INTRODUCTION
Studies comparing White non-Hispanics (WNHs) to ethnic minorities on tests of cognitive performance among individuals with dementia have consistently shown lower scores in memory, executive function, and overall cognitive performance among Hispanics, even when controlling for factors such as age, sex, education, and socioeconomic status (Boone, Victor, Wen, Razani, & Pontón, Reference Boone, Victor, Wen, Razani and Pontón2007; Castora-Binkley, Peronto, Edwards, & Small, Reference Castora-Binkley, Peronto, Edwards and Small2015; Espino & Lewis, Reference Espino and Lewis1998; Espino, Lichtenstein, Palmer, & Hazuda, Reference Espino, Lichtenstein, Palmer and Hazuda2004; Gasquoine, Reference Gasquoine1999; Guerrero-Berroa et al., Reference Guerrero-Berroa, Kluger, Schmeidler, Sailor, Lizardi, Golomb and Reisberg2014, Reference Guerrero-Berroa, Schmeidler, Raventos, Valerio, Berri, Carrion-Baralt and Sano2016). Among the elderly, one explanation for these differences in cognitive performance could be the greater reported prevalence of neurodegenerative disease, such as Alzheimer’s disease (AD). Among Medicare recipients over the age of 65, 11.5% of Hispanic elderly received an AD diagnosis versus 6.9% of WNH older adults (Alzheimer’s Association, 2016). Unmeasured factors, possibly cultural and/or psychological factors related to test taking, may also have resulted in poorer performance on a variety of cognitive measures in minorities, compared with majority Whites (Uzzell, Pontón, & Ardila, Reference Uzzell, Pontón and Ardila2007).
DeCarli et al. (Reference DeCarli, Reed, Jagust, Martinez, Ortega and Mungas2008) first observed in a cohort in Davis, California, that mild cognitive impairment (MCI) was associated with greater hippocampal volume (HPCv) loss in non-Hispanics compared to Hispanic elders even after accounting for various risk factors. More recently, Zahodne et al. (Reference Zahodne, Manly, Narkhede, Griffith, DeCarli, Schupf and Brickman2015) in the Washington-Heights-Inwood Columbia Aging Project, found HPCvs were lower among WNHs than Hispanics, despite finding higher memory and other cognitive scores among the WNH group. However, the Hispanic participants of these two studies had on average less than 8 and 7 years of educational attainment, respectively, compared with more than 13 and 14 years for the WNH group, suggesting that factors such as higher cognitive reserve among the WNHs resulted in better cognitive performance despite greater severity of neurodegeneration. A limitation of many previous studies is that differences in age, educational level, levels of distress (such as depression), and cultural factors are confounded, so that the impact of any one of these factors cannot be evaluated independently.
Regional brain atrophy, especially in medial temporal regions, representing the underlying severity of neurodegenerative disease, such as AD, is also strongly associated with the severity of memory and overall cognitive impairment (Brooks & Loewenstein, Reference Brooks and Loewenstein2010; Duara, Reference Duara, Loewenstein, Potter, Appel, Greig, Urs and Potter2008). These biomarkers of neurodegeneration can be used to distinguish cognitively normal (CN), mild cognitive impairment (MCI), and mild dementia patients (Frisoni, Fox, Jack, Scheltens, & Thompson, Reference Frisoni, Fox, Jack, Scheltens and Thompson2010; Vemuri et al., Reference Vemuri, Gunter, Senjem, Whitwell, Kantarci, Knopman and Jack2008) in different ethnic, linguistic, and demographic groups, using quantitative and semi-quantitative methods of analyzing regional brain atrophy in structural MRI scans (Carmichael et al., Reference Carmichael, Mungas, Beckett, Harvey, Tomaszewski Farias, Reed and DeCarli2012; Duara et al., Reference Duara, Loewenstein, Shen, Barker, Varon, Greig and Potter2013; Loewenstein et al., Reference Loewenstein, Acevedo, Potter, Schinka, Raj, Greig and Duara2009; Shen et al., Reference Shen, Loewenstein, Potter, Zhao, Appel, Greig and Duara2011; Varon et al., Reference Varon, Loewenstein, Potter, Greig, Agron, Shen and Duara2011). In cross-sectional and longitudinal studies of neurodegenerative conditions (Dong et al., Reference Dong, Nabizadeh, Caunca, Cheung, Rundek, Elkind and Wright2015; Scahill et al., Reference Scahill, Frost, Jenkins, Whitwell, Rossor and Fox2003), HPCv is widely used as a biomarker, in both Alzheimer and non-Alzheimer conditions. However, structural MRI biomarkers of neurodegeneration, in AD prone areas, such as the entorhinal cortex (EC), supramarginal gyrus, and precuneus, have not been found to be influenced by factors such as educational level, culture (Butters et al., Reference Butters, Young, Lopez, Aizenstein, Mulsant, Reynolds and Becker2008; Dickerson et al., Reference Dickerson, Stoub, Shah, Sperling, Killiany, Albert and deToledo-Morrell2011), and psychological states (such as depression). Therefore, neurodegeneration may more accurately reflect the underlying disease state. This suggests that structural MRI scans can be used to calibrate the severity of the underlying disease across cultural and linguistic groups in a relatively unbiased way.
