Introduction
Colorectal cancer (CRC) is the third most common cancer after lung cancer and breast cancer prevalent in both sexes worldwide with over 1·3 million new cases and 693,933 deaths estimated to have occurred in 2012 Reference Ferlay, Soerjomataram and Ervik1 compared to 1·8 million new cases and 881,000 deaths in 2018. Reference Bray, Ferlay, Soerjomataram, Siegel, Torre and Jemal2 Data from western-industrialised countries and global cancer statistics have shown that approximately one-third of CRCs cases occur in the rectum. Reference Bray, Ferlay, Soerjomataram, Siegel, Torre and Jemal2–Reference Tan, Lim Joon and Fitt4 As the risk of local recurrence is a key issue in rectal cancer, a multidisciplinary approach involving surgery, radiotherapy and chemotherapy is the mainstay of the management process. Reference McCarthy, Pearson, Fulton and Hewitt5,Reference Brown, Solomon, Mahon and O’Shannassy6 Radiotherapy a non-invasive method, combined with chemotherapy, that is, preoperative chemoradiotherapy (PCRT) has been the standard treatment of choice for resectable and/or locally advanced rectal cancers (LARC). Reference Rahbari, Elbers, Askoxylakis, Motschall, Bork and Büchler7,8 This approach has been reported to improve tumour downstaging which may allow tumour resectability, sphincter conservation Reference Rahbari, Elbers, Askoxylakis, Motschall, Bork and Büchler7 or non-operative management. Reference O’Neill, Brown, Heald, Cunningham and Tait9 Several randomised trials have demonstrated improved 5-year locoregional control rates of 91·9–94%. Reference Sauer, Becker, Hohenberger, Rödel, Wittekind and Fietkau10,Reference Gérard, Conroy and Bonnetain11
Radiotherapy generally aims to deliver prescribed radiation dose to target tumour volume (TV) while sparing adjacent normal surrounding tissues. Imaging in radiotherapy plays a crucial role for tumour localisation and target volume delineation (TVD) for radiotherapy treatment planning (RTP), verification of patient positioning for reproducibility and more recently for high dose delivery. Reference Pereira, Traughber and Muzic12 Currently, the mainstay of radiotherapy delivery technique for rectal cancer has been based on 3D conformal radiotherapy and advanced conformal techniques, namely, intensity-modulated radiotherapy (IMRT), volumetric-modulated arc therapy and image-guided radiotherapy (IGRT). Reference Wang and Zhe13 The advent of these techniques has demonstrated beneficial therapeutic ratio effects by maximising dose to the TV, reducing radiation toxicity to organs at risk (OAR), increasing the chance of local tumour control, and consequently, improving the quality of life. Reference Appelt and Sebag-Montefiore14 However, the success of these techniques requires excellent imaging quality for accurate visualisation and delineation of the TV and OAR. Reference Pereira, Traughber and Muzic12,Reference Wang and Zhe13
Lately, computed tomography (CT) imaging has been referred to as the gold standard for image-based conformal radiotherapy planning for all cancers. Reference Pereira, Traughber and Muzic12,Reference Schlegel15 Despite its advantages, a number of studies have reported significant inter- and intra-observer variation and overestimation of TVs with CT planning owing to its inadequate soft tissue contrast. Reference Tan, Lim Joon and Fitt4,Reference Buijsen, van den Bogaard and Janssen16
Recently, magnetic resonance imaging (MRI) is at the heart of rectal cancer staging and radiotherapy planning due to its superb soft tissue discrimination and multiplanar capabilities. Reference Glide-Hurst, Low and Orton17,Reference Dirix, Haustermans and Vandecaveye18 MRI in RTP is now well established to overcome the limitations of CT in rectal cancer RTP as it provides additional information that allows for detailed and accurate evaluation of the rectal wall and local tumour extent. Reference Wang and Zhe13 This could consequently result in smaller but accurate tumour contours thereby enabling dose constraint to the OAR and tumour dose optimisation. Reference Boldrini, Placidi and Dinapoli19 MRI can provide functional information in addition to anatomical imaging. Reference Pollard, Wen, Sadagopan, Wang and Ibbott20 The past decade has seen the rapid development of MRI from a complimentary modality to CT due to its geometric distortion and lack of electron density Reference Dirix, Haustermans and Vandecaveye18,Reference Schmidt and Payne21 to MRI-only simulation for radiotherapy planning. Reference Schmidt and Payne21,Reference Doemer, Chetty and Glide-Hurst22 Studies have been carried out using modified imaging sequences and distortion correction techniques to eliminate image distortion. Reference Schmidt and Payne21 Also, dose calculation in MRI has been enabled using synthetic or pseudo-CT density data. Reference Wang, Du, Qu, Chandarana and Das23,Reference Guerreiro, Burgos and Dunlop24 More recently, integrated MR with teletherapy units, that is, Cobalt or Linear accelerator has been developed to provide real time IGRT for adaptive treatment techniques. Reference Pollard, Wen, Sadagopan, Wang and Ibbott20 However, this imaging technology has not escaped some limitations, Reference Pollard, Wen, Sadagopan, Wang and Ibbott20 and importantly, there remains a paucity of evidence on its application in RTP.
