Cervical dystonia (CD) is a movement disorder characterized by muscle contractions causing sustained twisting movements and abnormal postures of the neck and head (Albanese et al., Reference Albanese, Bhatia, Bressman, DeLong, Fahn, Fung, Hallett, Jankovic, Jinnah, Klein, Lang, Mink and Teller2013). In almost all patients the CD is idiopathic. Once patients develop CD, this disorder is chronic. The natural course of idiopathic CD is that the symptoms usually progress in the first five years, after which they remain relatively stable (Albanese et al., Reference Albanese, Bhatia, Cardoso, Comella, Defazio, Fung, Hallett, Jankovic, Jinnah, Kaji, Krauss, Lang, King Tan, Tijssen and Vidailhet2023). Historically, CD has been considered a pure motor disorder. However, growing evidence suggests that non-motor symptoms such as depression, anxiety, and cognitive deficits are part of the phenotype (Aita et al., Reference Aita, Del Bene, Marotta, Pizer, Hawley, Niccolai, Walker, Gerstenecker, Martin, Clay, Crowe, Triebel and Hill2022; Ben-Shlomo et al., Reference Ben-Shlomo, Camfield and Warner2002; O’Connor et al., Reference O’Connor, Hevey, Burke, Rafee, Pender and O’Keeffe2023; Smit et al., Reference Smit, Kuiper, Han, Jiawan, Douma, van Harten, Oen, Pouwels, Dieks, Bartels and Tijssen2016). It is suggested that motor and non-motor symptoms are independent factors of the disease and can be related to the same underlying neuronal networks (Timmers et al., Reference Timmers, Kuiper, Smit, Bartels, Kamphuis, Wolf, Poll-The, Wassenberg, Peeters, de Koning and Tijssen2017). Abnormalities in connectivity in dystonia have been found in the cortico-striatal-thalamo-cortical networks (Allam et al., Reference Allam, Frank, Pereira and Tomaz2007; Brüggemann, Reference Brüggemann2021; Delnooz et al., Reference Delnooz, Pasman, Beckmann and van de Warrenburg2015; Stamelou et al., Reference Stamelou, Edwards, Hallett and Bhatia2012). These areas are also involved in cognitive functions, such as memory, mental speed, attention, and executive functions (Haber, Reference Haber2016). Executive functions incorporate aspects such as planning, cognitive flexibility, and inhibition (Gilbert & Burgess, Reference Gilbert and Burgess2008; Stuss & Alexander, Reference Stuss and Alexander2000; Stuss, Reference Stuss2011). Hence, given the involvement of the cortico-striatal-thalamo-cortical networks, impairments in these different cognitive functions can be expected in CD patients. Studies have shown conflicting results with some showing evidence for deficits in memory (Burke et al., Reference Burke, Monaghan, McCormack, Cogley, Pinto-Grau, O'Connor, Donohoe, Murphy, O'Riordan, Ndukwe, Hutchinson, Pender and O'Keeffe2020; Monaghan et al., Reference Monaghan, Cogley, Burke, McCormack, O'Riordan, Ndukwe, Hutchinson, Pender and O'Keeffe2021), and different aspects of executive functions (Bugalho et al., Reference Bugalho, Correa, Guimarães and Xavier2006; Foley et al., Reference Foley, Vinke, Limousin and Cipolotti2017; Scott et al., Reference Scott, Gregory, Wilson, Banks, Turner, Parkin, Giladi, Joint and Aziz2003), attention and psychomotor speed (Foley et al., Reference Foley, Vinke, Limousin and Cipolotti2017; Jahanshahi et al., Reference Jahanshahi, Rowe and Fuller2003; Monaghan et al., Reference Monaghan, Cogley, Burke, McCormack, O'Riordan, Ndukwe, Hutchinson, Pender and O'Keeffe2021) but others demonstrating intact executive functions (Burke et al., Reference Burke, Monaghan, McCormack, Cogley, Pinto-Grau, O'Connor, Donohoe, Murphy, O'Riordan, Ndukwe, Hutchinson, Pender and O'Keeffe2020; Monaghan et al., Reference Monaghan, Cogley, Burke, McCormack, O'Riordan, Ndukwe, Hutchinson, Pender and O'Keeffe2021), attention, and psychomotor speed (Burke et al., Reference Burke, Monaghan, McCormack, Cogley, Pinto-Grau, O'Connor, Donohoe, Murphy, O'Riordan, Ndukwe, Hutchinson, Pender and O'Keeffe2020). In their recent review, O’Connor et al. (Reference O’Connor, Hevey, Burke, Rafee, Pender and O’Keeffe2023) conclude that in CD patients, attention, and working memory are usually intact, but that there is evidence for deficits in social cognition (SC). Furthermore, there is conflicting evidence for minor problems regarding other cognitive domains, among which mental speed, memory, and executive functioning. The found cognitive deficits were thought to be linked to a dysfunctional striatum (Barahona-Corrêa et al., Reference Barahona-Corrêa, Bugalho, Guimarães and Xavier2011; Jahanshahi et al., Reference Jahanshahi, Rowe and Fuller2003) or a dysfunctional collicular-pulvinar-amygdala pathway (Burke et al., Reference Burke, Monaghan, McCormack, Cogley, Pinto-Grau, O'Connor, Donohoe, Murphy, O'Riordan, Ndukwe, Hutchinson, Pender and O'Keeffe2020) in CD patients. SC also depends on the same cortico-striatal-thalamo-cortical loop.
