INTRODUCTION
Impaired self-awareness, known as anosognosia or unawareness, is a disorder in which patients with brain damage fail to recognize the severity of deficits in motor, sensory, and cognition function (Hartman-Maier, Soroker, Ring & Katz, Reference Hartman-Maier, Soroker, Ring and Katz2002; Orfei et al., Reference Orfei, Robinson, Prigatano, Starkstein, Rüsch and Bria2007; Pia et al., Reference Pia, Spinazzola, Rabuffetti, Ferrarin, Garbarini and Piedimonte2013; Prigatano, Reference Prigatano2005). Unawareness has been extensively studied in patients with traumatic brain injury (Fischer, Gauggel & Trexler, Reference Fischer, Gauggel and Trexler2004; Prigatano, Reference Prigatano1996; Sherer, Hart & Nick, Reference Sherer, Hart and Nick2003), and its scope has been extended to the stroke population. (Cocchini, Beschin & Della Sala, Reference Cocchini, Beschin and Della Sala2018; D’Imperio, Bulgarelli, Bertagnoli, Avesani & Moro, Reference D’Imperio, Bulgarelli, Bertagnoli, Avesani and Moro2017; Fotopoulou, Pernigo, Maeda, Rudd & Kopelman, Reference Fotopoulou, Pernigo, Maeda, Rudd and Kopelman2010; Hartman-Maeir, Soroker & Katz, Reference Hartman-Maeir, Soroker and Katz2001; Jenkinson, Preston & Ellis, Reference Jenkinson, Preston and Ellis2011; Pia, Neppi-Modona, Ricci & Berti, Reference Pia, Neppi-Modona, Ricci and Berti2004; Spinazzola, Pia, Folegatti, Marchetti & Berti, Reference Spinazzola, Pia, Folegatti, Marchetti and Berti2008). It is commonly reported that the frequency and severity of unawareness are higher in patients with right hemisphere damage (RHD) than with left hemisphere damage (LHD) (Nurmi Laihosalo & Jehkonen, Reference Nurmi Laihosalo and Jehkonen2014; Orfei et al., 1973; Pia et al., Reference Pia, Neppi-Modona, Ricci and Berti2004). Additionally, the occurrence of unawareness is more frequent in the acute state (Starkstein, Jorge & Robinson, Reference Starkstein, Jorge and Robinson2010). However, a significantly higher percentage (40%) of patients with LHD show unawareness when the appropriate assessment tool is used (Cocchini, Beschin, Cameron, Fotopoulou & Della Sala, Reference Cocchini, Beschin, Cameron, Fotopoulou and Della Sala2009), and the unawareness remains beyond the acute phase (Jehkonen, Laihosalo & Kettunen, Reference Jehkonen, Laihosalo and Kettunen2006; Orfei et al., 1973). Because unawareness is multi-faceted, we need to explicitly or implicitly assess subtypes of unawareness using various measurement methods in the multiple domains (Cocchini, Beschin, Fotopoulou & Della Sala, Reference Cocchini, Beschin, Fotopoulou and Della Sala2010; Marcel, Tegnér & Nimmo-Smith, Reference Marcel, Tegnér and Nimmo-Smith2004), especially for the patients in the chronic stroke stage.
Here, we extend our understanding of unawareness by exploring the differences between chronic patients with RHD and LHD in evaluating task performance based on video recordings. Recent studies have shown that the evaluation of self-awareness is more accurate when using a video recording, in which the participants evaluate the movement after executing the actual task or answer questions from the third-person perspective (Fotopoulou, Rudd, Holmes & Kopelman, Reference Fotopoulou, Rudd, Holmes and Kopelman2009; Marcel et al., Reference Marcel, Tegnér and Nimmo-Smith2004). However, these studies have reported the effectiveness of video feedback on a single case of stroke or patients in acute settings; research with a larger group of patients with chronic stroke to assess unawareness via video-based self-observation is needed.
The frequency of spontaneous use of the more-affected limb during daily activities is higher in the patients with LHD than those with RHD, especially for individuals with a premorbid dominant right hand (Haaland et al., Reference Haaland, Mutha, Rinehart, Daniels, Cushnyr and Adair2012; Kim, Park, Han, Winstein & Schweighofer, Reference Kim, Park, Han, Winstein and Schweighofer2018; Mani, Przybyla, Good, Haaland & Sainburg, Reference Mani, Przybyla, Good, Haaland and Sainburg2014). This increased arm use can be viewed as a ’self-training' and leads to motor performance enhancement (Hidaka, Han, Wolf, Winstein & Schweighofer, Reference Hidaka, Han, Wolf, Winstein and Schweighofer2012). We hypothesized that increased arm use can also influence self-awareness due to the positive or negative experiences using the more-affected arm over time (Taub, Uswatte & Elbert, Reference Taub, Uswatte and Elbert2002). For example, patients with LHD who often use the more-affected arm may be more likely to underestimate their performance if they have more unsuccessful experiences. One recent study showed that patients with LHD following acute-to-subacute stroke tend to underestimate their ability during upper extremity movement (Fowler, Della Sala, Hart & McIntosh, Reference Fowler, Della Sala, Hart and McIntosh2018). In contrast, the patients with RHD tend to overestimate (Fowler et al., 1973), and a similar trend is found in both stroke (Marcel et al., Reference Marcel, Tegnér and Nimmo-Smith2004) and traumatic brain injury populations (Mizuno, Reference Mizuno1991; Prigatano, Reference Prigatano1996). The association between spontaneous use of the more-affected arm and self-evaluation based on video recording in patients with chronic stroke could provide further understanding of the nature of unawareness.
