm.
Diagnostics: Costae curved in the half of valve.
Petrodictyon patrimonii (F.A.S. Sterrenburg) F.A.S. Sterrenburg Figure 161
Reference: Lobban et al. (Reference Lobban, Schefter, Jordan, Arai, Sasaki, Theriot, Ashworth, Ruck and Pennesi2012): 306, pl. 66, figures 2 & 3.
Sample: CHB1-1 st1262.
Dimensions: 71.9 μm long, 26.1 μm wide.
Diagnostics: Valve oval with longitudinally straight/zigzag and lateral, slightly radiating costae.
Plagiodiscus martensianus Grunow & Eulensetin Figures 70, 162–164
References: Ruck & Kociolek (Reference Ruck and Kociolek2004): 44, pl. 51, figures 1–4; Lobban et al. (Reference Lobban, Schefter, Jordan, Arai, Sasaki, Theriot, Ashworth, Ruck and Pennesi2012): 307, pl. 61, figure 1.
Sample: TK28.
Dimensions: 70 μm long, 40 μm wide; striae 14 in 10 μm. Costae every 3–5 striae.
Diagnostics: Striae appearing continuously triseriate externally, but internally with a reticulate pattern of both virgae and vimines.
Comments: Our images of the flat sacs (palmulae) show the poration more clearly than in Paddock's images (Paddock, Reference Paddock1978). He describes the palmula thus: ‘The stem is hollow … and the blade is composed of two laminar layers, though so far it is not known whether the envelope formed by the lamina[e] is sealed.’ In the captions to his figures 5B and 5D (reproduced in (Round et al., Reference Round, Crawford and Mann1990: 641, figure h, i), he describes the stem and blade as ‘finely marked’, but here we can see more pattern in the poration and we can conclude that the palmula is a sac, not two separate laminae.
Surirella oblongoelliptica Hustedt ex Simonsen Figures 71, 165–168
References: Schmidt et al. (Reference Schmidt, Schmidt, Frike, Heiden, Müller and Hustedt1874–1959), pl. 309, figure 6; Simonsen (Reference Simonsen1987a): 51, pl. 59, figures 1–3; Navarro (Reference Navarro1983b): 396, figures 75 & 76 (misidentified as Surirella gemma).
Samples: Moen_02 #001, CHP-2 st1285, CHP-3 st1286.
Dimensions: 80.5–82.6 μm long, 34.0–35.4 μm wide.
Diagnostics: Valves linear-oval, slightly heteropolar, circlet flattened to a single longitudinal line.
Comments: Hustedt found this species from Miang Besar, Borneo but did not describe it validly (Schmidt et al., Reference Schmidt, Schmidt, Frike, Heiden, Müller and Hustedt1874–1959). Simonsen (Reference Simonsen1987a) validly published this species and briefly described it as having a valve, linear-oval, slightly heteropolar tip broadly rounded, about 135 μm long and 47 μm wide. Navarro (Reference Navarro1983b) found this taxon from Puerto Rico, but he identified as S. gemma. This is a first record from Micronesian waters.
Nomenclatural changes
Epithemia gibberula var. baltica (O. Müller) J.S. Park & Lobban, comb. nov.
Basionym: Rhopalodia gibberula var. baltica O.Müller, Hedwigia 38: p. 287, pl. 11, figures 3 & 4, 1899.
Epithemia gibberula var. tumida (Zanon) J.S. Park & Lobban, comb. nov.
Basionym: Rhopalodia gibberula var. tumida Zanon, Atti della Reale Accademia d'Italia, Memoria della Classe di Scienze Fisiche, Matematische e Naturali 536: pl. 4, figures 10 & 11, 1941.
Epithemia globosa (Hustedt) J.S. Park & Lobban, comb. nov.
Basionym: Rhopalodia gibberula var. globosa Hustedt, Archiv für Hydrobiologie Supplement 15: 58, pl. XIV, figure 15, 1938.
Synonym: Rhopalodia globosa (Hustedt) Moser et al., Reference Moser, Lange-Bertalot and Metzeltin1998: 215, pl. 67, figures 1, 2, 6.