SC refers to the ability to understand the behavior of others and to adapt behavior to social situations (Adolphs, Reference Adolphs2001). SC can be divided in three main aspects: perception of social information (e.g., facial expressions or prosody), understanding thoughts, intentions, and feelings of others (e.g., Theory of Mind (ToM)), and having empathy (Fiske & Taylor, Reference Fiske and Taylor2013). Perception of social information requires the direction of attention to socially relevant stimuli. The term “understanding” relates to attributing meaning to social information and is referring to ToM; the rational ability to understand what other people are thinking or feeling. Empathy is the term that is used for the process in which one actually shares the feelings of another person. Evidence was found for impaired perception of social-emotional information, such as poor recognition of facial expressions of disgust (Rinnerthaler et al., Reference Rinnerthaler, Benecke, Bartha, Entner, Poewe and Mueller2006) and detection of anger prosody (Nikolova et al., Reference Nikolova, Fellbrich, Born, Dengler and Schröder2011), as well as for poor performance in ToM tasks (Czekóová et al., Reference Czekóová, Zemánková, Shaw and Bareš2017; Lagravinese et al., Reference Lagravinese, Santangelo, Bonassi, Cuoco, Marchese, Di Biasio, Erro, Pelosin and Avanzino2021) in CD patients compared to controls. Rizzo et al. (Reference Rizzo, Martino, Avanzino, Avenanti and Vicario2023) show that especially negative emotions are recognized less accurately by CD patients and performance on ToM tasks is worse than in healthy controls (HCs). Empathy on the other hand seems to be intact in CD patients. These social cognitive deficits might be partly explained by deficits in non-SC, but the large variability between research methods hampers conclusions about this point across studies (Rizzo et al., Reference Rizzo, Martino, Avanzino, Avenanti and Vicario2023). O’Connor et al. (Reference O’Connor, Hevey, Burke, Rafee, Pender and O’Keeffe2023) conclude that SC is impaired in CD patients, but they have several concerns about the quality of the existing evidence. Sample sizes are generally small and studies use vastly different methods, making it difficult to compare results. None of the studies investigated all three aspects of SC, nor were they compared to the presence of possible impairments in a broad range of cognitive functions, in the same group of patients. Hence, the aim of the present study was to investigate a broad range of nonsocial cognitive and social cognitive functions to identify the specific (social and nonsocial) cognitive profile of CD patients and to investigate how this relates to disease characteristics, such as disease duration, disease severity and duration of treatment with botulinum toxin (BTX). Our hypotheses were that CD patients have nonsocial cognitive deficits in the domains of memory, psychomotor speed and executive functioning. In addition, we hypothesized that perception of social information and ToM ware impaired while empathy is intact in CD patients.
Method
Participants
Twenty-two adult patients (18 years and older) were recruited at the BTX clinic of the University Medical Center (UMCG) and the Martini Hospital in Groningen, the Netherlands. All patients were diagnosed with idiopathic chronic CD and the diagnosis was made by a neurologist, specialized in movement disorders. The study was carried out accordance with the Helsinki Declaration and the Medical Ethical Review Committee of the UMCG assessed and approved the study (MREC number 2013/001). Written informed consent was obtained from all participants. Exclusion criteria were intellectual disability, below cutoff performance on a symptom validity test (SVT), and other relevant neurological diseases. The HC group consisted of age-, gender-, and IQ-matched individuals with no history of dystonia or other relevant neurological or psychiatric diseases, head injury, or substance abuse. Due to logistical reasons, results of non-SC tests were available for a subset of the HC group only (n = 27 for the verbal memory test, n = 31 for the test for processing speed, n = 11 for the test for inhibitory control).
