Introduction
Head and neck squamous cell carcinomas (HNSCCs) are relatively aggressive, fast-growing tumours. Cancer treatment delays have been associated with increased mortality for various cancer types, with a stronger association in HNSCC. Reference Hanna, King and Thibodeau1 Prolonged time to treatment can occur in various ways including delays in referral from symptom onset, time from referral to speciality consultation and time to treatment initiation (TTI) from histologic diagnosis. TTI has been rising in the United States largely due to increasing care complexity. Reference Fareed and Galloway2 Prior to treatment, patients require dental assessment, multidisciplinary referrals and evaluations, diagnostic imaging and radiation planning. Each step presents an additional challenge that may lead to prolonged TTI.
The National Comprehensive Cancer Network (NCCN) guidelines recommend initiation of adjuvant post-biopsy radiation therapy (PORT) within 42 days of surgically managed HNSCC, and this benchmark is now a quality metric designated by the American College of Surgeons (ACS)/Commission on Cancer (CoC). Reference Caudell, Gillison and Maghami3,Reference Graboyes, Divi and Moore4 However, there is no national standard recommendation for TTI from histologic diagnosis for patients receiving curative intent (chemo)radiation therapy. It has been repeatedly demonstrated that increased TTI in HNSCC is correlated with decreased overall survival and increased risk of recurrence, but no specific cut-off point was determined. Reference Schoonbeek, Zwertbroek and Plaat5 Multiple studies have reported that a TTI greater than 60 days was associated with worse survival and greater risk of recurrence independent of other relevant factors. Reference Murphy, Galloway and Handorf6,Reference Liao, Schlecht and Rosenblatt7 Given the worse outcomes associated with prolonged TTI, we aimed to determine factors associated with delay at our institution using the 60-day benchmark.
Methods
This retrospective analysis conducted at a single centre institution was reviewed and approved by the study site institutional review board. Waiver of informed consent was obtained as no direct patient identifiers were collected.
Study population
Patients with a diagnosis of HNSCC who underwent curative-intent radiation therapy with or without concurrent chemotherapy were identified in EPIC. Patients age <18, missing timeline data, treated with primary surgical resection and those presenting with recurrence or distant metastatic disease were excluded. Data were collected on patients treated between September 2020 and September 2022 with the intention of characterising post-COVID trends and outcomes.
Data collection
Baseline patient characteristics were collected, including race, ethnicity, age, sex and insurance type. Cancer characteristics were collected, including tumour site, AJCC stage, tumour human papilloma virus (HPV) status and treatment details. Dates of biopsy, imaging and treatment initiation were recorded. Careful chart review was performed to determine the most important barriers to treatment initiation. Our variables are modelled after the key reasons representing barriers to timely PORT that were evaluated in an American Head and Neck Society-sponsored national quality improvement initiative. Reference Graboyes, Divi and Moore4
Definitions
The key reasons representing barriers to treatment initiation are defined in this study as follows: (1) Dental coordination/evaluation/treatment, (2) Care coordination (includes delay in radiation oncology referral placement or consultation, inadequate communication between otolaryngologists and radiation oncology at the time of care transition, delayed pathology reporting, inability to secure timely visits whether it was related to imaging or consults), (3) Post-biopsy issues (tracheostomy requirement, unexpected finding in biopsy, poor wound healing, prolonged length of hospital stay, discharge to skilled nursing facility), (4) Patient factors (patient indecision of facility, inadequate social support, lack of patient knowledge of healthcare steps to start treatment, lack of health insurance coverage or access and lack of transportation) and (5) Other (factors that do not fall in the above categories).
Outcomes
The study outcomes were to (1) determine the percentage of patients who experienced prolonged time to treatment initiation (TTI) defined as treatment initiation more than 60 days after histologic diagnosis and (2) determine the single most important key reason for a delay as defined above based on thorough chart review.
Statistical analysis
Statistical analysis was performed using SPSS, version 28 (IBM Corporation) and SAS, version 9.4 (SAS Institute) statistical software. Descriptive characteristics are presented as median for continuous parameters and frequency distributions for categorical parameters for all patient demographics and baseline characteristics. Mann–Whitney U test was utilised for differences in continuous measures, and Pearson’s Chi Square test was utilised for differences in categorical measures with α level of .05 used as the cut-off for statistical significance. Fisher’s exact test was used for differences in categorical measures when more than 20% of cells have expected frequencies < 5. A cut-off α level of .05 was used for statistical significance.