Currently, attention has also focused on the use of 18Fluorine-2-fluoro-2-deoxy-D-glucose or fluoro-2-deoxy-d-glucose-positron emission tomography/CT (FDG-PET/CT) combining functional and anatomical imaging to provide more detailed information on tumour metabolic activity in RTP. Reference Devic25 In contrast to morphological information provided by anatomical-base modalities, that is, MRI and CT, the advantages of PET/CT include: automatic creation of a delineation around the tumour thereby providing smaller but more accurate TVs. This allows for tumour dose escalation with minimal dose to the OAR, reduced interobserver variability, with the potential to alter treatment strategy. Reference Wang and Zhe13,Reference Buijsen, van den Bogaard and Janssen16,Reference Møller, Khalil, Knap, Muren and Hoffmann26 This sometimes detects new lesions thus minimizing geographical miss. PET/CT has been shown to supersede MR and CT imaging in many cancer staging. Reference Scarsbrook and Barrington27
The International Atomic Energy Agency (IAEA) suggests that RTP should always be based on the most accurate available assessment of tumour extent. Reference MacManus, Nestle and Rosenzweig28 In other words, the gold standard diagnostic modality for staging any cancer may be appropriate for its RTP. The only available systematic review which examined the benefits of MRI and PET over CT in TVD identified a paucity of evidence, indefinable optimal standard imaging sequence for MR rectal RTP and the methodological approaches with FDG-PET/CT TVD as major issues of concern. Reference Gwynne, Mukherjee and Webster29 The need to undertake a current systematic review is imperative due to the limited databases searched and also to assess what has been added to the evidence base since the previous version of the review.
The aim of this systematic review was to synthesise available data to provide a summary of evidence and compare the role of FDG-PET/CT and MRI with conventional CT imaging in TVD of patients with rectal cancer with regard to optimal TV definition and extent of interobserver variation (IOV).
Materials and Methods
Search strategy
An extensive systematic literature search was conducted from January 2005 to December 2018 in six electronic databases including MEDLINE PubMed, EMBASE, Cochrane library, CINAHL, Web of Science and Scopus, using the search terms rectal cancer, radiotherapy planning, FDG-PET/CT, MRI and CT with relevant synonyms. Search terms were classified using the PICO (patients or target condition, index tests, comparator and outcome) concept. Reference Higgins, Deeks, Higgins and Green30 Commencing search date from 2005 was to ensure that publications that may have been missed in the previous review possibly due to delayed period in publication are included in this study. Details of the search strategies are presented in Appendix 1 (Appendix 1 available as supplementary material).
Study selection
This systematic review was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement guidelines. Reference Liberati, Altman and Tetzlaff31
Studies were initially selected independently by one of the authors (EO) based on predefined eligibility criteria and then subsequently by one of the other authors (AN). The initial stage reviewed titles and abstracts of studies to select suitable articles and removed duplicates. Subsequent review focused on full text to identify eligible studies. Reference Liberati, Altman and Tetzlaff31 To be eligible, studies must include primary publications such as randomised studies, comparative observational studies or studies on CT, MR and PET/CT imaging for TVD for RTP of rectal cancer involving external radiotherapy treatment. Abstracts were excluded from the review in order to reduce publication bias partly due to high variability in their reliability as the full studies were not published. Reference Higgins, Deeks, Higgins and Green30,Reference Liberati, Altman and Tetzlaff31 The list with the exclusion criteria data is provided in Appendix 2 (Appendix 2 available as supplementary material).
Data extraction
A custom designed electronic data extraction form based on Cochrane collaboration Reference Higgins, Deeks, Higgins and Green30 was developed, pilot-tested on four included studies to identify any missing or surplus data and then refined accordingly. Corresponding study authors were contacted in case of missing or unclear and additional information such as the release of individual patient data. Data were primarily extracted by one of the authors (EO) and subsequently checked with two other authors. The extracted data included first author, year of publication, methods of study, participants characteristics, interventions and comparison (MRI, FDG-PET/CT and CT), and outcome measures of interest.