Materials
Cognitive test battery
Nonsocial cognition
Cognitive tests were selected that are widely used in clinical practice in the Netherlands, which also means that their psychometric characteristics and practical applicability are known well. Furthermore, these tests were selected based on the cognitive function they measure.
Estimation of premorbid, verbal IQ
The Dutch version of the National Adult Reading Test was used to estimate the premorbid verbal IQ (Schmand et al., Reference Schmand, Lindeboom and van Harskamp1992). In this test, participants are asked to read irregularly spelled Dutch words aloud. The number of correctly read words is counted and transferred to an estimated IQ score (mean = 100, sd = 15) according to the manual.
Memory
Verbal memory was measured with the Dutch version of the Rey Auditory Verbal Learning Test (Saan & Deelman, Reference Saan and Deelman1986). Fifteen unassociated words are read out five times in a fixed pace, and patients are asked to repeat as many words as possible after each trial, resulting in a maximum raw score of 75 (RAVLT IR). A delayed recall score is obtained after 20 minutes, with a maximum of 15 (RAVLT DR). The RAVLT Forgetting score is calculated by subtracting RAVLT DR from the score on the fifth trial. A score of 0 means that the same number of words was recalled after 20 minutes as was repeated in the fifth trial. Negative scores mean that more words were recalled, and positive scores mean that less words were recalled, that is, words were forgotten. Raw scores are used in the analysis.
Psychomotor speed
Psychomotor speed was assessed with the Trail Making Test (TMT, (Reitan, Reference Reitan1958)). In condition A of this paper and pen test, patients are asked to connect numbers in ascending order. In condition B, they are asked to alternate between numbers and letters. Completion time in seconds of part A is measured and used for analysis.
Executive functioning
Cognitive flexibility was assessed with the TMT B/A index, where completion time of condition B of the TMT is interpreted in relation to condition A. Inhibitory control was measured with the Hayling sentence completion test (Burgess & Shallice, Reference Burgess and Shallice1997). In this test, patients are asked to complete 15 sentences. In the first condition, patients are asked to add the last missing word to the sentence in order to complete it (e.g., “The captain left the sinking…” “ship”). In the second condition, the added word may not fit the rest of the sentence (e.g., “The captain left the sinking…” “sit”). The overall scaled score ranges from 1 to 10 with higher scores indicating better performance. Again, raw scores are used for the analysis. Planning was tested in the first condition of the zoo map subtest of the Behavioural Assessment of the Dysexecutive Syndrome (BADS-NL; (Wilson et al., Reference Wilson, Alderman, Burgess, Emslie and Evans1997)). Patients plan their way through a zoo while adhering to several rules and detailed instructions. Raw scores of this first condition were used for analysis with a maximum score of 8 for errorless performance.
Social cognition (SC)
Perception of social information
Facial Emotion Recognition was assessed with the Facial Expression of Emotion: Stimuli and Tests (FEEST; (Young et al., Reference Young, Perrett, Calder, Sprengelmeyer and Ekman2002)). This is a test for the recognition of anger, fear, surprise, sadness, disgust, and happiness. Correctly identified emotions are counted and scores range from 0 to 10 for each emotion with a total score range of 0–60. Raw scores are used in the analysis with higher scores indicating better performance.
Understanding thoughts and intentions of others
ToM was measured with two tests, The Faux -Pas (FP) test (Stone et al., Reference Stone, Baron-Cohen and Knight1998) and the Happé Cartoons (Happé et al., Reference Happé, Brownell and Winner1999). In the Happé Cartoons test, participants are presented 12 cartoons and asked what the artist intended to be the joke. In six of the cartoons the joke is based on a false belief of a character in the cartoon, requiring adequate ToM. In the other six cartoons the joke is based on a physical anomaly. Scores ranged from 0 to 3 points per cartoon: Three points for a full explanation, two points for a partial explanation, one point if only relevant details are mentioned, and zero points for incorrect or irrelevant answers. The total score is the sum of scores over all 12 cartoons and ranges from 0 to 36. The FP test consists of 10 short stories read aloud by the examiner, with 5 stories containing a FP. Afterward participants are presented with a copy of the story. Participants are then asked whether someone said something he or she should not have said (FP detection) and how the other person in the story may feel (empathy). The score for FP detection ranges from 0 to 10. The empathy score, denominating the number of times the subject correctly named the emotion felt by the victim of the FP, ranges from 0 to 5. Raw scores of both tests described are used for the analysis.