Some recent studies have begun exploring the extent to which depression, in particular, affects HPCv. Results have indicated that a depression diagnosis (n=248; 60 years and older) predicted a decrease in right HPCv within 4 years (Sawyer, Corsentino, Sachs-Ericsson, & Steffens, Reference Sawyer, Corsentino, Sachs-Ericsson and Steffens2012). A meta-analysis of 12 studies demonstrated that the total number of depressive episodes was significantly correlated to reduced right (but not left) HPCv (Videbech & Ravnkilde, Reference Videbech and Ravnkilde2004). Among 34 participants with depression, hippocampal and frontal lobe volumes were significantly lower than those without depression and these lower HPCvs were correlated with poorer performance in the Wisconsin Card Sorting Test, but not the Rey Auditory Verbal Learning Test. Severity of depression was also not statistically associated with HPCv (Frodl et al., Reference Frodl, Schaub, Banac, Charypar, Jäger, Kümmler and Meisenzahl2006).
In this study, we expected that there would be a relationship between HPCv and different levels of cognitive impairment at baseline among older adults participating in a longitudinal aging study. We also expected that using cognitive and functional measures, which were appropriately translated and were culture fair, and accounting for all other factors, such as age, educational level, and presence of depression, that equivalent levels of cognitive and functional impairment would be associated with equivalent levels of medial temporal atrophy in Hispanic and WNH ethnic groups.
METHODS
The current sample was recruited from a group of 498 subjects who were enrolled in the Florida Alzheimer’s Disease Research Center Clinical Core in Miami Beach, Florida, between 2005 and 2011. The study was approved by the Institutional Review Board at Mount Sinai Medical Center, Miami Beach. Either the subject and/or a legal representative provided informed consent. The analytic sample (n=165) was comprised of those with complete clinical data, who were self-reported as either Hispanic or non-Hispanic, and who had a structural MRI brain scan. Subjects underwent a comprehensive neuropsychological and clinical evaluation, which was conducted in English or Spanish, depending on their self-reported primary language (confirmed by the caregiver or another surrogate in bilingual subjects with MCI and AD).
All subjects received a comprehensive clinical evaluation including a formal CDR interview with an experienced bilingual psychiatrist (M.G.). The CDR score was established using an algorithm (Washington University Alzheimer’s Disease Research Center, 1999). This algorithm has previously been shown to correctly stage (AD vs. MCI vs. control) individuals 93% of the time (O’Bryant et al., Reference O’Bryant, Waring, Cullum, Hall, Lacritz, Massman and Doody2008). The CDR has been shown to be able to distinguish between participants with AD and controls when comparing English speakers and Spanish speakers. Even in the presence of education, age, and cultural differences, the CDR performed as intended in similar cohorts (Sano et al., Reference Sano, Mackell, Ponton, Ferreira, Wilson, Pawluczyk and Thal1997). All subjects were administered the Fuld Object Memory Evaluation (FOME) in their native language, one of the most widely accepted culturally and educationally fair tests for older adults, which has shown high sensitivity for mildly impaired Spanish-speaking patients (Loewenstein, Duara, Argüelles, & Argüelles, Reference Loewenstein, Duara, Argüelles and Argüelles1995) that are comparable to their English-speaking parts. The FOME has also been shown to be culturally fair in African-American and Hispanic groups (Rideaux, Beaudreau, Fernandez, & O’Hara, Reference Rideaux, Beaudreau, Fernandez and O’Hara2012).
Classification criteria were as follows: (a) criteria for CN (n=87) was a CDR Scale score of 0 (i.e., no reported cognitive or functional decline) and the FOME score of less than 1 SD below age and education related normative values; (b) criteria for amnestic MCI (aMCI) (n=42) was a CDR score of .5 and a FOME score of at least 1.5 SD below expected values; (c) criteria for dementia (n=36) was a CDR score of 1 and a FOME score at least 2.0 SD below expected values. Educational achievement was quantified by years of education. Subjects were also administered the Geriatric Depression Scale (GDS) (Yesavage et al., Reference Yesavage, Brink, Rose, Lum, Huang, Adey and Leirer1982) to measure reported levels of psychological distress.
MRI Scans
For volumetric studies, MRI scans were acquired using a proprietary three-dimensional volumetric protocol (MP-RAGE) on Siemens 1.5 Tesla machine. Volumetric analysis of MRIs of brain used a modification of the International Brain Atlases using Statistical Parametric Mapping, or IBASPM) [1920]. Normalized values for right and left HPCvs and ECv and inferior lateral ventricle (ILVv) volumes were obtained by dividing each regional volume by total intracranial volume, so as to adjust for variation in head size. In this study, we also used a sensitive visual rating system (VRS) that was developed to grade severity of atrophy of the hippocampus (HP) and EC.
In previous studies, we have shown that individual and summed HP and EC VRS scores are superior to volumetric measures for distinguishing subjects diagnosed as CN from aMCI and AD [21] better predicts progression from aMCI to dementia/AD, and to correlate with neuropsychological measures (Loewenstein et al., Reference Loewenstein, Acevedo, Potter, Schinka, Raj, Greig and Duara2009). VRS was also used to evaluate white matter hyperintensities (WMHs) on FLAIR sequences in four periventricular WMH regions, namely frontal, parietal, occipital, and temporal, as well as the centrum semiovale WMH region, using a “0” to “4” severity scale (Loewenstein et al., Reference Loewenstein, Acevedo, Potter, Schinka, Raj, Greig and Duara2009). The VRS system has been scored with high interrater reliabilities and all of our highly trained VRS raters were blinded to diagnostic category and ethnicity when scoring HPC and ERC on a coronal slice at the level of the mammillary body as WMH regions.