This study aimed to investigate whether patients with LHD and RHD following stroke showed a distinct capability for self-awareness of their upper extremity (UE) movement if they watched a video of their movement. Additionally, we sought to understand whether self-evaluation could be corrected in patients with chronic stroke if the clinician provided feedback. Finally, the relationships between unawareness and spontaneous use and between unawareness and impairment were explored. We hypothesized that there is a difference in self and clinical evaluation in patients with hemiparesis following stroke and that patients with LHD or RHD under- or overestimate the more-affected arm movement, respectively.
METHOD
Participants
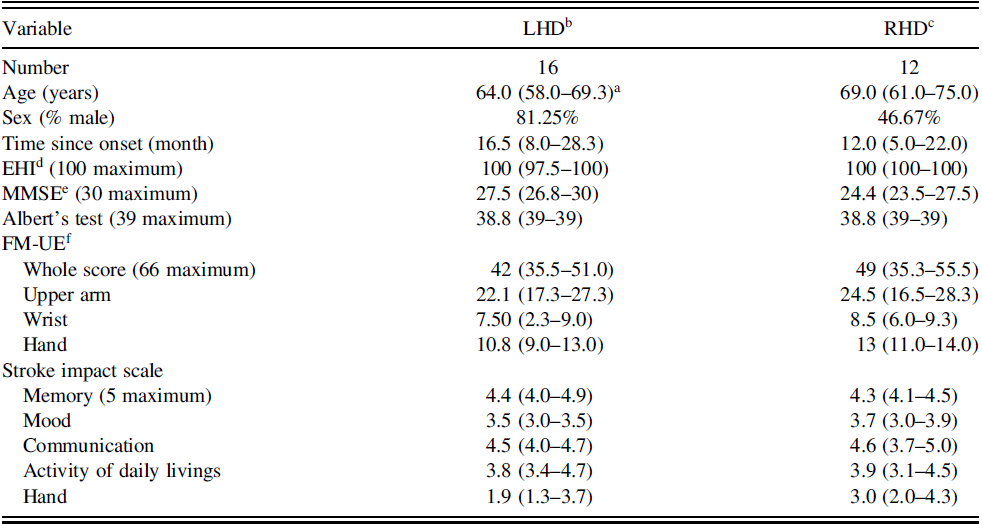
Twenty-eight patients with unilateral stroke (LHD, n = 16; RHD, n = 12) participated in this study. Participants with stroke were recruited from seven rehabilitation centers in South Korea. All participants had traditional physical and occupational therapy, on average, 40 min each, and they did not participate in other research interventions. The inclusion criteria were: (1) clinical diagnosis of a first or recurrent unilateral ischemic or hemorrhagic stroke in the chronic stage (at least 3 months after stroke onset) (Nakase, Yoshioka & Suzuki, Reference Nakase, Yoshioka and Suzuki2011); (2) age ≥21 years and impaired UE motor function, as indicated by the Fugl-Meyer upper extremity scale (FM-UE, ≥19 out of 66); (3) no unilateral sensory and motor neglect, as determined by line cancelation in the Albert test (Albert, Reference Albert1973; Blumenfeld, Reference Blumenfeld2002); (4) understanding and following the instructions of the experimenter (Fowler et al., Reference Fowler, Della Sala, Hart and McIntosh2018). Patients with a history of recent surgeries, pain, or orthopedic injuries affecting the movement of UE, or with severe cognitive deficits (MMSE < 18) (Lee, Cheong, Oh & Hong, Reference Lee, Cheong, Oh and Hong2009), communication problem (SIS – communication subscale <3.5), and severe spasticity were excluded. All participants with stroke were right-handed before the stroke, as measured using the Edinburgh Handedness Inventory (EHI). Their memory, mood, communication, and activities of daily livings were measured using the Stroke Impact Scale (SIS) (Duncan, Bode, Lai & Perera, Reference Duncan, Bode, Lai and Perera2003). The Ethics Committee of Jeonju University approved the study protocol (jjIRB-171115-HR-2017-1109), and all participants provided written informed consent for participation. They also signed an agreement to permit videotaping for the entire experiment. This study was conducted in accordance with the Declaration of Helsinki.
