Introduction
Computed tomography (CT) scans in the patient radiotherapy treatment position (CT simulation) are routinely acquired within radiotherapy departments as they are used for planning radiotherapy treatments. CT simulation is important as it provides the patient anatomical data for planning and delivering radiotherapy treatments. Reference Jonsson, Nyholm and Söderkvist1 Radiotherapy staff use their substantial experience of CT to inform scanning protocols which are designed to provide optimal patient experience and data collection.
Many radiotherapy departments within the UK do not use dedicated radiotherapy magnetic resonance imaging (MRI) within the pre-treatment pathway, with only 6% of radiotherapy patient treatments employing MRI guidance in 2018. Reference Speight, Schmidt, Liney, Johnstone, Eccles, Dubec, George, Henry and McCallum2 In most cases within the UK where MRI is utilised, it is in addition to the standard of care CT pathway. The majority of radiotherapy departments rely on scanners in diagnostic MRI departments as only a small number of radiotherapy centres have dedicated radiotherapy MRI equipment. Reference Speight, Schmidt, Liney, Johnstone, Eccles, Dubec, George, Henry and McCallum2 However, a rationale for increased use of MRI in radiotherapy is building through increased evidence of the benefit to patients, Reference Metcalfe, Liney and Holloway3 the development of new techniques such as magnetic resonance (MR)-only planning, Reference Bird, Henry, Sebag-Montefiore, Buckley, Al-Qaisieh and Speight4 and recommendations from national bodies. 5 Some specialist centres now acquire additional dedicated MRI scans with the patient in the radiotherapy treatment position (MR simulation), and this is likely to become more widespread as new techniques, such as MR-only planning, develop. Reference Bird, Henry, Sebag-Montefiore, Buckley, Al-Qaisieh and Speight4 However, as the MRI examination process is substantially different to CT simulation, both in terms of the resultant images and the method of acquisition, it is a challenge to directly relate patient CT and MR simulation experiences. It is vital that MR simulation process is optimised so that patient experiences are not compromised.
Studies of patient experiences undergoing diagnostic MRI scanning show that that patients can experience anxiety or claustrophobia prior to or during an MRI scan and that anxious patients are more likely to move resulting in motion artefacts which impairs the quality of the acquired data. Reference Evans, Taylor and Janes6–Reference Evans, Taylor and Beare8 It is therefore hypothesised that in the context of MR simulation, patient anxiety impacts on the image quality, limiting the potential benefit of MRI within radiotherapy, as well as negatively impacting patient treatment experience. However, while we can learn much from diagnostic imaging studies investigating patient experience, radiotherapy imaging differs due to the requirement for specialist immobilisation equipment and specific preparation and scanning protocols. Reference Bird, Henry, Sebag-Montefiore, Buckley, Al-Qaisieh and Speight4,Reference Bellhouse, Brown and Dubec9 These differences have the potential to significantly impact patient experience and as a consequence it is challenging to compare diagnostic MRI to MR simulation. To our knowledge, the only assessment of patient experience in MRI in radiotherapy in the literature assessed the tolerability of MR simulation for patients with lung cancer and found that one-third of patients had adverse anxiety during their scan, recommending that comfort should be a key consideration for optimising these scans. Reference Bellhouse, Brown and Dubec9
The results presented in this study are part of a wider study looking at MR-only planning for anal and rectal cancers, where dedicated MR simulation scans were acquired. This sub-study aimed to evaluate the patient experience of MR simulation for anal and rectal cancer patients when compared to their CT simulation, identifying the quality of patient experience, areas where patient experience could be improved and whether changes can be implemented which improve patient experience outcomes.
Method
This study is part of a wider MR-only radiotherapy study: ‘Mri-only treAtmeNT planning for Anal and Rectal cAncer radiotherapy’ (MANTA-RAY), research ethics committee reference: 18/LO/1298, ISRCTN Registry: ISRCTN82734641. MR simulation scans for guiding radiotherapy treatment planning were acquired between October 2018 and March 2020 at a single centre. Forty-six anal and rectal cancer patients (Table 1) who were due to undergo radical volumetric-modulated arc therapy (VMAT) external beam radiotherapy were consented to have a research MRI scan in addition to the standard of care imaging pathway. Exclusion criteria included contra-indications to MRI.