Campylodiscus giffenii (M.H. Giffen) Lobban & J.S. Park, nom. nov.
Basionym: Surirella scalaris M.H. Giffen, Nova Hedwigia,13: 284, figures 121–123, 1967.
Synonym: Campylodiscus scalaris (M.H. Giffen) Lobban & J.S. Park, Phytotaxa 351: 134, 2018. Illegit.
M. Guiry (personal communication, Sep. 2019) pointed out that our 2018 new combination was a homonym for C. scalaris Tempère & J.-J. Brun 1889, and we take this opportunity to correct that oversight.
Discussion
With addition of 105 diatom taxa in this study (excluding four species re-identified and as reported in Park et al., Reference Park, Lobban and Lee2018), 248 diatoms are known from Chuuk, although many taxa still remain unidentified or uncertainly identified. In comparison, Lobban (Reference Lobban2015a, Reference Lobban2015b) reported an accumulation of 271 new records in Guam. Of the 105 species in this study, 31 species are newly recorded for the Micronesian waters (Table 1). Most diatom species were benthic around Weno Island, despite the collection of the planktonic samples using a phytoplankton net. The presence of benthic diatoms from plankton samples might be related to the wind-driven shear current in shallow water. The water depth of sampling sites did not exceed 10 m. The water column in shallow water can be vertically mixed by Langmuir circulation caused by wind in shear. Langmuir supercells can suspend sediments and transport the resuspended particles offshore by inducing turbulence along the highly sheared oscillatory wave that serves to lift sediment grains from the seafloor into the water column (Gargett et al., Reference Gargett, Wells, Tejada-Martínez and Grosch2004). Langmuir circulations generally occur only for wind speeds greater than 3 m s−1 and appear within a few tens of minutes of wind onset (Talley et al., Reference Talley, Pickard, Emery, Swift, Talley, Pickard, Emery and Swift2011). Annual mean wind speed in Chuuk was 5.14 m s−1 (Ko et al., Reference Ko, Jeong and Kim2015), this is sufficient to produce Langmuir circulations and caused resuspension of benthic diatoms.
There were no euplanktonic diatoms except for Bacteriastrum furcatum and Chaetoceros cf. atlanticus var. skeleton, and both were observed from the passage of the barrier reef near open water. Coral reef environments are characterized by relatively stable temperature, low nutrients and high irradiance (Lobban & Jordan, Reference Lobban, Jordan, Stoermer and Smol2010). These environmental characteristics seem to have no advantages for planktonic diatoms compared with benthic ones. Strong light level can be a factor that inhibits the growth of phytoplankton (Moore & Villareal Reference Moore and Villareal1996), and nutrient acquisition is more favourable at the bottom than the water layer. In addition, the planktivorous zooplankton is highly abundant in coral reef systems (Nakajima et al., Reference Nakajima, Yamazaki, Lewis, Khen, Smith, Nakatomi and Kurihara2017), thus will act as a high grazing pressure for phytoplankton.
Although we collected the samples inshore, some freshwater taxa such as Gomphonema cf. lagenula, Diadesmis confervacea, Navicula gregaria, Caloneis macquariensis, Pinnularia cf. borealis and Surirella oblongoelliptica were observed. There are small streams near the sampling sites in Weno Island, and the freshwater species, observed from acid-cleaned specimens, were most likely dead. The freshwater diatoms of Micronesian islands have scarcely been studied (Zolan, Reference Zolan1981; Navarro & Lobban, Reference Navarro and Lobban2009 and unpublished observations by Lobban), but we do not expect great diversity because on these small islands the streams are short, shaded and ephemeral (Lobban et al., Reference Lobban, Daily, Daily, Hoshaw and Schefter1991).
Many new species and some new genera have been reported from Guam in the last decade and it is not surprising that some of them are turning up elsewhere in Micronesia as exploration gets underway in Chuuk, Yap, Palau and the Marshall Islands (e.g. here reported Hanicella moenia, Astrosyne radiata, Licmophora curvata and Gato hyalinus). At the same time, those islands are also revealing new species, even from the relatively small sampling effort, some not yet found in Guam despite much more extensive sampling (e.g. Divergita macinnisii Lobban and Licmophora complanata Lobban from the Marshall Islands (Lobban, Reference Lobban2021a, Reference Lobban2021b); several species from Yap (Lobban, Reference Lobban2021a)). While we have tended to assume a priori that all species are ubiquitous, an essentially untestable hypothesis, we are coming around to an assumption of regional endemicity as a starting point, recognizing that while there are many ubiquitous species, there are also different levels of endemicity both geographically and within genera (Lobban, in review).