Results
A total of 96 patients met the inclusion criteria, of which 83% were men with a median age of 64. 35.5% experienced TTI delay with a median of 73 days compared to 41.5 days in patients with no delay (p < 0.01). There was no difference in age (p = 0.08), gender (p = 0.78), race (p = 1.0), ethnicity (p = 1.0) and insurance type (p = 0.82) between patients with delayed TTI and those without (Table 1).
Table 1. Patient demographics between TTI delay and no delay group

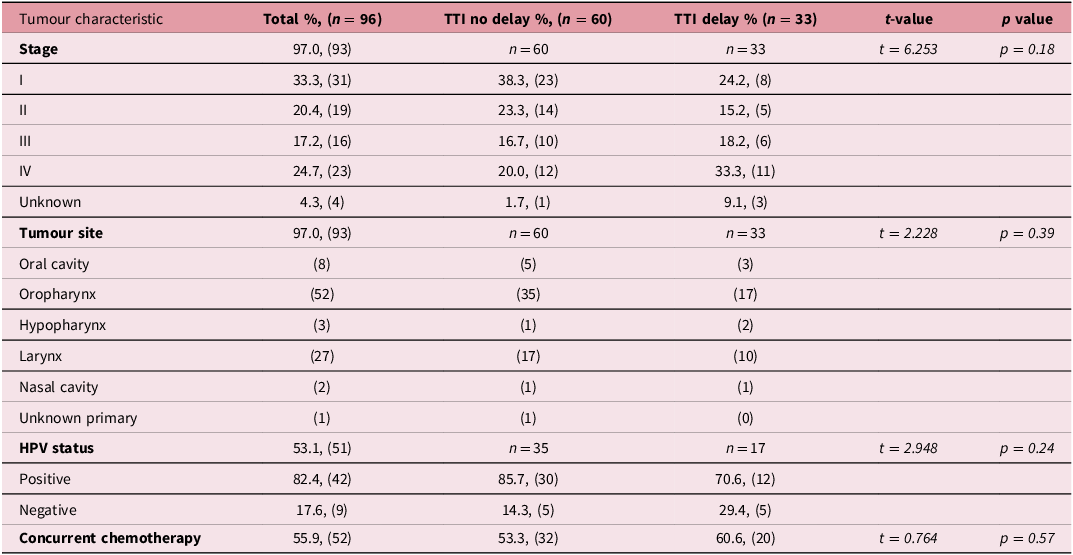
Tumour characteristics, including AJCC cancer stage, HPV status, tumour site and use of chemotherapy are presented in Table 2. There was no statistical difference in tumour site, HPV status and use of chemotherapy in patients with no delay compared to those with TTI delay. AJCC cancer stage trended towards statistical significance with higher stage tumours associated with an increased percentage of TTI delays (Stage 1 38.3% vs 24.2%, II 23.3% vs 15.1%, III 16.7% vs 18.1%, IV 20.0% vs 33.3%, unknown 1.67% vs 9.09%, p = 0.184)
Table 2. Tumour characteristics between TTI delay and no delay group
The single most important reason for delayed TTI was care coordination in the majority of cases (69.7%), followed by individual patient factors (18.2%) and dental coordination (9.09%) (Figure 1). Post-biopsy complications were not identified as a delay for any patient, and 3.03% of patients with prolonged TTI had another reason for delay that was not specified in the predetermined categories. Within the primary category of care coordination, one or more of the following factors contributed to prolonged TTI: pathology-related delays (47.8%), inability to secure timely appointments (43.5%), inadequate communication between specialities (21.7%) and radiation consult timing (21.7%). Within the primary category of individual patient factors, one or more of the following factors contributed to prolonged TTI: patient indecision (50%), social support factors (50%), patient knowledge of steps to start treatment (33.3%), transportation (33.3%) and insurance (16.7%). Only 63.6% of all patients had documentation of receiving dental care before radiation treatment. Table 3 shows examples of clinical cases highlighting the top key factors for delays in time to treatment initiation of (chemo)radiation.

Figure 1. Distribution of the most important reasons for delays in time to treatment initiation of radiation. Blue: care coordination; red: patient factors; green: dental coordination; purple: other.