Statistical Methods
Comparative demographic variables for the Hispanic and WNH diagnostic groups were analyzed using a series of analyses of variance (ANOVAs) with post-hoc Sidak tests used as a post-hoc tests of means following a statistically significant F value. Biological sex was analyzed by chi-square analyses. In this study, we explored the association of HPCv at baseline to different levels of cognitive impairment among older adults participating in a longitudinal aging study using a culture- and education-fair memory test (FOME), the Clinical Dementia Rating (CDR) scale, and a comprehensive clinical interview. There was not a statistically significant difference in age, educational levels and level of depression/stress between Hispanic and WNHs at each level of cognitive impairment, namely CN, MCI, and mild dementia. Hence, we were able to evaluate the extent that cultural differences between the two ethnic groups influenced the relationship between severity of cognitive impairment and level of brain atrophy represented by structural MRI biomarkers, independent of the aforementioned demographic and language factors.
Our primary analyses was conducted using a series of 2 × 3 (ethnicity by diagnosis) ANOVAs for different brain regions where the main effect for ethnicity, the main effect for diagnosis and the ethnicity by diagnosis interaction term was investigated. Age, education, and GDS scores were entered as covariates into statistical models. To protect against spurious errors of inference associated with conducting multiple F-tests, the individual test-wise alpha (and criterion for statistical significance) was set at p<.01 for each F-test. p-Values less than p<.05 but >p<.01 were treated as approaching statistical significance. Lowering the test-wise p-value is preferable to approaches such as the Bonferroni correction, which results in reduced statistical power and enhanced Type 2 errors (failing to reject a false null hypotheses). A power analysis (Faul, Erdfelder, Buchner, & Lang, Reference Faul, Erdfelder, Buchner and Lang2009; Faul, Erdfelder, Lang, & Buchner, Reference Faul, Erdfelder, Lang and Buchner2007) assuming 75 Hispanic and 90 WNH subjects comprising six comparative groups confirmed the ability to detect an 0.05 alpha with 99.9%, and 99.6% power to detect a 0.01 alpha, using the means for left HPCvs with a relatively large clinically significant effect size of .40. Human data included in this manuscript were obtained in compliance with the Helsinki Declaration.
RESULTS
Table 1 shows the mean values for demographic and clinical variables for the overall sample. In Table 1, analysis of covariance (ANCOVA) results indicated a statistically significant difference across the groups for age [F(5,159)=11.21; p<.000], and education [F(5,159)=3.52; p=.005]. Among the clinical diagnostic measures significant differences across groups were found on the Mini Mental State Examination (MMSE) [F(5,159)=49.93; p<.001; Figure 1a], FOME [F(5,159)=61.99; p<.001; Figure 1b], and CDR Sum of Boxes [F(5,159)=64.79; p<.001; Figure 1c]. Post-hoc analysis on the GDS did not reveal significant differences across the six groups. Performance on neuropsychological tests revealed significant differences across groups for digit span forward [F(5,159)=7.04; p<.000], digit span backward [F(5,159)=5.23; p<.000], Trails A [F(5,159)=10.73; p<.000], Trails B [F(5,159)=24.40; p<.000], and category fluency [F(5,159)=29.58; p<.000].
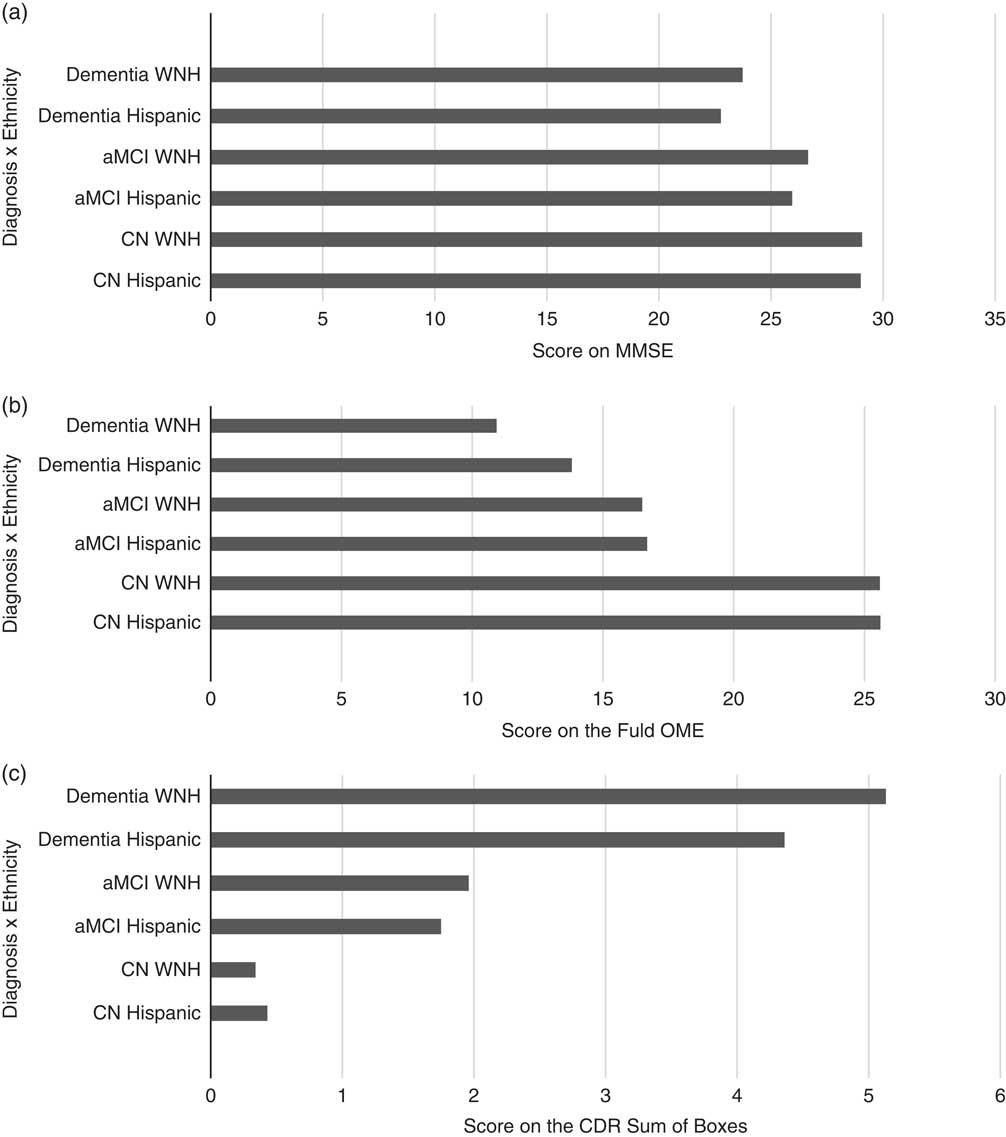