It is tempting, in floristic studies such as this to compare species richness or species diversity of the list to date with the findings from other places but, apart from the question of the amount of effort put into different regions, the more important reason to avoid such comparisons is that the method in our floristic studies is to present micrographic documentation of each species as evidence for each hypothesis of identity. We have included some taxa here identified only to genus because of limited sampling, whereas Lobban's records papers (Lobban et al., Reference Lobban, Schefter, Jordan, Arai, Sasaki, Theriot, Ashworth, Ruck and Pennesi2012; Lobban, Reference Lobban2015a) report only taxa identified to species during the progress of a long-term study. The state of knowledge of tropical marine diatoms is still very preliminary, even in a few relatively well-studied places, and taxonomy is still in a state of flux as new studies uncover previously hidden diversity. Our studies in Guam have shown repeatedly that there are lookalike species that either cannot be distinguished in LM or that have a striking feature, thought to be unique, that leads to the similar species being overlooked. The documentation and arguments we provide for species records claims are essential for the continued utility of the work, so that findings can be reinterpreted as needed when new information is available. Lists with little or no documentation, even by very experienced authors (e.g. Giffen's (Reference Giffen1980) list for Mahé, Seychelles), become outdated. The question of regional endemicity in benthic marine diatoms has scarcely been addressed but it is becoming clear, from our work in progress based on the approach used by Williams & Kociolek (Reference Williams, Kociolek and Ebach2017), that at least some genera have strongly regional floras. Large-scale ecological studies such as those by Kryk et al. (Reference Kryk, Bąk, Gorecka, Riaux-Gobin, Bemiasa, Bemanaja, Li, Dąbek and Witkowski2020) and Risjani et al. (Reference Risjani, Witkowski, Kryk, Yunianta, Krzywda, Safitri, Sapar, Dąbek, Arsad, Gusev, Rudiyansyah and Wróbel2021) are able to make comprehensive comparisons of species diversity by including many incompletely identified species (essentially ‘operational taxonomic units’). Kryk et al. (Reference Kryk, Bąk, Gorecka, Riaux-Gobin, Bemiasa, Bemanaja, Li, Dąbek and Witkowski2020: 161), said of their results for Madagascar, ‘Amongst all 332 taxa, only 35% have been identified to the species level. Many of the taxa identified to the generic level may turn out to represent taxa new to science after further SEM examination and DNA sequencing.’ The ratio is probably still lower for the studies in Micronesia but we have not yet tried to distinguish and tally the unidentified species.
There remains much work to be done on the Chuuk flora, both with the relatively few samples collected already and the need for more systematic collections. The marine flora to date continues to show strong similarities to the much better-known flora of Guam while also revealing species not yet reported from there, and for all Micronesian islands there are still few mangrove and sediment biofilm samples.
Data
The diatom samples were deposited in the Library of Marine Samples, Korea Institute of Ocean Science & Technology, Republic of Korea and Guam Diatom Herbarium, USA.
Acknowledgements
We would like to thank Dr Choi, Y. U. for supporting the sampling at the Korea South Pacific Ocean Research Center in Weno Island, Chuuk.
Authors’ contributions
JSP, KWL and CSL collected the samples. JSP and CSL analysed and observed the samples. JSP and CSL wrote the manuscript. KWL and SWJ arranged the figures and gave constructive comments for the manuscript. All authors read and approved the final manuscript.
Financial support
This work was supported by the KIOST projects ‘The development of the technologies for the production, application, and evaluation of small molecules from marine organisms’ (PEA0021), ‘Exploration of new marine biological/genetic resources and rare metal resources in the Area beyond national jurisdiction of the West Pacific’ (PEA0024), and by the Ministry of Science & ICT (NRF-2017M3A9E4072753).