Table 3. Representative clinical cases highlighting top key factors for delays in time to treatment initiation of (chemo)radiation
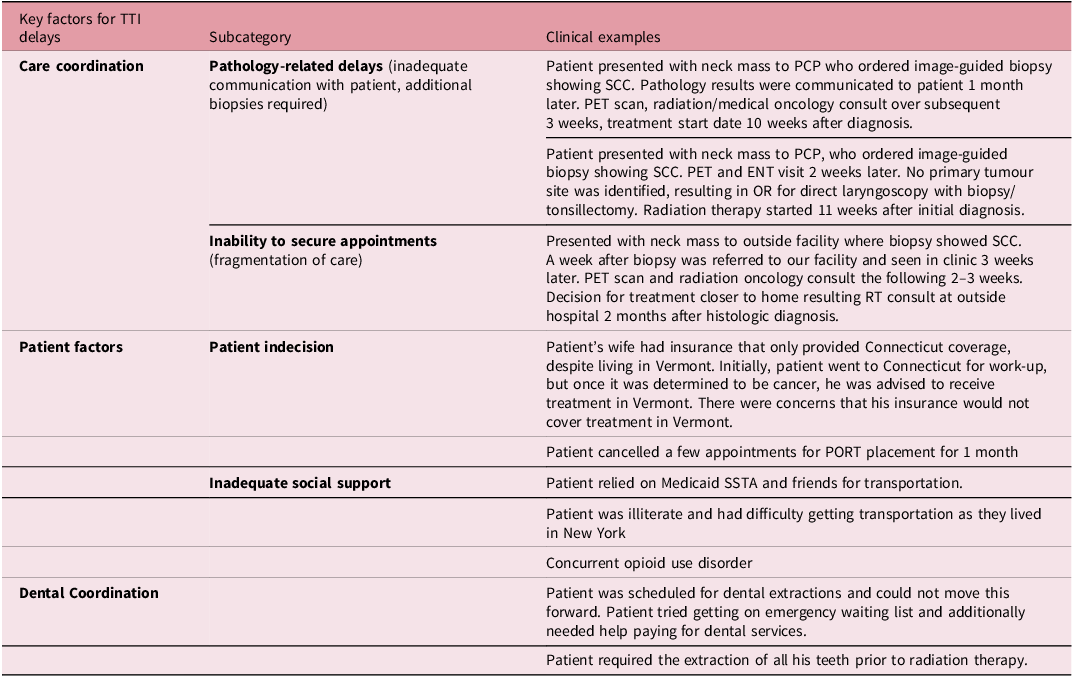
PCP, primary care provider; TTI, time to treatment initiation; SCC, squamous cell carcinoma; OR, operating room; PET, positron emission tomography; SSTA, Special Service Transportation Agency.
Discussion
The NCCN recommends PORT take place within 6 weeks of primary surgical treatment of HNSCC since delays beyond 6 weeks lead to decreased overall survival. Reference Caudell, Gillison and Maghami3,Reference Graboyes, Garrett-Mayer and Ellis8–Reference Rygalski, Zhao and Eskander12 Furthermore, there has been an exploration of the barriers that contribute to delays in PORT. Reference Graboyes, Halbert and Li13,Reference Sawaf, Virgen and Renslo14 However, there is no official recommended guideline for the time from diagnosis to treatment initiation in patients receiving curative intent (chemo)radiation. Several studies have reported that a TTI greater than 60 days is associated with worse survival and a greater risk of recurrence independent of other relevant factors Reference Murphy, Galloway and Handorf6,Reference Liao, Schlecht and Rosenblatt7 . We found that over one-third of patients in our study experienced prolonged TTI, which is similar to rates reported in the literature. Reference Murphy, Galloway and Handorf6
Factors associated with prolonged TTI in HNSCC that have been studied include demographics, tumour site and stage, insurance type and primary treatment modality. A recent systematic review of 52 studies assessing determinants of delay found that race (non-Caucasian), facility type (academic setting), treatment modality (primary radiation) and type of insurance (Medicaid) were associated with delays in TTI. Reference Schoonbeek, Zwertbroek and Plaat5 This is in contrast to our study, which showed no difference in race, insurance or gender between those who experienced prolonged TTI versus those who did not. Similar to their study which found advanced tumour stage to be related to prolonged TTI, we did find a trend towards significance in delays for patients with increasing AJCC stage. This likely reflects the increased complexity and treatment planning required for advanced tumours.
