Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Whitfield, M.
1975.
An improved specific interaction model for seawater at 25°C and 1 atmosphere total pressure.
Marine Chemistry,
Vol. 3,
Issue. 3,
p.
197.
Leyendekkers, J.V.
1975.
The effect of pressure on the chemical potentials of seawater components, on ionic species in seawater, and on calcite saturation levels.
Marine Chemistry,
Vol. 3,
Issue. 1,
p.
23.
Duedall, Iver W.
Bowman, Malcolm J.
and
O'connors, Harold B.
1975.
Sewage sludge and ammonium concentrations in the New York Bight apex.
Estuarine and Coastal Marine Science,
Vol. 3,
Issue. 4,
p.
457.
Sedgwick, Roy
and
Arthur, D.R.
1976.
A natural pollution experiment: The effects of a sewage strike on the fauna of the Thames estuary.
Environmental Pollution (1970),
Vol. 11,
Issue. 2,
p.
137.
Hampson, B.L.
1976.
Ammonia concentration in relation to ammonia toxicity during a rainbow trout rearing experiment in a closed freshwater-seawater system.
Aquaculture,
Vol. 9,
Issue. ,
p.
61.
Wickins, J.F.
1976.
The tolerance of warm-water prawns to recirculated water.
Aquaculture,
Vol. 9,
Issue. ,
p.
19.
Duedall, I.W.
O'Connors, H.B.
Parker, J.H.
Wilson, R.E.
and
Robbins, A.S.
1977.
The abundances, distribution and flux of nutrients and chlorophyll a in the New York Bight apex.
Estuarine and Coastal Marine Science,
Vol. 5,
Issue. 1,
p.
81.
Millero, Frank J
1977.
The use of the specific interaction model to estimate the partial molal volumes of electrolytes in seawater.
Geochimica et Cosmochimica Acta,
Vol. 41,
Issue. 2,
p.
215.
Khoo, K. H.
Culberson, Charles H.
and
Bates, Roger G.
1977.
Thermodynamics of the dissociation of ammonium ion in seawater from 5 to 40�C.
Journal of Solution Chemistry,
Vol. 6,
Issue. 4,
p.
281.
Whitfield, M.
1978.
The hydrolysis of ammonium ions in sea water-experimental confirmation of predicted constants at one atmosphere pressure.
Journal of the Marine Biological Association of the United Kingdom,
Vol. 58,
Issue. 3,
p.
781.
WINKLER, MATTHEW M.
and
GRAINGER, JAMES L.
1978.
Mechanism of action of NH4Cl and other weak bases in the activation of sea urchin eggs.
Nature,
Vol. 273,
Issue. 5663,
p.
536.
Alderson, R.
1979.
The effect of ammonia on the growth of juvenile Dover sole, Solea solea (L.) and turbot, Scophthalmus maximus (L.).
Aquaculture,
Vol. 17,
Issue. 4,
p.
291.
Alabaster, J. S.
Shurben, D. G.
and
Knowles, G.
1979.
The effect of dissolved oxygen and salinity on the toxicity of ammonia to smolts of salmon, Salmo salar L..
Journal of Fish Biology,
Vol. 15,
Issue. 6,
p.
705.
Brownell, Charles L.
1980.
Water quality requirements for first-feeding in marine fish larvae. I. Ammonia, nitrite, and nitrate.
Journal of Experimental Marine Biology and Ecology,
Vol. 44,
Issue. 2,
p.
269.
Johansson, Olof
and
Wedborg, Margareta
1980.
The ammonia-ammonium equilibrium in seawater at temperatures between 5 and 25�C.
Journal of Solution Chemistry,
Vol. 9,
Issue. 1,
p.
37.
Millero, Frank J
1981.
The ionization of acids in estuarine waters.
Geochimica et Cosmochimica Acta,
Vol. 45,
Issue. 11,
p.
2085.
Dickson, A.G.
and
Whitfield, M.
1981.
An ion-association model for estimating acidity constants (at 25°C and 1 ATM total pressure) in electrolyte mixtures related to seawater (ionic strength < 1 mol kg−1 H2O).
Marine Chemistry,
Vol. 10,
Issue. 4,
p.
315.
PULLIN, R.S.V.
and
KUO, C.-M.
1981.
Advances in Food-producing Systems for Arid and Semiarid Lands.
p.
899.
Poxton, M.G.
and
Allouse, S.B.
1982.
Water quality criteria for marine fisheries.
Aquacultural Engineering,
Vol. 1,
Issue. 3,
p.
153.
Turner, David T.
and
Bower, Carol E.
1982.
Removal of ammonia by bacteriological nitrification during the simulated transport of marine fishes.
Aquaculture,
Vol. 29,
Issue. 3-4,
p.
347.
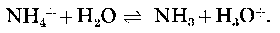 The toxicity of ammonium salts to freshwater life has been shown to be strongly dependent on the pH in a manner that is consistent with un-ionized ammonia (NH3) being the most lethal fraction (see, for example, Wuhrmann & Woker, 1948; Downing & Merkens, 1955; Lloyd & Herbert, i960; Hemens, 1966; and Brown, 1968). These papers and many others have been thoroughly reviewed (EIFAC Technical Paper no. 11, 1970, Kemp, Abrams & Overbeck, 1971). The free base (NH3) has a relatively high lipid solubility because it carries no charge and is therefore able to diffuse quite readily across cell membranes (Fromm & Gillette, 1968).
The toxicity of ammonium salts to freshwater life has been shown to be strongly dependent on the pH in a manner that is consistent with un-ionized ammonia (NH3) being the most lethal fraction (see, for example, Wuhrmann & Woker, 1948; Downing & Merkens, 1955; Lloyd & Herbert, i960; Hemens, 1966; and Brown, 1968). These papers and many others have been thoroughly reviewed (EIFAC Technical Paper no. 11, 1970, Kemp, Abrams & Overbeck, 1971). The free base (NH3) has a relatively high lipid solubility because it carries no charge and is therefore able to diffuse quite readily across cell membranes (Fromm & Gillette, 1968).