Quality assessment
The Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) Reference Whiting, Rutjes and Westwood32 tool, a domain-based approach was used to assess the quality of included studies in this review. The quality assessment addressed risk of bias (internal validity or methodological quality) and concerns about applicability (external validity or directness) for each eligible study. Signalling questions in each of the four domains were rated with answer options low, high or unclear.
Data analysis
Extracted data were processed where necessary before presentation in tables. A narrative synthesis approach was adopted to summarise and synthesise findings from all included studies. The primary outcome measured was the summary statistics (means) for FDG-PET/CT, MRI and CT delineated TVs while the secondary outcome was interobserver variability.
Results
Study selection
A total of eight studies were identified for inclusion in the review. The literature search identified a total of 1448 studies. After removing duplication, 827 studies remained. Of these, 805 studies were excluded based on the eligibility criteria after reading their titles and abstract. Another nine studies were discarded after detailed screening of the full text. Five additional studies were excluded as full text of the study was unavailable. No studies were identified by checking the reference list neither were unpublished relevant studies available. A PRISMA flow diagram summarising the selection process is presented in Figure 1.
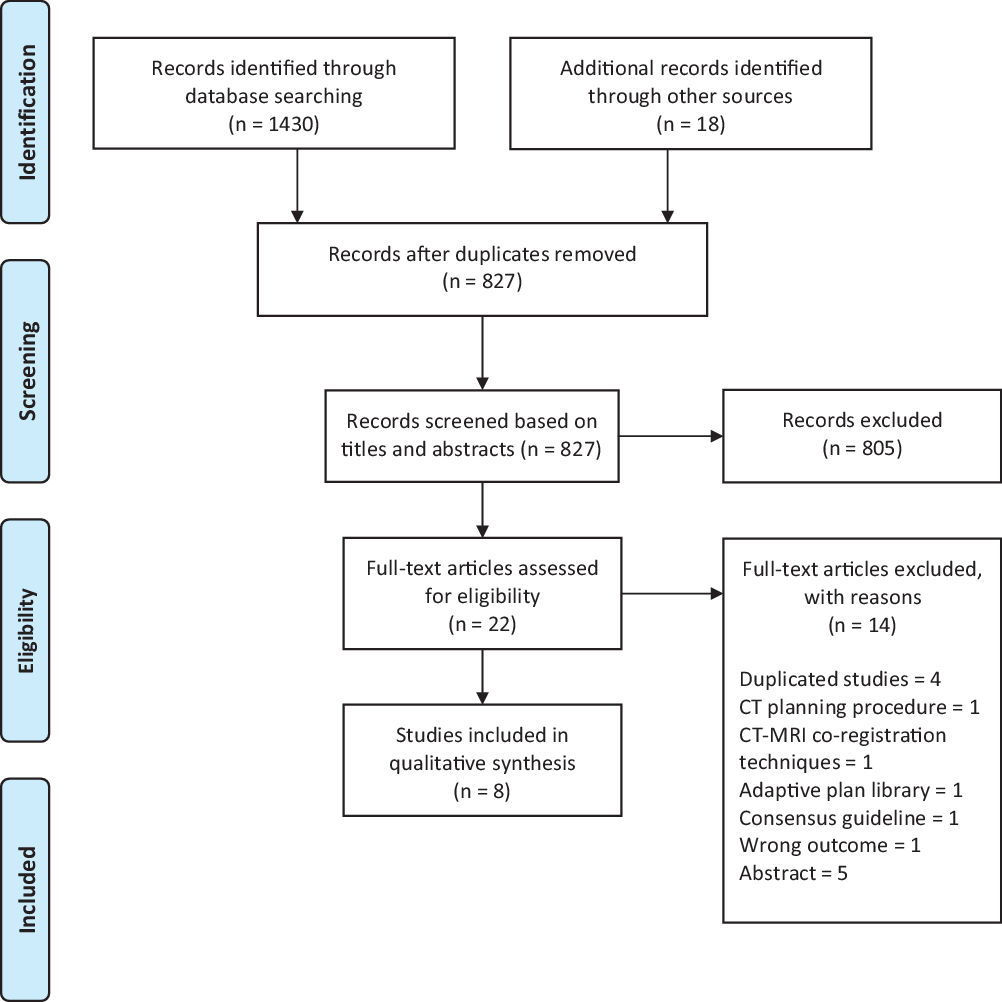
Figure 1. PRISMA flow diagram summarising the study selection process.