While many studies have evaluated factors such as patient demographics and tumour features associated with prolonged TTI, not many have evaluated the barriers resulting in those delays. Reference Liao, Schlecht and Rosenblatt7,Reference Shaikh, Morrow and Stokes9,Reference Fortin, Bairati and Albert15 This is likely due to the difficulty in obtaining specific information, such as care coordination details through large databases. Our study is unique because we also investigated factors related to the provider, hospital system and the patient as determinants of prolonged TTI in a rural setting. We found the primary reason for delays in TTI at our institution was related to care coordination. Under the category of care coordination, pathology-related delays and inability to secure appointments were the most prevalent factors. Pathology-related delays included inadequate patient contact or communication regarding pathology results. Pathology-related delays were not due to a delay in pathologists determining tumour histology. Inability to secure appointments could be at any point during the care continuum, including with the otolaryngologist, radiation oncologist or medical oncologist, radiology for scans or the operating room for biopsies. To address this issue, our institution has begun implementing an initiative for early introduction of a nurse navigator in patient care, which has helped facilitate more timely appointments. Additionally, fixed slots for radiology appointments have been created for cancer patients to obtain expedited scans. Another potential solution which has shown a reduction in treatment delay is the development of multidisciplinary clinics where a patient is seen by surgery, dental, radiation oncology and medical oncology at their consultation visit Reference van Huizen, Dijkstra and van der Laan16 .
Individual patient factors were the second most common reason for a TTI delay identified in our study. While we reported that approximately 20% of patients experienced issues with either indecision, social support, knowledge of treatment steps, insurance or transportation, this number may actually be greater. One unique aspect of our institution is its geographic catchment area, providing care to many patients living in rural areas of Vermont and upstate New York. This leads to patient indecision about where to receive their primary treatment and potential transportation issues due to the distance from our centre. These types of patient factors are often underreported and therefore may not be adequately addressed. Quality improvement should be aimed at better documentation of these factors and providing the appropriate support and resources.
Dental coordination was not as frequently associated with prolonged TTI as we had anticipated, representing only 10% of delays. However, less than two-thirds of patients had documentation of receiving dental evaluation prior to treatment initiation. Therefore, presumably a proportion of patients begin radiation treatment without dental care, which potentially increases their risk of subsequent osteonecrosis. The omission of dental care is likely multifactorial including lack of insurance coverage, inability to secure timely appointments or insufficient knowledge of the importance of dental evaluation prior to treatment. We have started partnering with community dentists to ensure patients without insurance have a resource for dental evaluation. A future study on whether lack of dental evaluation increases the risk of osteoradionecrosis would be important, and the findings could potentially be used to advocate for more access to dental care in HNSCC.
Our study has limitations inherent to its retrospective design including potential for selection bias and incomplete data available for review. Selecting the primary reasons for treatment delay is inherently subjective as there are often multiple causes, and the reviewer must determine the most important reason. We assessed different patient characteristics such as race, ethnicity, age and gender. However, there may be other patient characteristic variables that were not captured that can influence treatment delay. Furthermore, our patient population is limited to a single institution in a rural setting that may not be generalisable to other institutions in different regions.
Conclusion
Approximately one-third of HNSCC patients in this retrospective study had prolonged TTI beyond 60 days. Primary reasons identified for treatment delays included care coordination and lack of patient social support. These results prompted quality improvement initiatives at our institution including early connection with a nurse navigator and partnering with a community dentist to support patients with barriers to dental care. The design and results of this study could be used as a guide for other institutions to improve TTI and optimise care for patients with HNSCC undergoing definitive (chemo)radiation therapy.
Acknowledgements
We express our sincere gratitude to the Divisions of Haematology and Oncology, Otolaryngology and Radiation Oncology at the University of Vermont Medical Centre for their invaluable expertise and assistance in both the various stages of our study and the manuscript writing process.
Financial support
None.
Competing interests
Authors 1, 2 and 3 do not declare any conflicts of interest. Authors 4 and 6 are employed by the Division of Haematology and Oncology at the University of Vermont Medical Centre. Authors 5, 9 and 10 are employed by the Division of Otolaryngology at the University of Vermont Medical Center. Authors 7 and 8 are employed by the Division of Radiation Oncology at the University of Vermont Medical Centre.