Fig. 1 a: Bar graph demonstrating the mean Mini-Mental State Examination (MMSE) scores for Hispanic and white non-Hispanic (WNH) subjects with a cognitive classification of cognitively normal (CN), amnestic mild cognitive impairment (aMCI), or dementia. There are significant differences between the cognitive groups (F[5,159]= 49.93; p<.000). b: Among the clinical diagnostic measures, significant differences across groups were found in the Fuld Object Memory Evaluation (FOME) [F(5,159)=61.99; p<.000]. c: Among the clinical diagnostic measures significant differences across groups were found in the Clinical Dementia Rating (CDR) Sum of Boxes (SOB) [F(5,159) =64.79; p<.000].
Table 1 Demographic and clinical variables for Hispanic and White non-Hispanic (WNH) participants in cognitively normal (CN), amnestic mild cognitive impairment (aMCI), and Dementia Groups

Note. Means with different alphabetic superscripts are statistically significant using the Sidak post-hoc test of means. Means with identical alphabetic superscripts were found not to be significantly different.
CN=cognitively normal; WNH=White non-Hispanic; aMCI=amnestic mild cognitive impairment; FOME=Fuld Object Memory Evaluation; MMSE=Mini Mental State Examination; CDR SOB=Clinical Dementia Rating Sum of Boxes; GDS=Geriatric Depression Scale; DSF=Digit Span Forward; DSB=Digit Span Backward; Trails=Trail Making Test; CatFl=Category Fluency.
*p<.05.
**p<.01.
***p<.001.
The results of the post-hoc analyses among the clinical diagnostic groups are demonstrated within Table 1 with alphabetic superscripts. Means with identical alphabetic superscripts were found not to be significantly different, whereas different alphabetic superscripts indicate significantly different scores. For example, the CN group had similar age between Hispanic and WNH (as indicated by “a”), but were significantly different from the other groups (as indicated by “b”). A chi-squared analysis revealed significant differences among the groups [χ2(5, N=159)=16.39; p<.006] with the clinically normal groups containing significantly more female participants than the aMCI groups.
With regard to language of testing, 93% of CN Hispanic participants were tested in Spanish and 7% in English; 93% of the Hispanic aMCI group were tested in Spanish and 7% in English; 97% of the WNH aMCI participants were tested in English and 3% in Spanish; and 96% of the Hispanic participants with dementia were tested in Spanish and 4% in English. All (100%) of the WNH CN and WNH with dementia participants were tested in English. Significant differences were found between language of testing among the Hispanic aMCI group for digit span forward scores [F(1,24)=7.09; p<.015], and among the WNH aMCI participants for Trails A [F(1,33)=30.52; p<.000] and category fluency [F(1,33)=4.17; p<.05]. There were no significant differences between language of testing for the other groups.
Table 2 depicts the results of a series of 2 × 3 (ethnicity by diagnosis) ANOVAs for different brain regions where the main effect for ethnicity, the main effect for diagnosis, and the ethnicity by diagnosis interaction term was investigated. Age, education, and GDS scores were entered as covariates into statistical models. Following a statistically significant F, group means were examined using the Sidak procedure (p<.05).
Table 2 Volumetric variables across groups, adjusted

Note. All analyses were adjusted for age, education, and Geriatric Depression Scale depression score.
CN=cognitively normal; WNH=White non-Hispanic; aMCI=amnestic mild cognitive impairment; HPCv, Hippocampal volume; ECV, entorhinal cortex volume; ILV, inferior lateral ventricle; WMH, white matter hyperintensity; L, left; R, right.
*p<.05; **p<.01; ***p<.001
HPCv
Means for regional volumes showed significantly lower mean scores in bilateral HPCv from CN to dementia, with HPCvL [F(5,159)=20.87; p<.000], HPCvR [F(5,159)=18.69; p<.000] (Table 2; Figure 2a). Smaller means were noted for the HPCv among WNHs compared with Hispanics. No diagnosis by ethnicity interaction was noted for HPCv.