Table 1 summarizes the general characteristics of the participants with stroke. All participants with stroke were in either early chronic phase (3–6 months, four participants in each group) or late chronic phase (>6 months), and they had mild-to-moderate impairment on their UE (median score of FM-UE: 42 vs. 49 for LHD and RHD, respectively). There were no significant differences in age (t = .112, p = .912), cognitive function (Mini-Mental State Examination, W = 97, p = .160), time since stroke onset (W = 83, p = .551), degree of hand preference (EHI, W = 54, p = .120), and visuomotor neglect between the LHD and RHD groups (Albert’s test, W = 67.5, p = .722). In addition, the two groups did not differ in the physical impairments measured by the arm, wrist, and hand sub-items of FM (all variables, p > .05). Memory, mood, communication, ADLs, and hand function (measured by SIS) did not differ between the groups (all variables, p > .05).
Table 1. General characteristics of the participants (N = 28)
a Median (interquartile).
b LHD, Left Hemisphere Damage.
c RHD, Right Hemisphere Damage.
d EHI, Edinburgh Handedness Inventory.
e MMSE, Mini-Mental State Exam.
f FM-UE, Fugl-Meyer Upper Extremity scale.
None of difference between the RHD and LHD groups were found in the above variables.
Clinical Measurements
Actual amount of use test assessing the self-evaluation error
The actual amount of use test (AAUT) was developed by Taub et al. to measure an individual’s spontaneous use of the more-affected limb during ADL, for example, opening a file folder, picking up a photo album, or turning the pages of a photo album (Taub & Uswatte, Reference Taub, Uswatte, Frank and Elliott2004; Uswatte & Taub, Reference Uswatte, Taub, Struss, Winocur and Robertson1999). The AAUT contains 17 items; however, we only used the first 14 items because the last three items are related to general, unpurposeful movements, for example, gesturing or posture (Han et al., Reference Han, Kim, Chen, Lai, Lee and Osu2013; Kim et al., Reference Kim, Park, Han, Winstein and Schweighofer2018). We conducted AAUT in two ways: (1) a spontaneous use condition (sAAUT), in which the participants did not notice that they were undertaking the AAUT. We used a customized scenario that prompted the use of the more-affected arm within our experimental environment; therefore, the participant naturally chose this arm to complete the tasks; (2) a forced use condition (fAAUT), in which participants were asked to use their more-affected arm (Han et al., 1973; Sterr, Freivogel & Schmalohr, Reference Sterr, Freivogel and Schmalohr2002). A video camera recorded the entire experimental process, and the recording was used to score the AAUT.
Since participants with stroke noticed the AAUT test after the forced use condition, they performed sAAUT only once before the fAAUT on the first visit day to prevent a behavioral bias (Figure 1). Next, patients performed the fAAUT twice repeatedly across testing days (fAAUT1, test; fAAUT2, retest). The fAAUT scoring was completed by patients and a well-trained and standardized clinician. The sAAUT scoring was performed by a clinician. The quality of movement (QOM) scale was used to evaluate movements, ranging from 0 to 5, with 0 = participants unable to move the more-affected arm to complete the activity, and 5 = normal performance as before stroke. Scoring included 0.5 units, for example, 0.5, 1.5, 2.5, 3.5, and 4.5. for a more specific evaluation.