Study characteristics
Only one Reference Withofs, Bernard and Van der Rest33 out of the eight studies selected for the review mentioned the study design used. Reference Roels, Slagmolen and Nuyts34–Reference Kilic, Catli, Ulger and Kapucu40 Two of the included studies were conducted using prospective design. The included studies involved 261 patients with LARC. All patients were planned for PCRT with majority (87·5%) to undergo long-course preoperative chemoradiotherapy. Of the six studies that provided detailed participants characteristics, 83/199 were females, with ages between 26 and 89 years. Only one of the eight included studies was multicentre while others were single-centre studies. The characteristics of the included studies are summarised in Table 1. Data for the outcome variables, TV and IOV, are provided in Tables 2–4. A meta-analysis though initially planned was not feasible due to heterogeneity across and between the included studies.
Table 1. Characteristics of studies included in the review
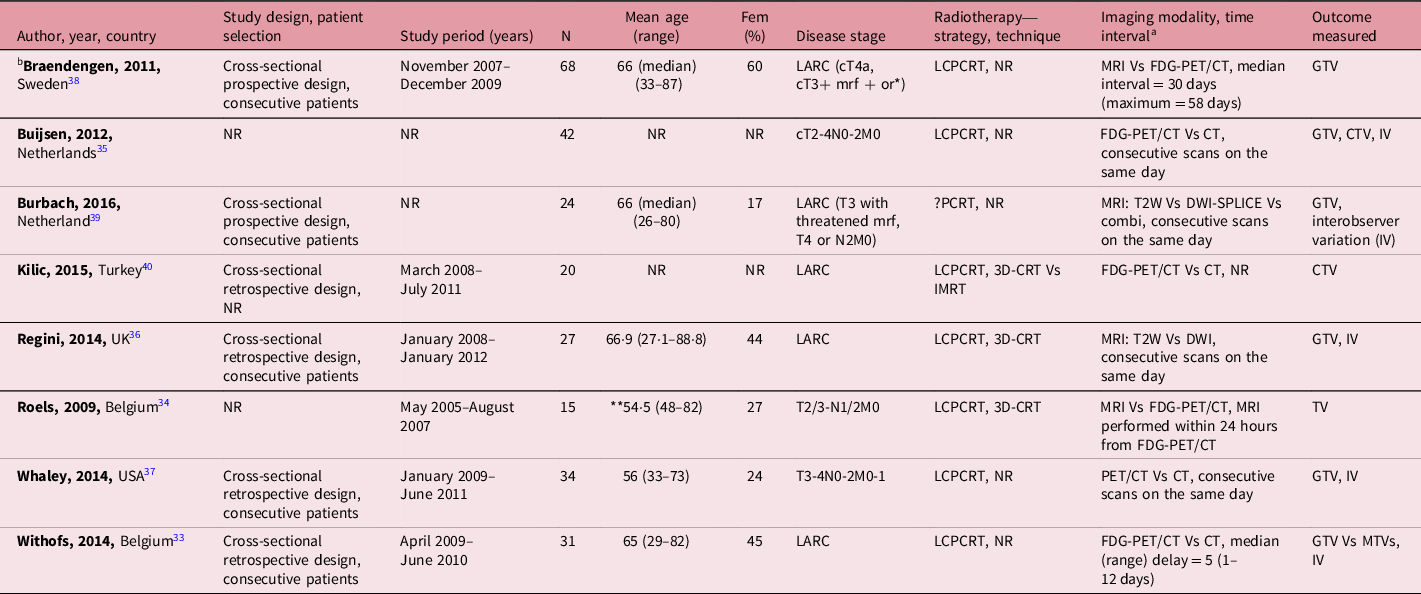
Notes: aTime interval between imaging modalities.
bMulticentre study.
or*, or with radiologically malignant lateral lymph nodes outside the mesorectum.
**54·5, combined groups calculation.
Abbreviations: LARC, locally advanced rectal cancer; mrf, mesorectal fascia; N, number of patients with rectal cancer; Fem, female; LCPCRT, long-course preoperative chemoradiotherapy; NR, not reported; MRI, magnetic resonance imaging; Vs, versus; FDG-PET/CT, fluoro-2-deoxy-d-glucose positron emission tomography/computed tomography; CT, computed tomography; IV, interobserver variation; PCRT, perioperative chemoradiotherapy; T2W, T2-weighted image; DWI, diffusion-weighted image; 3D-CRT, 3D conformal radiotherapy; IMRT, intensity-modulated radiotherapy; MTVs, metabolic tumour volumes; GTV, gross tumour volume; CTV, clinical target volume; TV, tumour volume; combi, combination.
Risk of bias within studies
The quality assessment with regards to risk of bias within individual studies were judged ‘low risk of bias’ in three studies and ‘at risk of bias’ in five studies as three studies were judged unclear in one domain. Reference Buijsen, van den Bogaard and van der Weide35,Reference Braendengen, Hansson, Radu, Siegbahn, Jacobsson and Glimelius38,Reference Burbach, Kleijnen and Reerink39 One study Reference Withofs, Bernard and Van der Rest33 was judged high risk in one domain, while Kilic et al. Reference Kilic, Catli, Ulger and Kapucu40 was judged unclear in three domains. In terms of applicability, all included studies had an overall judgement of ‘low concern’ (see Figure 2).