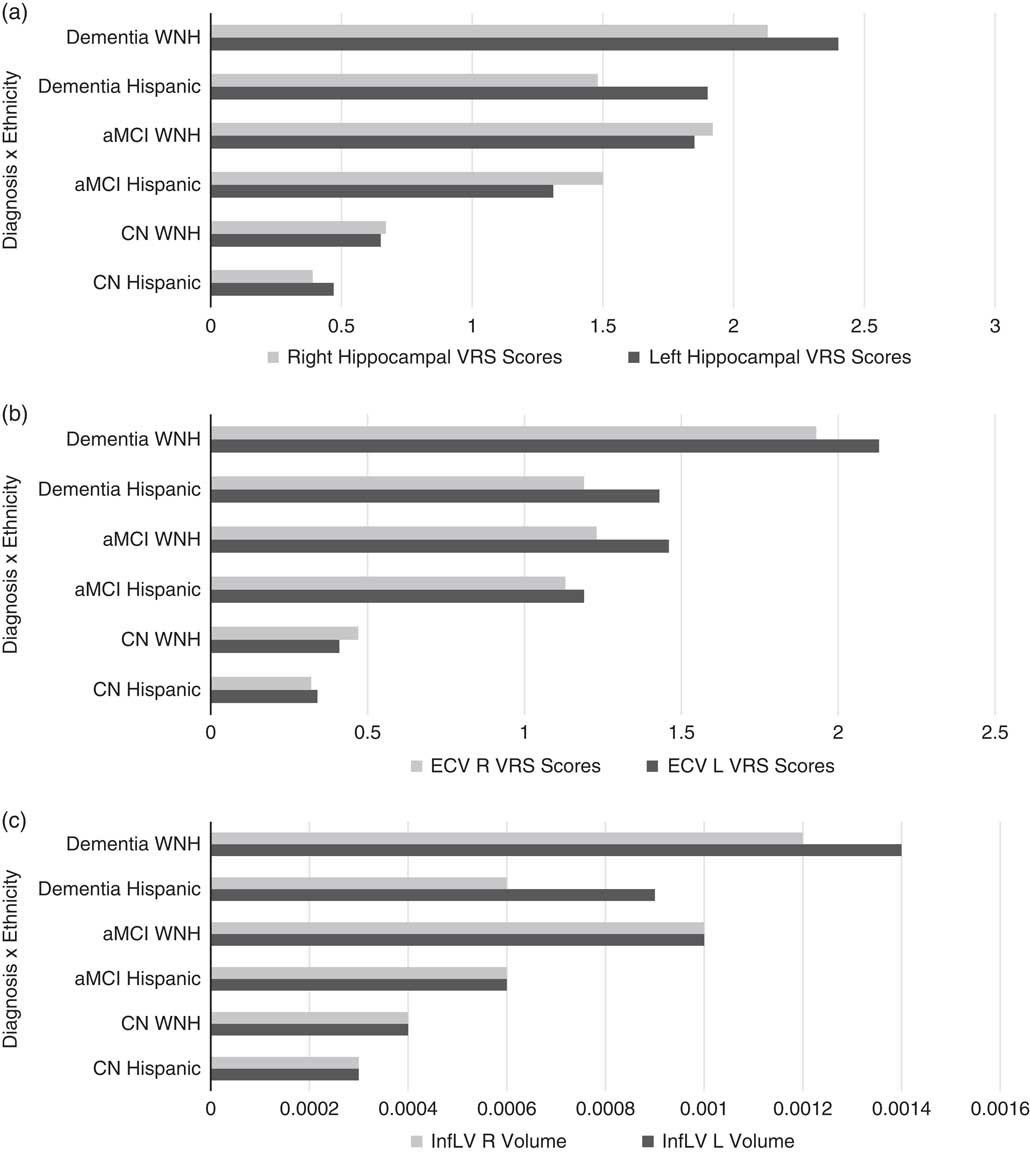
Fig. 2 a: Means for regional visual rating scale (VRS) scores showed a progressive decline in bilateral hippocampal volume (HPCv) cognitively normal (CN) to dementia, with HPCv left (L) [F(159)=20.87; p<.000], HPCv right (R) [F(5,159)=18.69; p<.000]. Smaller means for the hippocampus (HP) among WNHs compared with Hispanics indicate a higher level of atrophy among WNHs. b: Means for regional volumes and regional visual rating scale (VRS) scores showed a progressive decline in bilateral entorhinal cortex volume (ECv) from CN to dementia, with ECv L [F(5,159)=20.04; p<.000] and ECv R [F(5,159)=13.97; p<.000]. c: A main effect for ethnicity was noted for bilateral inferior lateral ventricle volume (ILVv): ILVv L [F(2,162)=13.46; p<.000] and ILV R [F(2,162)=10.51; p<.000]. A main effect for diagnosis was noted for bilateral ILVv: ILVv L [F(5,159)=14.03; p<.000] and ILV R [F(5,159)=13.80; p<.000]. An interaction effect for diagnosis and ethnicity approached significance for bilateral ILVv: ILVv [F(2,162)=3.60; p<.049] and ILVv R [F(2,162)=3.07; p<.03]. Overall, there were larger means for bilateral ILVv among WNH, compared with Hispanics. InfL V, ILV.
ECv
Means for regional volumes (Table 2; Figure 2b) showed significantly decreasing means in bilateral ECv from CN to dementia, with ECv left (L) [F(5,159)=20.04; p<.000] and ECv right (R) [F(5,159)=13.97; p<.000]. Smaller means were noted for the ECv among WNHs compared with Hispanics. No diagnosis by ethnicity interaction was noted for the ECv volume.