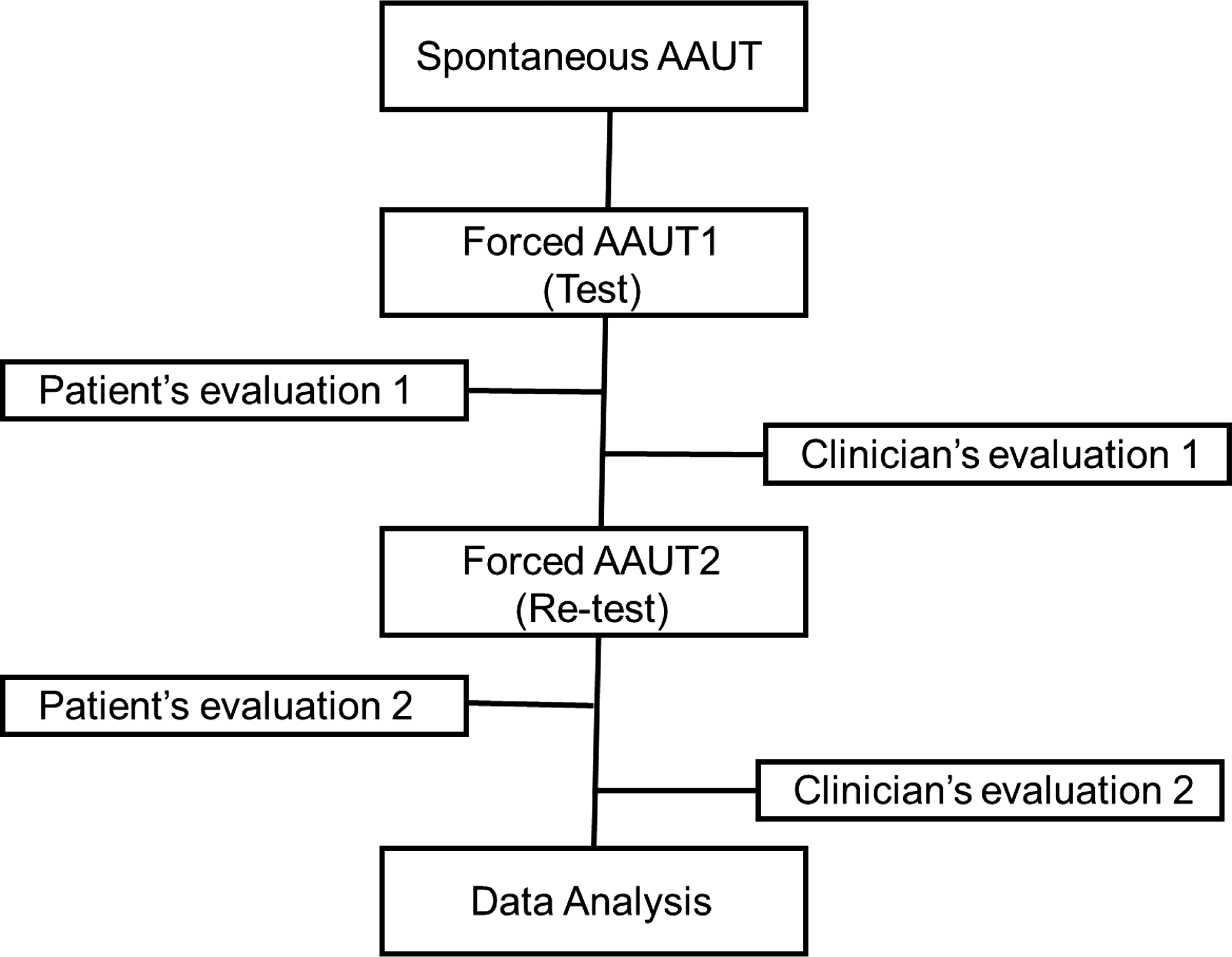
Fig. 1. Experimental procedure.
The difference between a patient’s self-evaluation and the clinician’s evaluation of the fAAUT was defined as the self-evaluation error. This had a potential range of −5 to +5, with zero representing total agreement, and positive and negative scores representing overestimation and underestimation of the more-affected arm when performing tasks, respectively. Cut-off scores of +0.22 and −0.22, calculated by the mean discrepancy between the fAAUT1 and fAAUT2 of the clinician’s scores plus or minus two standard deviations, were used to determine the range for correct estimation. This cut-off allowed for a small proportion of evaluation errors that would occur in the self-awareness judgments of a human being (Della Sala, Cocchini, Beschin & Cameron, Reference Della Sala, Cocchini, Beschin and Cameron2009). A mean self-evaluation error greater or smaller than these cut-off scores indicated an over- or underestimation of UE performance, respectively (Fowler et al., Reference Fowler, Della Sala, Hart and McIntosh2018). The mean score between the cut-off scores was defined as the correct estimation.
Impairment and use of the more-affected arm
The FM-UE was developed to measure the severity of a patient’s disability and the outcomes of medical rehabilitation. The maximum FM score was 66, indicating a normal motor ability (Fugl-Meyer, Jääskö, Leyman, Olsson & Steglind, Reference Fugl-Meyer, Jääskö, Leyman, Olsson and Steglind1975). FM showed the excellent intra- and inter-rater reliability (ICC > .98) (See et al., Reference See, Dodakian, Chou, Chan, McKenzie, Reinkensmeyer and Cramer2013). The Actual spontaneous use of the more-affected arm was measured using sAAUT described above. A trained evaluator scored how well the subjects used their more-affected arm and hand by watching the videotape. Lower and higher scores indicated less and more use of the more-affected arm, respectively.
Procedure
Participants with stroke underwent two days of experimental evaluation with a one-week interval between evaluations to prevent any AAUT learning effect. On the first day, the experimenter explained the purpose of the study and obtained informed consent before the experiment started. First, the participants explicitly underwent FM-UE and SIS tests, and each item in the AAUT was naturally mixed with these tests via a customized scenario. Therefore, participants were unaware of the sAAUT. Immediately after sAAUT, the participants were instructed to repeat the same activity (fAAUT1, test). A trained and standardized evaluator graded the actual movement by watching the video (clinician’s evaluation 1; Figure 1).
On the second day, participants viewed their actual movement via video recordings and assigned their QOM scores (patient’s self-evaluation 1). Immediately after this, the participants were informed of the clinician’s score so that they could compare the scores. Next, the participants performed the fAAUT (fAAUT2, retest) and were instructed to rate their actual performance (patient’s self-evaluation 2). Once the participants had completed their self-evaluation, the clinician scored the second fAAUT (clinician’s evaluation 2). Self-evaluation errors 1 and 2 were calculated as the patient’s self-evaluation 1 or 2 minus the clinician’s evaluation 1 or 2, respectively.