Symptom validity
We used the Amsterdamse Korte Termijn Geheugen Test (AKTG) as a measure of symptom validity (Schmand & Lindeboom, Reference Schmand and Lindeboom2004). In this test, patients were asked to recognize three previously read words in a list of five words. The total score is the sum of correctly recognized words over 30 trials, ranging from 0 to 90. The cutoff score indicating suboptimal effort is 85. Patients who scored below the cutoff were excluded from the analysis.
Assessment of symptoms of depression and anxiety
Symptoms of depression and anxiety were assessed with the Hospital Anxiety and Depression Scale (HADS) by Zigmond and Snaith (Reference Zigmond and Snaith1983). This self-report questionnaire yields one score for symptoms of depression and one for symptoms of anxiety. Raw scores from 0 to 7 are interpreted as normal, 8–10 as indications of mild symptoms of anxiety or depression, 11–14 as moderate and 15–21 as severe.
Motor assessment
Dystonia severity was determined in an independent assessment by an expert (MS) of a video based on the motor severity subscale of the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS; (Consky et al., Reference Consky, Basinski, Belle, Ranawaya and Lang1990)). It is based on the physical findings and consists of six items that can result in a maximum score of 35, with higher scores indicating higher dystonia severity.
Statistical analysis
All statistical analyses were performed with IBM SPSS Statistics 28. Histograms of scores on dependent variables were visually inspected to judge the degree of normal distribution of the scores. Based on this inspection, we decided to used nonparametric tests (Mann–Whitney U tests) in the analysis. Cohen’s d was used to determine the effect sizes (ESs) for the between group comparisons. For Cohen’s d an effect of 0.2 is considered small, 0.5 moderate, and ≥ 0.8 large. Spearman’s correlation coefficients were calculated within the CD group to assess whether educational level, HADS anxiety or depression scores, or disease severity were associated with results on any of the cognitive tests.
Results
Group characteristics
Twenty-two idiopathic CD patients were included but two patients scored below the cutoff on the AKTG and were excluded from further analysis. Patient data were compared to an age-, gender-, and IQ-matched HC group (n = 40). Yet, the HC group had a higher educational level compared to the patient group. Demographic characteristics are shown in Table 1. TWSTRS scores indicate that the patients in this sample on average had CD of medium severity. HADS scores indicate normal levels of symptoms of depression and anxiety for the group. Yet, there were five patients with mild symptoms of depression, two patients with mild symptoms of anxiety, and two patients with moderate symptoms of anxiety. Based on their files, patients did not have other psychiatric disorders. One quarter of our patients were treated with antidepressants and 6 were treated with benzodiazepines.
Table 1. Group characteristics of the CD group and HC group
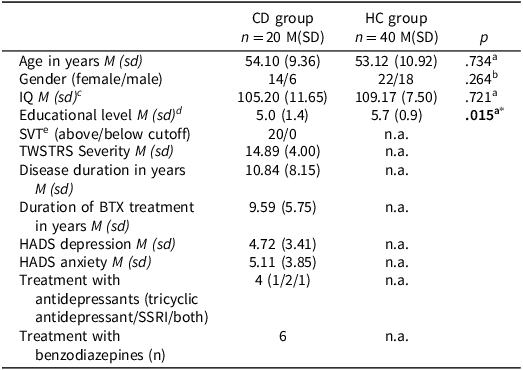
SSRI = Selective Serotonin Reuptake Inhibitor.
TWSTRS Severiy: Toronto Western Spasmodic Torticollis Rating Scale severity score; Disease duration: time since diagnosis; Duration of BTX treatment: time since start treatment with botulinum toxin injections.
a Independent-samples t-test.
b Chi-square test.
c Estimated with the Dutch version of the National Adult Reading Test (Schmand et al., Reference Schmand, Lindeboom and van Harskamp1992).
d Educational level: (1) primary school, (2) finished primary school, (3) unfinished secondary school or special education, (4) finished secondary school, (5) finished vocational training, (6) finished college education, and (7) university degree.
e Amsterdamse Korte Termijn Geheugen Test (AKTG; (Schmand & Lindeboom, Reference Schmand and Lindeboom2004)).