Bilateral ILVv
An increase in means for bilateral ILVv was noted in Table 2 (and Figure 2c) from CN to dementia, with ILVv L [F(5,159)=14.03; p<.000], and ILVv R [F(5,159)=13.80; p<.000]. A main effect for ethnicity was noted for bilateral ILVv, with ILVv L [F(2,162)=13.46; p<.000] and ILV R [F(2,162)=10.51; p<.000], with larger means for bilateral ILVv among WNH, compared with Hispanics. An ethnicity by diagnosis interaction effect approached significance for bilateral ILVv, with ILVv L [F(2,162)=3.60; p<.049] and InfLV R [F(2,162)=3.07; p<.03].
ANOVA for VRS measures (Table 3) showed a significant main effect for diagnosis, for bilateral HP and EC regions, but only a trend for WNHs.
Table 3 Visual rating scale variables across groups, adjusted

All analyses were adjusted for age, education, and GDS depression score.
CN=cognitively normal; WNH=White non-Hispanic; aMCI=amnestic mild cognitive impairment; HPCv=hippocampal volume; ECV=entorhinal cortex volume; WMH=white matter hyperintensity; L=left; R=right.
*p<.05; **p<.01; ***p<.001
Hippocampal VRS:
Means for these measures demonstrated a progressive increase in scores (indicating greater atrophy) from CN to dementia, with HPCv L [F(5,159)=2061; p<.001] and HPCv R [F(5,159)=14.69; p<.001]. Means for this region demonstrated consistently higher scores for each of these regions among WNHs compared with Hispanics, although no diagnosis by ethnicity interaction was found.
EC VRS
Means for these measures demonstrated a progressive increase in scores from CN to dementia, with EC L [F(5,159)=14.79; p<.000] and EC R [F(5,159)=9.91; p<.000]. The main effect for ethnicity was not statistically significant for this region nor was the interaction term.
WMH VRS
Means for these measures demonstrated a progressive increase in scores from CN to dementia, with WMH L [F(5,159)=5.06; p <.007, and WMH R [F(5,159)=4.30; p<.015. The main effect for ethnicity was statistically significant for the right WMH region [F(2,162) 6.89; p<.023] as well as the left WMH region [F(2,162) 5.24, p<.010]. Means for these regions demonstrated consistently higher scores among WNHs compared with Hispanics.
DISCUSSION
In the current study, we compared Hispanic to WNH individuals who were carefully diagnosed as CN, MCI, or dementia. We also evaluated regional brain volumes and the severity of white matter disease, all of which can be used as indices of the severity of degenerative or mixed degenerative and vascular brain diseases. We demonstrated the expected finding of increasing cognitive and functional impairment, in parallel with lower hippocampal and EC volumes, greater ILVvs (all indicating greater neurodegeneration in the temporal lobes), in both Hispanic and WNH ethnic groups. Moreover, the VRS also showed a trend for the Hispanic group to have less ventricular dilatation and for lower severity of WMH, the latter of which most likely reflects non-specific manifestations of degenerative and vascular brain diseases.
To determine level of cognitive impairment, we used the most culture- and education-fair memory test (FOME and the CDR scale) available, coupled with a comprehensive clinical interview with the participants and informants. Age, educational levels, and level of depression/stress were not significantly different between Hispanic and WNHs at each level of cognitive impairment, namely CN, MCI, and mild dementia. As a result of the statistically non-significant demographic and depression measures, we were able to evaluate the extent that cultural differences between the two ethnic groups, including the associated demographic, psychological, and language factors, influenced the relationship between severity of cognitive impairment and level of neurodegeneration as represented by structural MRI biomarkers.
In this study, the impairment of episodic memory was assessed by the FOME, which has been found to have high cultural validity in the MCI and later stages of AD (Loewenstein et al., Reference Loewenstein, Duara, Argüelles and Argüelles1995). However, performance on all cognitive tests can be influenced by sociodemographic and culture factors as well as psychological states, such as depression and anxiety. The concept of cognitive reserve was developed to explain the ability of individuals with higher levels of education and/or occupational and life experience to perform better on cognitive tests than would be expected for the level of underlying brain disease, regardless of the etiology of the disease (Stern et al., Reference Stern, Gurland, Tatemichi, Tang, Wilder and Mayeux1994).
Individuals who are representative of a dominant culture in which the test was developed, may also have an advantage over those who belong to a non-dominant culture. For example, it is well known that the MMSE has poor sensitivity and specificity for identifying cognitive impairment among minority populations (Boone et al., Reference Boone, Victor, Wen, Razani and Pontón2007; Gasquoine, Reference Gasquoine1999; Guerrero-Berroa et al., Reference Guerrero-Berroa, Schmeidler, Raventos, Valerio, Berri, Carrion-Baralt and Sano2016) even after accounting for other factors, such as age and educational level. Individuals with greater cognitive reserve may also perform at a higher level on cognitive tests and functional measures, than would otherwise be expected for the severity of their underlying neurodegenerative or vascular brain disease. In this study, no difference in cognitive reserve between the two ethnic groups would be expected, based on our findings that no statistical difference in age and education level was present between the Hispanic and WNH participants within each diagnostic group, a finding consistent with other studies (Espino et al., Reference Espino, Lichtenstein, Palmer and Hazuda2004; Gasquoine, Reference Gasquoine1999; Guerrero-Berroa et al., Reference Guerrero-Berroa, Kluger, Schmeidler, Sailor, Lizardi, Golomb and Reisberg2014, Reference Guerrero-Berroa, Schmeidler, Raventos, Valerio, Berri, Carrion-Baralt and Sano2016; Uzzell, Ponton, & Ardila, Reference Uzzell, Ponton and Ardila2013).