Statistical Analysis
The Shapiro-Wilk test was used to assess the normal distribution of all data. A one-sample t-test was used to identify the self-evaluation error and a paired t-test was used to detect test-retest differences in the evaluation of patients, clinicians, and self-evaluation errors. A linear mixed-effects analysis with categorical variables was used to assess the differences in self-evaluation between tests (first and second evaluation) and groups (LHD and RHD). As fixed effects, we entered the test and group into the model and set each subject as a random effect. First, we started with the model with a random intercept and sequentially extended models by adding groups, tests, and the interaction between groups and tests. Log-likelihood ratio tests (if the models were nested) or Akaike Inclusion Criterion (AIC; if the models were not nested) were used for model comparison. Next, a visual inspection of residuals versus fits plots and Q-Q plots was performed to check the residuals for normality and the presence of outliers (Lang et al., Reference Lang, Strube, Bland, Waddell, Cherry-Allen and Nudo2016; Varghese & Winstein, Reference Varghese and Winstein2020). There were no severe outliers, and departures from normality were mild. In addition, effect size was computed using partial omega square, with 0.01, 0.06, and 0.14 considered as small, medium, and large effect sizes, respectively. The chi-squared test was used to detect within-group differences in over-, under-, and correct estimations. Associations between the self-evaluation errors and other characteristic variables, including age, stroke onset, emotional control, MMSE score, and FM-UE score, were examined using Pearson or Spearman’s rank correlation coefficients, depending on the data distribution. Statistical significance was set at .05, and all results were reported as mean ± standard deviation (SD) or median. All statistical analyses were performed using the statistical package version 3.6.2 (Team R Development Core, 2018).
RESULTS
Patients and Clinicians Differently Evaluate Upper Extremity Movement
The QOM scores of fAAUT during test and retest for both patients and clinicians are shown in Table 2. The patient’s self-evaluation and clinician’s evaluation did not significantly differ between test to retest in both the LHD group (paired t-test, t = −.627, p = .540; t = −1.441, p = .170, for patient’s and clinician’s evaluation, respectively) and RHD group (t = −1.229, p = .245; t = .457, p = .656, for patient and clinician’s evaluation, respectively). Additionally, there was no difference in self-evaluation error between test to re-test in both groups, even after performance feedback was provided (paired t-test, t = .072, p = .943; t = −1.403, p = .188 for LHD and RHD, respectively). The one-sample t-test revealed that the self-evaluation errors were not significantly different from zero in the RHD group (t = −.413, p = .688; t = .822, p = .428 for test and retest, respectively). In contrast, the self-evaluation errors were significantly lower than zero during test (t = −2.728, p = .016) and retest (t = −3.988, p = .001) in the LHD group.
Table 2. Scores of Patient and clinician, and self-evaluation errors in LHD and RHD groups

a Mean ± SD.
* p < .05.
*** p < .001 by one-sample t-test to confirm that evaluation gap was different from zero.
The Influence of Stroke Side on Self-evaluation Error
Next, we conducted a linear mixed model analysis with group (LHD and RHD) and tests (test and re-test) as the fixed factors and each individual as a random factor to analyze the effect of group and clinician feedback on self-evaluation error. The best-fit mixed-effects model included the effect of group and random intercept of patients because the model fit improved by adding group compared with the random intercept of patients alone. (AIC = 131.05 vs. 126.35, Log-likelihood test, χ2 = 6703, p = .009). Adding tests and the interaction between group and test did not improve the model fit. The best-fit model revealed that participants in the LHD group had a lower self-evaluation error in test and retest, indicating, on average, they underestimated their performance more than patients in the RHD group (t = 2.350, p = .019, partial ω2 = .18) (Figure 2a). Furthermore, we analyzed the frequencies of correct, under-, and overestimation scores in each group across tests. The chi-square test revealed that the number of patients who underestimated their performance was significantly greater in the LHD group than in the RHD group at retest (χ2 = 9.049, p = .003), and marginally greater at test (χ2 = 3.114, p = .07) (Figure 2b; overall, more than 80% of the LHD group underestimated their performance throughout test and retest). On the contrary, patients with RHD tended to overestimate, especially during retest (χ2 = 7.428, p = .006).