Nonsocial cognition
Table 2 provides an overview over the tests for non-SC. CD patients scored significantly lower on the RAVLT IR and DR, but they did not forget more words than the HC group. Patients needed significantly more time to complete the TMT A and B compared to HCs, but TMT B/A did not indicate disproportionate slowing on B. Patients scored also lower on the Hayling and zoo map test. The ESs showed moderate to large differences between the groups. A substantial number of patients in our sample was treated with benzodiazepines, which are known to decrease cognitive speed. Therefore, we repeated the analysis without patients who used these drugs (n = 14), the results remained unchanged.
Table 2. Results for nonsocial cognition, Mann–Whitney U tests and effect sizes for differences
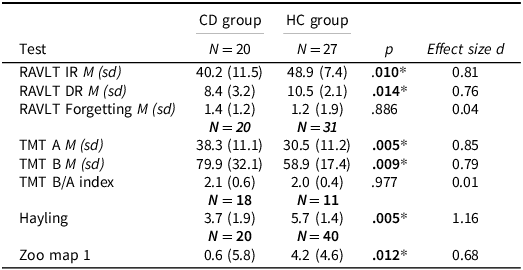
RAVLT IR = Rey Auditory Verbal Learning Test Immediate Recall, RAVLT DR = Rey Auditory Verbal Learning Test Delayed Recall, TMT A = Trail Making Test condition A, TMT B = Trail Making Test condition B, TMT B/A = Trail Making Test condition B in relation to A.
Social cognition
In Table 3, the descriptive statistics of the SC measures for the two groups and the between group comparisons are shown. There were no significant differences between both groups and ESs are generally small.
Table 3. Results for social cognition, Mann–Whitney U tests and effect sizes for differences
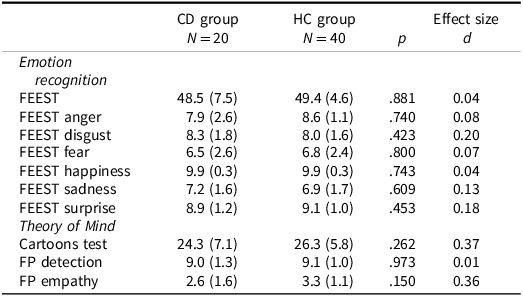
FEEST = Facial Expression of Emotion: Stimuli and Tests, FP = faux pas.
Relation between cognitive tests, educational level, HADS anxiety and depression scores, and disease characteristics
There were no significant correlations, that is, dystonia severity, disease duration, and duration of treatment with BTX were unrelated to the cognitive tests (Table 4). Educational level was significantly correlated to the RAVLT Forgetting score, the TMT A, the Cartoons test, and FP empathy scores. HADS anxiety scores were significantly correlated to the total score of the Hayling sentence completion test.
Table 4. Spearman’s rho correlation (p) between disease severity, disease duration, duration of BTX treatment, and cognitive tests
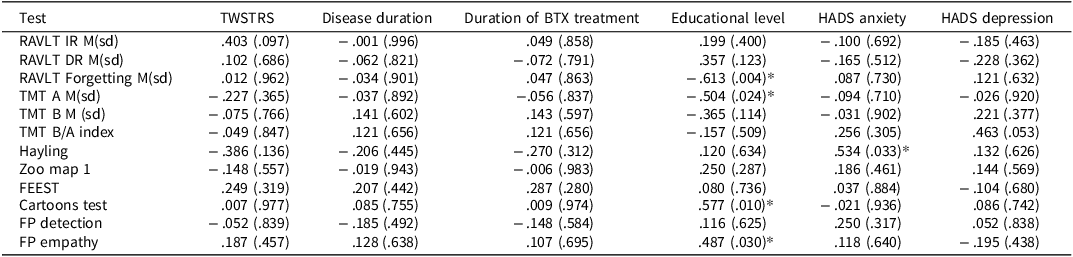
FEEST = Facial Expression of Emotion: Stimuli and Tests, FP = Faux Pas, RAVLT IR = Rey Auditory Verbal Learning Test Immediate Recall, RAVLT DR = Rey Auditory Verbal Learning Test Delayed Recall, TMT A = Trail Making Test condition A, TMT B = Trail Making Test condition B, TMT B/A = Trail Making Test condition B in relation to A.
Educational level: (1) primary school, (2) finished primary school, (3) unfinished secondary school or special education, (4) finished secondary school, (5) finished vocational training, (6) finished college education, and (7) university degree.
*Significant correlation.