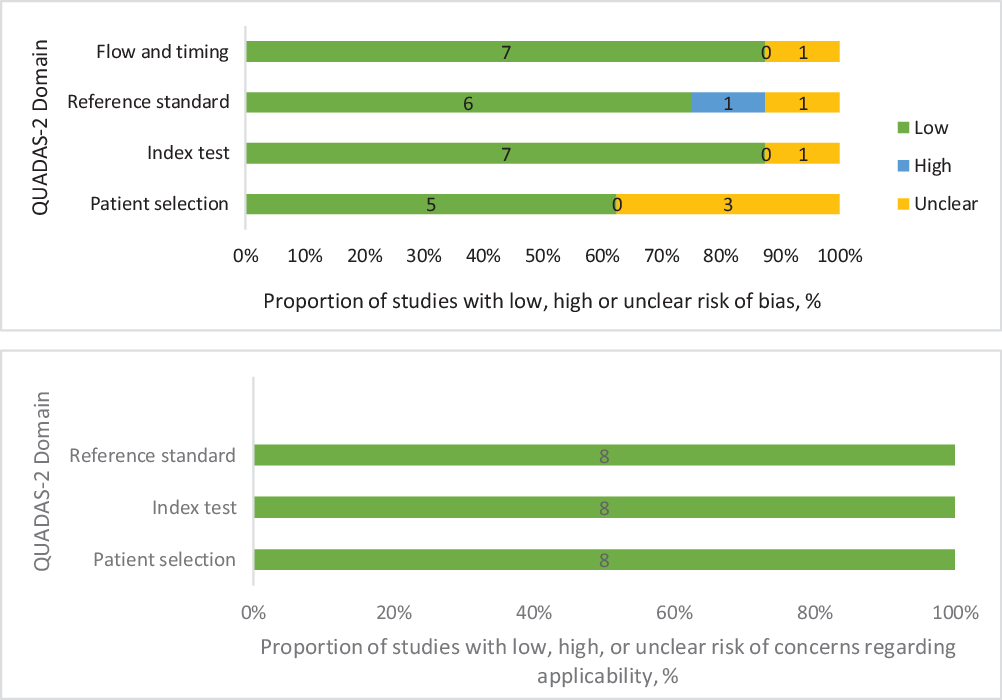
Figure 2. Recommended graphical display for QUADAS-2 results of risk of bias and concern regarding applicability of the included studies.
Narrative synthesis
Relating to the TV outcome, all studies included in the review were classified into three different groups based on the imaging tests (see Tables 2 and 3). Two studies compared MR with PET/CT imaging including a total of 83 patients. Reference Roels, Slagmolen and Nuyts34,Reference Braendengen, Hansson, Radu, Siegbahn, Jacobsson and Glimelius38 Their results revealed that MRI significantly delineate larger TVs than PET/CT. PET/CT was also reported to provide additional information to standard delineation method as new lesions were identified in 15% of patients. Reference Braendengen, Hansson, Radu, Siegbahn, Jacobsson and Glimelius38 The same authors in an attempt to co-register MR with PET/CT gross tumour volume (GTV), found that the volume increased with a median of 11% above the standard GTV-MRI.
A comparison between two MRI sequences, T2W and Diffusion Weighted Imaging (DWI), was reported in two studies which included 51 patients (Table 2). Although both studies found that DWI delineated smaller GTV volumes when compared with T2W, only one study found a statistically significant difference in volumes between the two approaches. Reference Burbach, Kleijnen and Reerink39 The same study further showed that DWI consistently and significantly delineated the smallest volumes compared to co-registered T2W–DW and T2W sequences. The mean differences were: T2W versus DWI = 19·85 mL (p < 0·0001); T2W versus T2W–DWI = 7·16 mL (p < 0·0001) and DWI versus T2W–DWI = 12·69 mL (p < 0·0001).