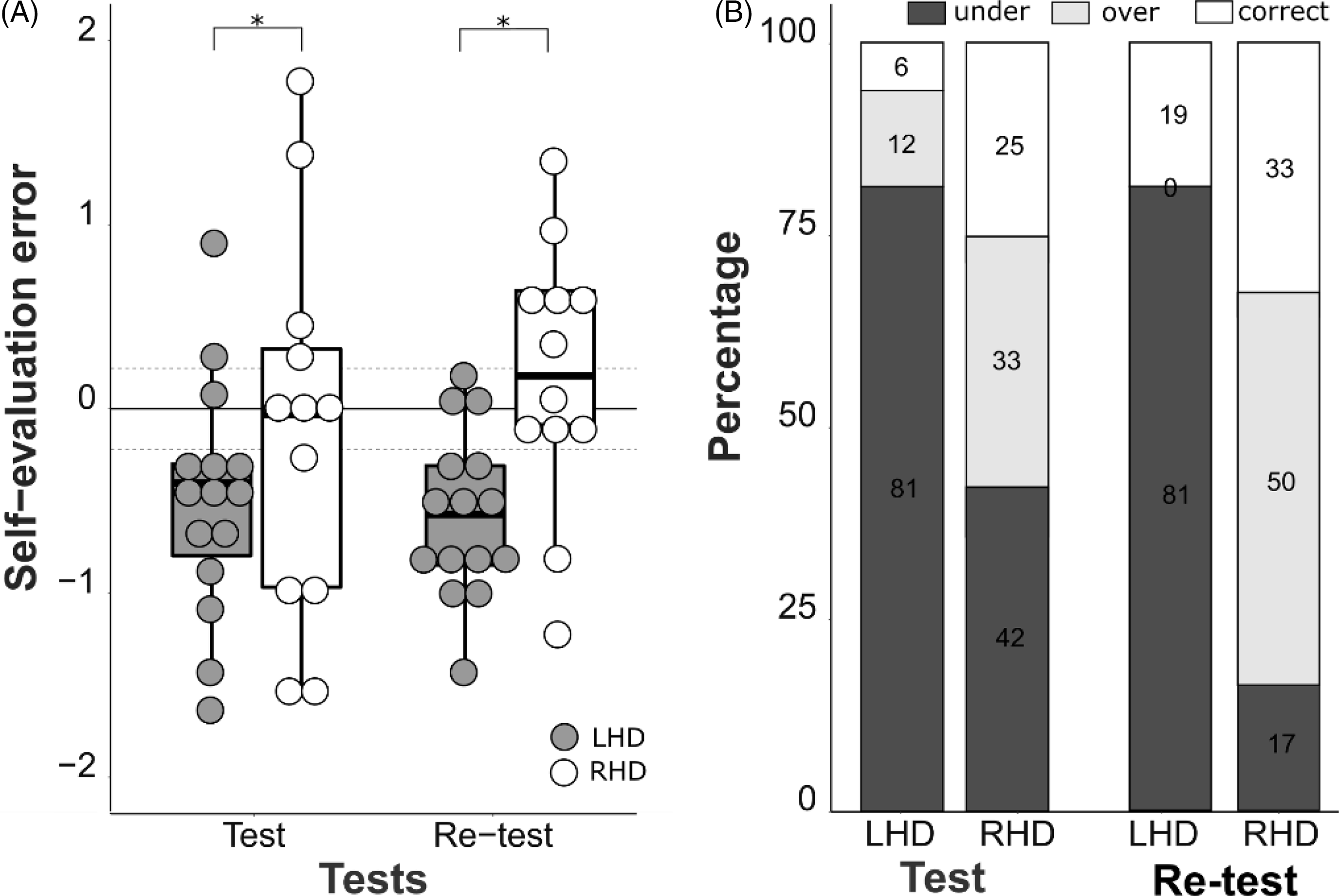
Fig. 2. The self-evaluation errors between LHD and RHD groups during test and retest 2. (A) Self-assessment error in the patients with LHD was lower than in the patients with RHD (mixed-effect model, t = 2.350, p = .019). They consistently underestimated performance, while patients with RHD did not. Self-evaluation errors did not change across tests for both groups (t = .899, p = .368). (B) Proportion of under-, correct, and overestimation in patients with RHD and LHD. The number of patients underestimated their performance was significantly greater in the LHD group than in the RHD group (chi-square test, χ2 = 3.114, p = .07 and χ2 = 9.049, p = .003 for test and re-test, respectively). Overall, more than 80% of the patients with LHD underestimated while the patients with RHD evenly performed under-, over-, and correct estimations. LHD: left hemisphere damaged group, RHD: right hemisphere damaged group.

Fig. 3. Correlation plots of self-evaluation error and impairment (A) and use (B). Patients with LHD and RHD did not show an association between self-evaluation error and impairment, as well as between self-evaluation error and spontaneous use of the more-affected arm.
Relationship Between Self-evaluation Error and Impairments and Spontaneous Affected Limb Use
Overall FM score was not significantly correlated with self-evaluation error in either group (r = −.022, p = .935 for LHD and ρ = .047, p = .884 for RHD) (Figure 3a). Nor of arm, wrist, and hand sub-items of FM were correlated with self-evaluation error (all p > .05). Additionally, the use of the more-affected arm was not significantly correlated with self-evaluation error (LHD, r = −.265, p = .320 RHD, r = −.067, p = .835) (Figure 3b). These results indicated that self-evaluation errors occurred in patients with chronic stroke regardless of motor impairment score and more-affected arm use during ADL. Exploratory analyses found no correlations between self-evaluation errors and age, onset months poststroke, or MMSE scores in either group.