Discussion
This is the first study in which a broad range of cognitive functions, both non-SC and SC, was investigated in one and the same group of idiopathic CD patients. We found worse performance on tests for non-SC (verbal learning and memory, psychomotor speed, and inhibitory control and planning as part of executive functioning) compared to HCs but no evidence for impaired SC in patients with CD. The cognitive impairments in our patient group were not related to disease duration, disease severity or BTX treatment.
The sample in the present study is comparable to previously studied samples concerning age, years of education and the distribution of men and women in the sample. Concerning the sample size, there are studies with larger samples (Barahona-Corrêa et al., Reference Barahona-Corrêa, Bugalho, Guimarães and Xavier2011; Burke et al., Reference Burke, Monaghan, McCormack, Cogley, Pinto-Grau, O'Connor, Donohoe, Murphy, O'Riordan, Ndukwe, Hutchinson, Pender and O'Keeffe2020; Monaghan et al., Reference Monaghan, Cogley, Burke, McCormack, O'Riordan, Ndukwe, Hutchinson, Pender and O'Keeffe2021), studies with comparable sample sizes (Czekóová et al., Reference Czekóová, Zemánková, Shaw and Bareš2017; Lagravinese et al., Reference Lagravinese, Santangelo, Bonassi, Cuoco, Marchese, Di Biasio, Erro, Pelosin and Avanzino2021; Nikolova et al., Reference Nikolova, Fellbrich, Born, Dengler and Schröder2011; Rinnerthaler et al., Reference Rinnerthaler, Benecke, Bartha, Entner, Poewe and Mueller2006), but also studies with smaller samples (Jahanshahi et al., Reference Jahanshahi, Rowe and Fuller2003; Scott et al., Reference Scott, Gregory, Wilson, Banks, Turner, Parkin, Giladi, Joint and Aziz2003). The results of the present study are in line with earlier research concerning memory and executive dysfunction in CD patients. A recent meta-analysis (Aita et al., Reference Aita, Del Bene, Marotta, Pizer, Hawley, Niccolai, Walker, Gerstenecker, Martin, Clay, Crowe, Triebel and Hill2022) has shown that memory disorders are frequently found in adult dystonia patients, including CD. Burke et al. (Reference Burke, Monaghan, McCormack, Cogley, Pinto-Grau, O'Connor, Donohoe, Murphy, O'Riordan, Ndukwe, Hutchinson, Pender and O'Keeffe2020) found a lower memory composite score of several memory tests in CD patients, but specifically for the RAVLT, which was also administered in our study, they found no difference between patients and controls. The authors argue that repetition of information in the RAVLT might aid patients in encoding this information. This is supported by their finding that patients were impaired on other (verbal and visual) memory tests which do not include repetition of information. In our study, we also included a score of the RAVLT of how many words were forgotten, in relation to the number of encoded words. Here, we saw no difference between patients and HCs. This means that CD patients can remember and retrieve encoded information. Apparently, patients with CD primarily have an encoding problem only, without disproportional forgetting of what was encoded. Hence, this encoding problem is not so much a memory deficit, but, given that the words are presented with a specific pace and that encoding may be enhanced by organizing the unrelated words, may be mainly attributed to the lower information processing speed and executive deficits for which we found evidence in this patient group. This would also be in line with the function of the cortico-striatal-thalamo-cortical networks.