Four papers reported on PET/CT and CT in TVD (Table 3). Three of these articles found that GTV volumes delineated with CT were significantly larger; mean volume varied from 40·6 cm3 to 102·8 cm3 compared with PET/CT delineated volumes. Reference Withofs, Bernard and Van der Rest33,Reference Buijsen, van den Bogaard and van der Weide35,Reference Whaley, Fernandes and Sackmann37 PET/CT based TVs extended outside the routinely used clinical standard CT-TVs in about 29–83% of patients, as shown by two of those papers. Reference Buijsen, van den Bogaard and van der Weide35,Reference Whaley, Fernandes and Sackmann37 Whaley et al. Reference Whaley, Fernandes and Sackmann37 also found out that treatment plan was altered in five patients (17%) by PET/CT. The only study where PET/CT delineated larger volumes, 51·74 cm3, than CT, 46·26 cm3 (p = 0·043), measured the clinical target volume (CTV) which encompasses both GTV and an additional margin to cover for microscopic tumour spread. Reference Kilic, Catli, Ulger and Kapucu40
Comparisons of different PET/CT volume delineation methods demonstrated that auto-delineated (contoured) volumes were significantly smaller than subjective interpretation performed using visual inspection or manual delineation. Reference Buijsen, van den Bogaard and van der Weide35 Whereas, gradient-based (GR) auto-contouring method defined smaller TVs than signal-to-background ratio (SBR) auto-contouring method. Reference Roels, Slagmolen and Nuyts34 In a study to compare six different functional volume delineation methods, Withofs et al. Reference Withofs, Bernard and Van der Rest33 reported that all the delineated volumes were significantly different.
Closer inspection of Tables 2 and 3 shows that of the six papers on FDG-PET/CT compared with either MRI or CT, only two studies reported a change in treatment plan due to new lesions identified by FDG-PET/CT.
Five papers reported on IOV for TVs delineation (see Table 4). None of the studies on MRI sequences observed significant volume differences between observers and between modalities, indicating delineation similarities and consistencies for T2W, DWI and T2W–DWI, respectively. Reference Regini, Gourtsoyianni and Cardoso De Melo36,Reference Burbach, Kleijnen and Reerink39 Two articles investigated IOV in GTV between PET/CT and CT and found significantly improved interobserver agreement with PET/CT in comparison with CT (p < 0·001) and in addition, Buijsen et al. found the best interobserver agreement in auto-delineated PET/CT (p < 0·001). Reference Buijsen, van den Bogaard and van der Weide35,Reference Whaley, Fernandes and Sackmann37 Conversely, Withofs et al. Reference Withofs, Bernard and Van der Rest33 reported different interobserver variability between the various metabolic tumour volumes and GTV, and this was significant (p < 0·0001).
Discussion
This section provides a discussion on the findings of this review. The review demonstrates that TVs delineated on DWI-MR and FDG-PET/CT images are smaller compared to CT and T2W-MRI images. This implies that unnecessary inclusion of adjacent normal tissues in the planned TV was avoided. Further findings showed that PET/CT can aid adequate tumour coverage and can identify new lesions missed by MRI and CT.
Comparison of these findings with evidence from the previous review seems to be consistent only with data obtained from PET/CT and CT studies. Reference Gwynne, Mukherjee and Webster29 It has been reported that a potential effect of PET/CT is either an increase or decrease in size of TVs. Reference MacManus, Nestle and Rosenzweig28 Existing guidelines such as the Radiation Therapy Oncology Group consensus guidelines Reference Gay, Barthold and O’Meara42 on pelvic structures that may be included in a defined rectal TV should consist of the demonstrable tumour bed or GTV and the CTV which covers the GTV, mesorectum, pelvic lymph nodes, perirectal and presacral regions. It also includes the planning target volume (PTV) which covers the CTV, and an appropriate margin to account for variation in size, shape, position due to physiological mobility of structures in the pelvic region, and set-up error. Reference Boldrini, Placidi and Dinapoli19,Reference Gay, Barthold and O’Meara42 The previous review Reference Gwynne, Mukherjee and Webster29 and this current review identified few studies where PET/CT-TVs were significantly greater than CT-TVs. However, contrary to larger PET/CT-GTVs in the previous review, Reference Gwynne, Mukherjee and Webster29 larger PET/CT-CTV values were reported in this current review. Several lines of evidence have established that PET/CT delineates smaller GTVs which practically approximate the true tumour. Reference Wang and Zhe13,Reference Buijsen, van den Bogaard and Janssen16,Reference Møller, Khalil, Knap, Muren and Hoffmann26,Reference MacManus, Nestle and Rosenzweig28,Reference Bhatnagar, Subesinghe, Patel, Prestwich and Scarsbrook43 A possible explanation for the larger PET/CT-CTV result from this study is likely related to the inclusion of proximal and/or metastatic lymph nodes missed on CT in the CTV. Reference MacManus, Nestle and Rosenzweig28