DISCUSSION
This study found hemispheric differences in self-evaluation errors based on video recordings among chronic stroke survivors. Video-based self-observation has several benefits in evaluating performance. For example, individuals have a more generalized awareness of their movement from the third-person perspective (Besharati, Kopelman, Avesani, Moro & Fotopoulou, Reference Besharati, Kopelman, Avesani, Moro and Fotopoulou2015), and ‘offline’ playback can be paused easily frame by frame for participants to evaluate carefully over a relatively long period, compared with the ‘online’ awareness interviews or questionnaires (Besharati et al., Reference Besharati, Kopelman, Avesani, Moro and Fotopoulou2015; Fotopoulou et al., Reference Fotopoulou, Rudd, Holmes and Kopelman2009). In addition, participants might be more comfortable evaluating past poor performance ‘offline’, which may prevent a reduction in self-esteem. (Marcel et al., Reference Marcel, Tegnér and Nimmo-Smith2004). With the video-based feedback, we found that chronic stroke patients with LHD significantly underestimated their performance, while patients with RHD under-, over-, or correctly estimated their movement performance. These findings are in line with previous research showing the underestimation of LHD (Fowler et al., Reference Fowler, Della Sala, Hart and McIntosh2018), and the overestimation of RHD during the bimanual UE tasks in acute-to-subacute phases (Marcel et al., Reference Marcel, Tegnér and Nimmo-Smith2004).
One possible explanation for the hemispheric differences in self-evaluation is the frequency of use of the more-affected arm. For example, patients with LHD tended to use their impaired right arm more than patients with RHD who used their impaired left arm, because right-hand preferences exist even after stroke (Mani et al., Reference Mani, Przybyla, Good, Haaland and Sainburg2014). Consequently, patients with LHD experienced more errors or unsuccessful movements than those with RHD. In contrast, patients with RHD experienced fewer errors or unsuccessful movements because they did not attempt to move their more-affected left arm (nondominant arm). Repeated negative consequences, such as spilling hot coffee or dropping dishes when using a more-affected arm, might cause disappointment in patients with stroke (Taub et al., Reference Taub, Uswatte and Elbert2002; Uswatte & Taub, Reference Uswatte, Taub, Struss, Winocur and Robertson1999), which may lead to performance underestimation. However, patients with RHD use their intact dominant right arm approximately four times more frequently than move their more-affected left arm (Haaland et al., Reference Haaland, Mutha, Rinehart, Daniels, Cushnyr and Adair2012). Therefore, they might not have experienced enough movement attempts to determine their performance levels. This association between frequency of movement and unawareness is also observed during bipedal actions when patients in the acute phase have fewer opportunities to attempt bipedal tasks in the hospital (Marcel et al., Reference Marcel, Tegnér and Nimmo-Smith2004).
Another hypothesis to explain self-evaluation errors is the general superiority of the right hand for motor skills, while the left hand is regarded as the weaker counterpart. The ADL tasks that we used were bimanual tasks requiring equal contribution from both hands (Han et al., 1973; Kim et al., Reference Kim, Shin, Lim, Jung, Oh and Kim2019); however, each hand had a specific role in the tasks. For example, holding a paper with the left hand and writing a pen with the right hand. Typically, skilled movements are accurately performed using the dominant right arm (Haaland & Harrington, Reference Haaland and Harrington1996; Sainburg, Reference Sainburg2002). Consistent with the Dunning-Kruger’ effect, such that overestimation is observed in the least skilled arm and hand, and vice versa (Mahmood, Reference Mahmood2016; Schlösser, Dunning, Johnson & Kruger, Reference Schlösser, Dunning, Johnson and Kruger2013), patients with LHD who have better motor skills in their dominant right hand are more likely to underestimate their performance. In addition, they might not be satisfied with their performance when compared to their abilities before stroke. Consequently, a performance underestimation will occur.
Additionally, under- and overestimation in patients with LHD or RHD are associated with hemispheric asymmetry in the regulation of emotions and attention. The right hemisphere is more likely to process emotional material than the left hemisphere (Wager, Phan, Liberzon & Taylor, Reference Wager, Phan, Liberzon and Taylor2003). Patients with RHD often show alexithymia, which is an impairment of decoding, expressing, and awareness of feelings, and an attention deficit (Carson et al., Reference Carson, MacHale, Allen, Lawrie, Dennis, House and Sharpe2000; Spalletta, Ripa, Bria, Caltagirone & Robinson, Reference Spalletta, Ripa, Bria, Caltagirone and Robinson2006). Thus, the patients with RHD in our study might be less concerned about their errors or unsuccessful use of the more-affected arm. In contrast, patients with LHD express catastrophic reactions to their disease and are often in a low mood (Carson et al., 1973; Fowler et al., Reference Fowler, Della Sala, Hart and McIntosh2018); therefore, they may have more concerns about their errors and unsuccessful trials made by the more-affected arm in this study. Interestingly, there was no difference in self-evaluation error even after accurate feedback from clinicians (self-evaluation error at test: −0.74 ± 1.09, self-evaluation error at retest: −0.76 ± 0.76, p > .05 in LHD, and self-evaluation error at test: −0.13 ± 1.09, self-evaluation error at retest: 0.18 ± 0.76, p > .05 in RHD). Our participants obtained the clinician’s evaluation before they performed the fAAUT on the second day. However, this one-time feedback was not strong enough to change the participants’ self-evaluations. Evaluation of the AAUT with 14 different ADLs using the QOM scale was too complex for our participants; therefore, there was a possibility that the patients did not fully understand or accept the one-time feedback from the clinician. Therefore, continuous and repeated reporting of actual scores may be necessary to reduce self-evaluation errors and improve movement self-awareness.