The lower score on tests for psychomotor speed was in line with previous findings of Monaghan et al. (Reference Monaghan, Cogley, Burke, McCormack, O'Riordan, Ndukwe, Hutchinson, Pender and O'Keeffe2021). The TMT has a motor component, just as the tests used by Monaghan et al. (Reference Monaghan, Cogley, Burke, McCormack, O'Riordan, Ndukwe, Hutchinson, Pender and O'Keeffe2021). It is possible that motor symptoms have interfered with the TMT. Yet, the results were not significantly correlated to disease severity or disease duration. Additionally, executive deficits, especially deficits in inhibitory control and cognitive flexibility, have been described in earlier studies with CD patients (Aita et al., Reference Aita, Del Bene, Marotta, Pizer, Hawley, Niccolai, Walker, Gerstenecker, Martin, Clay, Crowe, Triebel and Hill2022; Bugalho et al., Reference Bugalho, Correa, Guimarães and Xavier2006; Foley et al., Reference Foley, Vinke, Limousin and Cipolotti2017; Scott et al., Reference Scott, Gregory, Wilson, Banks, Turner, Parkin, Giladi, Joint and Aziz2003). In line with these studies, our study shows that deficits in inhibitory control seem to be part of the CD phenotype. However, cognitive flexibility, another aspect of EF, was unimpaired in our study, which contradicts findings by Bugalho et al. (Reference Bugalho, Correa, Guimarães and Xavier2006) and Scott et al. (Reference Scott, Gregory, Wilson, Banks, Turner, Parkin, Giladi, Joint and Aziz2003). Bugalho et al. (Reference Bugalho, Correa, Guimarães and Xavier2006) measured cognitive flexibility with the Wisconsin Card Sorting Test (WCST;(Berg, Reference Berg1948)), and Scott et al. used a similar test from the Cambridge Neuropsychological Test Automated Battery (CANTAB). Though the WCST has been shown to correlate with the TMT (Kortte et al., Reference Kortte, Horner and Windham2002), the two tests approach cognitive flexibility at different levels of control, and one might argue that the WCST measures a more complex, strategic, level than the TMT (Spikman et al., Reference Spikman, Kiers, Deelman and Van Zomeren2001). This might explain the difference between results of the present study and those of Bugalho et al. (Reference Bugalho, Correa, Guimarães and Xavier2006); Foley et al. Reference Foley, Vinke, Limousin and Cipolotti(2017), also found impaired cognitive flexibility, but this was measured with the TMT B only. In the present study, patients’ performance on the TMT B was worse compared to HCs as well, but especially in patients with movement disorders it is important to correct the performance for slower motor performance. This can be done by using the TMT B/A score, as was done in the present study. Aita et al. (Reference Aita, Del Bene, Marotta, Pizer, Hawley, Niccolai, Walker, Gerstenecker, Martin, Clay, Crowe, Triebel and Hill2022) have linked executive deficits to basal ganglia dysfunctions. Other studies pointed more specifically to the striatum and the cortico-striatal-thalamo-cortical networks (Barahona-Corrêa et al., Reference Barahona-Corrêa, Bugalho, Guimarães and Xavier2011; Foley et al., Reference Foley, Vinke, Limousin and Cipolotti2017; Scott et al., Reference Scott, Gregory, Wilson, Banks, Turner, Parkin, Giladi, Joint and Aziz2003). So, with regard to non-SC, our results point to slower information processing speed and deficits in two aspects of executive functioning, namely inhibitory control and planning, as main characteristics, which may also (partly) determine lower encoding in memory, but the intact forgetting ratio shows that encoded information can be successfully retained in memory.
SC also, at least partly, depends on this same cortico-striatal-thalamo-cortical loop, but to our surprise we did not find support for our hypothesis that CD is associated with deficits in SC. This is not in line with the results from previous studies and the recent review by O’Connor et al. (Reference O’Connor, Hevey, Burke, Rafee, Pender and O’Keeffe2023). Previous studies point toward impaired SC in CD patients, but the results are conflicting at times and this aspect of cognition has not yet been investigated sufficiently in CD patients. Previous studies have investigated separate aspects of SC in CD patients, but never multiple aspects within one and the same study. Evidence has been found for impaired recognition of disgust in facial emotional expressions, but not for overall impairment in emotion recognition (Rinnerthaler et al., Reference Rinnerthaler, Benecke, Bartha, Entner, Poewe and Mueller2006), as well as impaired ToM (Czekóová et al., Reference Czekóová, Zemánková, Shaw and Bareš2017; Lagravinese et al., Reference Lagravinese, Santangelo, Bonassi, Cuoco, Marchese, Di Biasio, Erro, Pelosin and Avanzino2021). In the present study, we assessed multiple aspects of SC in the same group of CD patients. We did not find evidence for impairments in overall emotion recognition, nor for an impairment in specific emotions. This is largely in line with the findings of Rinnerthaler et al. (Reference Rinnerthaler, Benecke, Bartha, Entner, Poewe and Mueller2006) who also did not find overall emotion recognition to be impaired. However, they found lower scores for recognition of disgust in patients, but their group was not entirely comparable as it contained not only patients with CD but also with blepharospasm. This condition might explain lower scores on such a visual task because of an inability to keep the eyes open for a sufficiently long timespan to identify the stimulus.