Although both MRI findings are consistent with the literature, this present review found two studies that compared T2W-MRI with DW-MRI, whereas the earlier review reported three studies which compared MRI with CT and co-registered MRI-CT. The two studies in this review revealed that despite the differences in scanner quality and resolution, the standard T2W-MRI delineate larger TVs than DW-MRI-based approach. Even when compared with fused DW–T2W-MRI, DWI delineated the smallest volumes of the three approaches. A possible explanation for this might be based on the functional characteristics of DWI, that is, its ability to distinguish tumours from healthy tissues upon restricted diffusion which enhances tumour visibility. Reference Kaur, Choi and You44,Reference Nguyen, Soyer, Fornès, Rousset, Kianmanesh and Hoeffel45
For studies that compared FDG-PET/CT and MRI, the results presented here have not previously been described. The findings showed that larger GTVs were defined with MRI than FDG-PET/CT; however, there are apparent potential for result bias as possible interferences of some of the imaging procedures adopted could not be ruled out. For example, in one of the studies rectal contrast was used to improve tumour conspicuity, Reference Roels, Slagmolen and Nuyts34 whereas Regini et al. Reference Regini, Gourtsoyianni and Cardoso De Melo36 argued that this could have affected an overestimation of TV leading to larger volume delineated with MR. The observed larger volume with MRI could be attributed to the low resolution 1·5 T MR scanners used, no correction for geometric distortions and potential variations in the anatomical shape of the tumour due to physiological fillings in the rectum and bladder between both modalities. Furthermore, as revealed by Withofs et al., Reference Withofs, Bernard and Van der Rest33 DICOM transfer of delineated TVs to the treatment planning system could probably have introduced added volume alterations.
On the IOV outcome, the results of this review showed interobserver delineation consistencies between T2W and DWI MRI sequences despite greater T2W volumes. However, a better interobserver agreement was found with PET/CT while PET/CT auto-delineation provided the best concordance compared to CT. These later findings are in line with those of previous review. An implication of this is that PET/CT and DW-MRI are not only reliable but also accurate for TVD. In contrast to earlier findings, however, observers with different levels of background were involved in the delineation process in the reports cited in this review (see Table 4). This finding may help us understand that both radiation oncologists and therapeutic radiographers should be able to take part in tumour delineation, so they can carry out their roles effectively and deliver high quality care. Experience acquired through training, peer-review and supervision can play an important role. Reference Buijsen, van den Bogaard and van der Weide35,Reference Burbach, Kleijnen and Reerink39
Contrary to expectations, this review also highlights important issues, some of which are similar to those discussed in the previous review. First, the findings demonstrate a paucity of data since the last review. By contrast to previous review, specifically no published data comparing MRI and CT, and co-registered MRI and CT were identified, thereby restricting the review to limited comparisons. Furthermore, only two studies have attempted to investigate MRI sequences appropriate for rectal cancer delineation; two studies compared MRI with FDG-PET/CT while four studies have investigated PET/CT and CT. Second, gold standards, such as contouring method and imaging sequence, to validate and standardise TVD in PET/CT and MRI, respectively, are still lacking. Third, none of the included studies reported on rectal and bladder filling. Overestimation of CT volumes as observed in this review could possibly result from faeces in close proximity to the tumour. Reference Tan, Lim Joon and Fitt4,Reference Gwynne, Mukherjee and Webster29 Finally, the use of FDG-PET/CT results in a change of treatment plan in about one out of six patients. Reference Whaley, Fernandes and Sackmann37,Reference Braendengen, Hansson, Radu, Siegbahn, Jacobsson and Glimelius38
This systematic review reveals that both DW-MR and PET/CT imaging can likely benefit rectal cancer patients. Although this finding to an extent is in consonance with the previous review and other evidence, it provides added advancement with functional DW-MRI as against the suggested standard T2W-MRI for rectal cancer treatment planning. Reference Wang and Zhe13,Reference Gwynne, Mukherjee and Webster29 An implication of this finding is the possibility that both functional imaging modalities can approximately define the boost volumes, that is, GTVs for dose escalation for local tumour control with minimal radiation-toxicity to OAR. However, in the context of this present era of advanced conformal radiotherapy, for example, IMRT, and coupled with the current shift from anatomical concept such as GTV, CTV and PTV to biological targeting concept, the combination of findings provide some support for the preliminary evidence that of the three modalities, PET/CT provides the best efficacy for image acquisition and TVD in rectal cancer radiotherapy planning. The biological target volume concept is fast becoming an important aspect in rectal cancer treatment as its major aims addresses boost volume, identifying new lesions as regard to nodal involvement and distant metastasis to avoid geographic misses, and dose painting, that is, heterogenous dose delivery to different regions of the tumour.