Our results, however, point to a significant decline in volumes and increasing atrophy of the HP and the EC from CN state to dementia, in both Hispanics and WNHs, as in accordance with previous reports (DeCarli et al., Reference DeCarli, Reed, Jagust, Martinez, Ortega and Mungas2008; Loewenstein et al., Reference Loewenstein, Acevedo, Potter, Schinka, Raj, Greig and Duara2009; Zahonde et al, 2015). Enlargement of an inferior lateral ventricle indicates atrophy in the regions surrounding this structure, including the HP, parahippocampal gyrus, lingual and inferior temporal gyri, amygdala, and related white matter structures. All of these aforementioned brain regions have been associated with episodic memory function.
HPCv is widely used as a biomarker in cross-sectional and longitudinal studies of neurodegenerative conditions, such as AD. Non-neurodegenerative conditions, such as depression, stress, and the use of steroid medications are also known to impact HPCv, and should be taken into consideration when HPCv is used as a biomarker in studies of neurodegenerative disease (Brown, Rush, & McEwen, Reference Brown, Rush and McEwen1999). The volume of the HP and the EC has been shown to have a positive association with performance on delayed recall tests (Sheline, Wang, Gado, Csernansky, & Vannier, Reference Sheline, Wang, Gado, Csernansky and Vannier1996), whereas the volume of the lateral ventricle, especially the inferior aspects of the lateral ventricle, has a negative association with the scores on cognitive tests.
We also found that across diagnostic groups there was greater neurodegeneration (i.e., with regard to ILVvs) among WNHs compared with Hispanics. The trend toward a diagnosis by ethnicity interaction effect found in bilateral ILVvs confirmed that Hispanic participants tend to have less enlargement in these regions, which is an important factor for degeneration in this structure. Similar findings have been reported previously by DeCarli et al (Reference DeCarli, Reed, Jagust, Martinez, Ortega and Mungas2008) and Zahodne et al. (Reference Zahodne, Manly, Narkhede, Griffith, DeCarli, Schupf and Brickman2015), although in contrast to these previous reports, the differences in severity of neurodegeneration between Hispanics and WNHs, with regard to the present study, could not be explained by differences in age or education between the two ethnic groups.
The level of neurodegeneration in each diagnostic group (and especially in aMCI and dementia groups) was greater in WNHs than Hispanics, even though the two ethnic groups were statistically non-significant equivalent both in cognitive and functional performance (Table 2). Because the severity of neurodegeneration, as measured on structural MRI scans is a biologically based measure, it is highly likely that it is less biased with regard to ethnicity, culture, level of depression, anxiety, or other psychological states relative to cognitive measures. Our findings point to the likelihood that Hispanics in this study may have performed worse on cognitive tests than would have been expected, given that they had, on average, a lesser degree of neurodegeneration than WNH and yet performed at the same level as WNHs. This bias appears to be unrelated to differences in cognitive reserve and may be attributable to other cultural differences, such as the approach to test-taking between the two ethnic groups.
In cross-cultural studies, the use of neuropsychological tests scores, as an index of the severity of underlying disease process in the brain may be subject to nuances of language and individual cultural attitudes, even when accounting for the effects of cognitive reserve, based on educational levels, and when using validly translated and back-translated tests (Ostrosky-Solis, Ramirez, & Ardila, Reference Ostrosky-Solis, Ramirez and Ardila2004; Rosselli & Ardila, Reference Rosselli and Ardila2003; Tappen, Rosselli, & Engstrom, Reference Tappen, Rosselli and Engstrom2010). These cultural factors include differences in the motivation for the speed, rigor, and precision in interpreting a given task and providing a required response (Ardila, Reference Ardila2013). The requirement that a participant should engage in optimal performance for a task may be alien or unfamiliar to certain cultures and, as such, these cultural factors may result in a participant trivializing the process of test taking. The impact of these cultural factors may partially or fully account for reported higher prevalence and earlier age of onset among minority groups of conditions such as AD, the diagnosis of which, especially in epidemiological studies, depends strongly on performance on cognitive tests.
Measurement bias in cognitive testing may result in errors in the accuracy of diagnostic testing and cognitive classification may result in overtreatment of certain conditions, such as AD, and under-recognition and -treatment of other conditions, such as depression, stress, and anxiety in minority groups. These differences in neuropsychological performance, with better scores among WNHs, has been suggested by Ardila (Reference Ardila2013), to be related to the fact that “speed, competitiveness, and high productivity are important cultural values in literate Anglo-American society, but that is not true in other cultural groups. The detached professional relationship usually adopted in neuropsychological testing may also be alien and even condescending in certain cultures, in which close interpersonal relationships are valued” (pp. 7–8).
Our findings suggest that psychometrically valid clinical protocols may be influenced by additional cultural variables. Our findings of equivalent or better cognitive scores on Digit Span Forward and Category Fluency test among WNH, than among Hispanics, despite greater severity of overall neurodegeneration, suggests that language and cultural factors may provide an advantage to WNHs. Similar findings were reported previously by Mungas, Reed, Farias, and DeCarli (Reference Mungas, Reed, Farias and DeCarli2009) and Zahodne et al. (Reference Zahodne, Schofield, Farrell, Stern and Manly2015), who found that HPCv was larger among Hispanics than in WNHs, whereas WNHs scored better on memory and non-memory measures.