Self-evaluation errors were not correlated with FM-UE or sAAUT scores. Previous studies have shown that actual motor ability is associated with self-evaluation; patients with more severe impairments tend to overestimate their movement and vice versa in acute-to-subacute stroke (Fowler et al., Reference Fowler, Della Sala, Hart and McIntosh2018). However, our patients exhibited chronic stroke impairments and ADL tasks in the AAUT focused on bimanual upper extremity movements rather than general gross movements. Therefore, over two-thirds of our participants the LHD group underestimated their movement, leading to no correlation between self-evaluation errors and impairment.
Furthermore, we did not find a significant relationship between self-evaluation error and the actual use of the more-affected arm. This might indicate that correct or overestimation of movements does not necessarily guarantee more use of the more-affected arm. Interestingly, use of the more-affected arm in sAAUT was similar between groups (1.60 ± 1.15, 1.60±.97 ± for LHD and RHD groups, respectively), while self-evaluation was not. Because AAUT consists of bimanual tasks and is typically tested in a laboratory setting over a relatively short period, arm use might not be fully measured. Other methods, such as an accelerometer, to assess the frequency of use of the more-affected arm over a longer time, may be necessary to monitor movements in the real world and fully elucidate the association between self-estimation error and spontaneous arm use.
This study had several limitations. First, we did not directly use patients’ brain imaging data; therefore, we did not know the exact lesion size and location, such as assessing lesions in the anterior and medial prefrontal cortex or medial temporal lobe, which control self-awareness (Berti et al., Reference Berti, Bottini, Gandola, Pia, Smania and Stracciari2005; Stuss, Reference Stuss, Prigatano and Schacter1991). Second, we excluded patients with severe motor and cognitive impairments and language and neglect dysfunctions. In addition, three participants in our study experienced recurrent strokes. Because we tried to distinguish the difference between RHD and LHD groups in over-and underestimation of performance, the participants had to have some motor function on their upper extremity (UE). Therefore, we recruited poststroke survivors with mild-to-moderate impairment and made a homogenous group.
Finally, all participants reported being right-handed before stroke onset; therefore, our results cannot be generalized to patients who are premorbidly left-handed. Magnetic resonance imaging showing the location of lesions in patients with LHD and RHD should be obtained and voxel-based lesion-symptom mapping between neural substrates and self-evaluation error should be performed in future studies. In addition, patients who are left-handed before stroke onset should be included.
From a clinical perspective, we argue that therapists should be aware of the possibility of patient self-evaluation errors. When patients underestimate their movements, therapists should provide verbal encouragement to let them know that their movements in the more-affected limb are sufficient for executing ADL. In these instances, video recording can be helpful in providing more objective information (Marcel et al., Reference Marcel, Tegnér and Nimmo-Smith2004). In addition, patients who overestimate their movements should be supported to notice their limitations so that they can successfully protect themselves from unexpected dangerous situations, including spilling hot coffee or dropping fragile dishes. However, it often upsets patients to see themselves fail while performing a task on a videotape; therefore, a therapeutic alliance should be created with patients before therapy to overcome self-evaluation errors (Prigatano, Reference Prigatano2005).
CONCLUSION
This study investigated the self-evaluation errors in more-affected arm movements between patients with chronic stroke hemiparesis and clinicians when performing bimanual ADL. Self-evaluation errors existed, but were not correlated with age, FM-UE score, ability to control emotions, or onset months poststroke. Furthermore, the LHD and RHD groups differed in their self-evaluation tendency; patients with LHD underestimated their upper extremity movements, while patients with RHD did not show consistent self-evaluation errors. Taken together, therapists are required to recognize the impaired self-awareness in patients. Furthermore, they should develop personalized rehabilitation strategies to overcome any unawareness with respect to the brain lesion side. Using this therapeutic approach, patients can be guided to achieve better results and faster progress in motor re-learning.
AUTHOR CONTRIBUTIONS
S.C and C.S.K wrote the paper; S.C and C.S.K designed the research; S.C. performed the experiments; S.C, C.S.K, J.W., and K.P analyzed the data; J.W. and K.P edited the paper.
FINANCIAL SUPPORT
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2017R1C1B5076731).
CONFLICTS OF INTEREST
The authors have nothing to disclose.