Another implication from these pooled findings is that the use of a sole imaging modality especially anatomical T2W-MR or CT imaging to delineate TVs may no longer be tenable in practice. However, with additional findings from PET/CT, it seems that multimodality which provides both metabolic and morphological information about the tumour as provided by PET/CT may be considered superior than single modality imaging in TVD. One of the included studies in this review Reference Roels, Slagmolen and Nuyts34 co-registered FDG-PET and MRI GTVs but observed a mismatch of approximately 50% between both modalities. A few studies have been conducted with PET/MRI modality in meningioma and head and neck tumours radiotherapy planning. Reference Thorwarth, Henke and Muller46,Reference Thorwarth, Leibfarth and Mönnich47 However, despite this promising hybrid modality, questions remain and further studies are needed to determine how best to overcome the mismatch of the hybrid modality.
Furthermore, as it has been suggested, appropriate multimodality imaging (integrated or non-integrated) may be used for complementary purposes rather than substituting existing methods Reference Glide-Hurst, Low and Orton17,Reference Devic25,Reference Bhatnagar, Subesinghe, Patel, Prestwich and Scarsbrook43 as absolute reliance on PET/CT may result in some geometrical misses especially in the evaluation of local tumour extent where MRI is indispensable. Reference Wang and Zhe13,Reference MacManus, Nestle and Rosenzweig28
Strengths and limitations
The strengths of this review were the comprehensive and credible search strategy, acknowledgement of potential biases, inclusion of primary studies and use of the recommended QUADAS-2 quality assessment tool. However, there were also limitations as follows:
First, the quality of the included studies varied. Although there was no concern regarding the applicability of the studies; however, few of the studies were subjectively judged as ‘at risk of bias’ due to poor reporting and TVs not being delineated independently and blindly by observers. Second, majority of included studies were retrospective studies, often susceptible to unknown biases and hypothetical depiction of the results which might have impacted the reliability of the study conclusion. Reference Devic25 Third, nearly all the studies reviewed were single-centred with small sample sizes. This may have hampered the precision of the review. Fourth, none of the imaging modalities used in the reviewed studies were part of randomised controlled trials. A possible reason for this may be due to associated ethical challenges. Reference MacManus, Nestle and Rosenzweig28 Fifth, exclusion of non-English language publications may have resulted in missed studies which could have contributed to the outcome of the review. Reference Coughlan and Cronin48 Sixth, five potentially eligible studies could not be retrieved. Finally, this review identified only eight studies since the last review in 2012. A limitation of this is possible threat to the generalisability of the findings. Notwithstanding, this study offers valuable insights into the amount of attention in the research literature that has been paid to imaging for TVD in rectal cancer despite the advances in imaging techniques and technology in external RTP.
Conclusion
This study evaluated the role of FDG-PET/CT and MRI in conjunction with conventional CT imaging in TVD in external radiotherapy planning of rectal cancers. This systematic review confirmed that FDG-PET/CT and DW-MRI delineated smaller TVs and showed better interobserver agreement compared to CT and T2W-MRI. FDG-PET/CT provided additional information by identifying new lesions missed by MRI and CT which resulted in a change of treatment plan for almost one in six patients with rectal cancer. The future of rectal cancer radiotherapy planning lies more with FDG-PET/CT and DW-MR imaging. The use of a single imaging modality, namely, CT, T2W-MR or PET-CT to delineate TV in rectal cancer radiotherapy planning is not acceptable; multimodality imaging should be advocated. Consequently, the findings from this systematic review do support strong recommendations of FDG-PET/CT and MRI combination for treatment planning of external radiation therapy for rectal cancer. Nevertheless, deciding on appropriate imaging modality for rectal cancer TVD should be a joint responsibility of a radiotherapy team or a designated body such as The European Society for Radiotherapy and Oncology and the IAEA.
Implications for further research
In total, 23 studies (previous = 15 and current reviews = 8) have been identified but the total number of patients so far enrolled (n ≤ 500) is too small to provide a basis for the generalisation of findings. Larger prospective multicentre studies, in particular, cross-sectional study designs as recommended by the Cochrane collaboration are needed to investigate these imaging modalities and patients’ outcome, that is, treatment and survival outcome.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1460396921000388.
Acknowledgements
The authors are grateful to all the academic staff of the School of Health and Social Work, University of Hertfordshire, United Kingdom, who offered support and/or valuable comments on this work. Furthermore, the support of the library staff of the school in organising and executing the search strategy is appreciated.
This study was extracted from a thesis conducted in partial fulfilment for the award of a master’s degree in Medical Imaging and Radiation Sciences at the University of Hertfordshire, United Kingdom. The first author would like to thank the Board of the University College Hospital, Ibadan, Nigeria, for granting the study leave to complete the master’s degree programme.
Financial support
No financial support from any sources of funding
Conflicts of interest
The authors declare none.