In our study, it should be noted that an inspection of HPCv means across diagnostic groups consistently showed lower means for the WNH group, despite the lack of an overall ethnicity or ethnicity by diagnostic group interaction (which may have reflected the lack of statistical power given modest cell sizes). More importantly, the trend toward less inferior lateral ventricular dilatation, particularly in Hispanic aMCI and mildly demented participants, as well as main ethnicity effect showing less WMH for the Hispanic group as a whole is consistent with the notion that the Hispanic group had less brain pathology.
The similarities between our findings of less neurodegeneration in Hispanics in Miami and other Hispanic cohorts in California-Davis (DeCarli et al., Reference DeCarli, Reed, Jagust, Martinez, Ortega and Mungas2008) and in New York (Zahonde et al., 2015) suggests the possibility that the same unmeasured sociocultural factors influenced these interactions and is an issue worthy of further collaborative research.
Future studies should examine factors such as the level of acculturation, education, effort/motivation, and attitudes toward test taking on cognitive performance on standardized tests. Acculturation rating scores, amount of time educated outside of the United States, and the frequency of English spoken when growing up has been found to be correlated with neuropsychological test performance among ethnically diverse groups (Razani, Burciaga, Madore, & Wong, Reference Razani, Burciaga, Madore and Wong2007). In future studies, controlling for acculturation when examining relationships between cognitive performance and brain atrophy will have important implications to diagnostic accuracy among Hispanic elderly as well as understanding ethnic differences in cognitive manifestations of neuropathology in dementia.
Also, ethnic differences may be better understood if the participants’ language experience is taken into account. For example, many Hispanic immigrants are bilingual, and it has been well documented that bilingualism could have an effect on the aging process (Schweizer, Ware, Fischer, Craik, & Bialystok, Reference Schweizer, Ware, Fischer, Craik and Bialystok2012). Similar to other ethnic minorities, Hispanics have also been found to have a higher prevalence of AD and are diagnosed at later stages and at younger ages than WNHs (O’Bryant et al., Reference O’Bryant, Xiao, Edwards, Devous, Gupta, Martins and Barber2013; O’Bryant, Humphreys, Schiffer, & Sutker, Reference O’Bryant, Humphreys, Schiffer and Sutker2007; Tang et al., Reference Tang, Cross, Andrews, Jacobs, Small, Bell and Mayeux2001). Medical conditions, such as diabetes mellitus, vascular disease, and depression, which have a higher prevalence among Hispanics (Bell-McGinty et al., Reference Bell-McGinty, Butters, Meltzer, Greer, Reynolds and Becker2002; Livney et al., Reference Livney, Clark, Karlawish, Cartmell, Negrón, Nuñez-Lopez and Arnold2011), may also have a bearing on the relatively poorer performance on cognitive tests among this group and is worthy of further research.
In conclusion, epidemiological studies have shown differences in cognitive performance between Hispanics individuals with and without dementia, with lower performance in memory, executive function, and overall cognitive performance among Hispanics than WNHs, even after controlling for factors such as age, biological sex, education, and socioeconomic status (Castora-Binkley et al., Reference Castora-Binkley, Peronto, Edwards and Small2015; Chin, Negash, & Hamilton, Reference Chin, Negash and Hamilton2011). We expanded upon these previous findings by demonstrating that the differences detected in structural brain imaging between Hispanics and WNHs occurred despite the finding that scores on ostensibly culture-fair tests of memory (the FOME) and functional impairment (the CDR scores) in each diagnostic group of Hispanic versus non-Hispanic participants. We have used regional brain volumes as independent, and potentially more valid indices of underlying disease states than performance on traditional neuropsychological assessments and functional assessments.
It is important to note, however, in the absence of amyloid and imaging or CSF markers, it is difficult to determine the extent to which MRI findings were associated with AD versus other or concomitant pathologies. For example, there were significantly increased WMHs for WNH as a whole, which might indicate more severe Alzheimer’s pathology or conversely more independent vascular pathology, but still does not explain the equivalence of memory and functional scores within the Hispanic and non-Hispanic diagnostic subgroups. This naturally raises the issue of what are the implications for the field. While it would be impractical to revise all existing neuropsychological measures in the field, these findings speak to the vital importance of establishing appropriate culture and language specific normative databases for clinical use, appropriate test–retest and other psychometric properties.
Consideration of neuroimaging measures, such as VRS could help better ensure that those with brain pathology are not included in normative samples as well as considering measures of effort that may reveal individual differences in test engagement. Bilingualism and acculturation are also important factors that merit further attention. There is an emerging literature showing the potential value of cognitive stress tests that are more sensitive to the earliest manifestations of neurodegenerative disease in different ethnic and cultural groups than traditional measures (see Loewenstein et al., Reference Loewenstein, Curiel, Greig, Bauer, Rosado, Bowers and Duara2016; Loewenstein, Curiel, Duara, & Buschke, Reference Loewenstein, Curiel, Duara and Buschke2017). Use of this literature can aid clinicians to incorporate culturally sensitive measures in the neuropsychological batteries when assessing cognitive decline among multicultural groups, thereby improving diagnostic accuracy and treatment efficacy.
ACKNOWLEDGMENTS
Funding: NIA, NIH, Bethesda, MD, Grant # 1P50AG025711-05 and 1P50AG047266-01. None of the authors have any conflicts of interest to disclose.