We also did not find evidence for impairments in ToM tasks, which contradicted the findings of Czekóová et al. (Reference Czekóová, Zemánková, Shaw and Bareš2017). They used the same FP test that was used in the present study. However, their version was twice as long, so an effect of fatigue and/or deficits in sustained attention cannot be ruled out. Czekóová et al., also found that the understanding of the FP situation was impaired, while the detection was intact. In accordance with the manual of the FP test, detection of the FP was based on two questions in the present study (1) Did anyone say something they shouldn't have said or something awkward? (2) If yes: Who said something they shouldn't have said or something awkward?). Questions about understanding the inappropriateness, the intentions, and the belief were not included. It might be that these questions would also have been impaired in the present sample. However, the shortened version of the FP test, that has been used in the present study, has been shown sensitive enough to detect social cognitive deficits in other patients groups, such as patients with traumatic brain injury (Spikman et al., Reference Spikman, Timmerman, Milders, Veenstra and Van der Naalt2012) or subarachnoid hemorrhage (Buunk et al., Reference Buunk, Spikman, Veenstra, van Laar, Metzemaekers, van Dijk, Meiners and Groen2017). Lagravinese et al. (Reference Lagravinese, Santangelo, Bonassi, Cuoco, Marchese, Di Biasio, Erro, Pelosin and Avanzino2021) have confirmed the results by Czekóová et al. and added that CD patients with tremor have more pronounced SC deficits than CD patients without tremor. They used different SC tests compared to our study and related the results to scores on cognitive screenings (Mini Mental State Examination, MMSE, and Montreal Cognitive Assessment, MoCA). They found that in CD patients with tremor, the SC scores were related to executive functioning as measured by the MoCA. In the current study, non-SC (including executive functioning) was measured with neuropsychological tests instead of a screening instrument, and results showed (among others) worse processing speed and executive functioning compared to HCs. Based on our data, it seems that there is a clear dissociation between SC and processing speed and executive functioning.
Levels of symptoms of anxiety and depression were normal on a group level, but a quarter of our patients had mild symptoms of depression and 20% of our patients had mild to moderate levels of symptoms of anxiety. Also, 20% of our patients were treated with antidepressants and 30% used benzodiazepines. Especially this last group of drugs is known to decrease cognitive speed. Yet, when we repeated the analysis without patients who used benzodiazepines (n = 14), the results remained unchanged for non-SC as well as SC.
A strong point of our study is the use of a SVT. To our knowledge, other studies have not made use of these kinds of tests and excluding patients who scored below the cutoff, might partly explain differences between our results and results of previous studies. This test was included because the motor symptoms can be distracting and/or painful, which can prevent patients to perform at their best. In that case, patients would not pass the test and would consequently be excluded from the study. Not excluding those patients can lead to an inflation of found cognitive deficits. Yet, our results have to be viewed in the light of limitations. One limitation of our study is the relatively small sample size of CD patients. Based on the inclusion of ESs in our study however, we are confident that our findings are not due to lack of statistical power. The ESs for the differences between patients and HCs on tests of SC were small, which leaves it unlikely that a larger sample size might have resulted in a significant difference. Furthermore, we found moderate to large differences on the measures of non-SC, pointing to sufficient statistical power. Our patient sample had a slightly lower educational level than the HC group. Educational level was significantly correlated to the results or the TMT A. As correlation do not indicate causal relationships, this does not necessarily mean that the found difference between patients and controls is entirely due to differences in educational level. Rather, it might be that faster processing speed enables patients and controls to complete higher level of education. A factor that might influence the results is the time within the BTX treatment cycle. Previous studies have assessed patients prior to the injections, when dystonic symptoms are at peak, thus the results might have been confounded by discomfort caused by the motor symptoms. Yet, like in our own study, there were no significant correlations between SC measures and motor symptoms in these previous studies. Allam et al. (Reference Allam, Frank, Pereira and Tomaz2007) found evidence for a slight improvement in sustained attention in focal dystonia patients after BTX treatment. This might account for the lower scores on the FP test in the study by Czekóová et al. (Reference Czekóová, Zemánková, Shaw and Bareš2017), but neither we, nor previous studies report measures of sustained attention in addition to SC measures. Future studies should consider this point in the study design.
In conclusion, we confirmed that idiopathic CD is not solely a movement disorder, with deficits in different aspects of non-SC, mainly psychomotor speed, encoding in memory and two executive subfunctions, inhibitory control and planning in patients with idiopathic CD compared to HCs. A strong point in the current research design is the assessment of the three major aspects of SC in the same patients. With this setup, however, we did not find evidence of affected SC, so it seems that the cognitive profile of patients with idiopathic CD is mainly characterized by impairments in non-SC.
Acknowledgments
The authors of this publication are members of the European Reference Network for Rare Neurological Diseases – Project ID no 739510.
Funding statement
None.
Competing interests
None.