Table 1. Demographics of study patients including responders and non-responders

MRI scans were acquired on a 1.5 T Siemens Aera (Siemens Healthineers, Airlanger, Germany), with radiotherapy radiographers positioning patients on the MRI scanner couch and diagnostic MRI radiographers leading the scanning session. Patients were routinely set up ‘head first’ to match their CT simulation, but were offered a ‘feet first’ scan if they indicated prior to consenting that they found MRI claustrophobic. Patient preparation was also matched between patient’s CT and MR simulations and included a bladder filling protocol and immobilisation indexed to an in-house built radiotherapy flat top couch (knee block and ProStep for rectal cancers, knee block for anal cancers). Buscopan (20 mg) was administered intravenously to patients to reduce muscle motion within the bowels five minutes prior to the MRI. Headphones were placed over the ears of the patients who were given a choice of music or no music to listen to during the examination. MR compatible coil bridges were used to keep the MRI coils from touching and consequently deforming the patient skin position. Axial T2-SPACE (sampling perfection with application optimised contrasts using different flip angle evolution), T2 and DWI (diffusion weighted imaging) MRI scans were acquired.
In order to reduce the inconvenience to the patients, an attempt to schedule the CT and MRI appointments on the same day was made. However, this was not possible for some patients and in these cases the MRI scan was scheduled for a time when the patient had a clinical appointment prior to or during their first two weeks of treatment. Consequently, the mean time between patient CT and MR simulation appointments was 15·1 days (range: 0–43 days), where CT simulation was always carried out first.
An audit of MR examination time was carried out by calculating the difference in acquisition time between the first and last acquired sequences from scan data collected from picture archiving and communication system (PACS). No measures of time prior to or after MR sequence acquisition, for example, time for patient set up, were acquired as this was outside the scope of this study. Examination time was calculated for all patients, including those who did not respond to the questionnaire to allow a complete assessment of scan duration between cohorts. The number of scanning sessions that were terminated prior to completion was noted and removed from the sample prior to calculation.
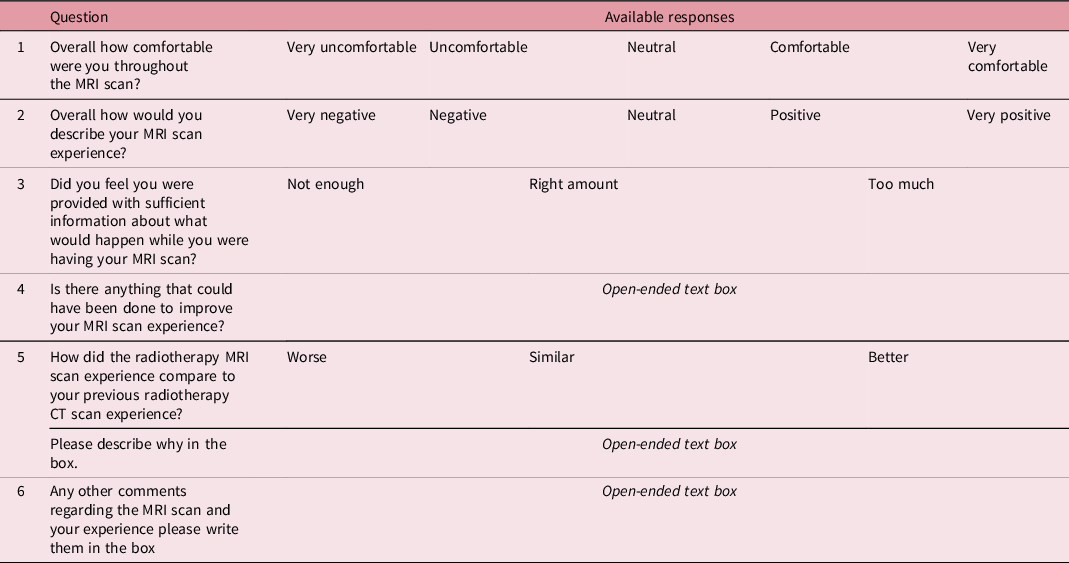
The questionnaire was co-designed by the local patient and public involvement group to ensure its suitability for assessing patient experience. We chose to use a locally designed questionnaire, rather than a validated questionnaire from the literature, as it allowed us to concisely ask the specific questions we felt were required to achieve the aims of this study, which were to compare CT and MR simulation. The questionnaire (Table 2) consisted of both multiple choice and free-text questions. The content of the questions was designed to assess similarity of the MR simulation compared to the CT simulation and to establish options for further improvement of the patient experience. Multiple choice questions allowed patients to rate aspects of the MRI scan on a Likert scale, as shown in Table 2. Patient experience questionnaires were provided to participants in a paper format directly after their MRI scan, this was provided with a stamped addressed envelope for ease of returning.
Table 2. The questions and available responses in the questionnaire provided
Quantitative and qualitative analysis was performed on the responses of the first 33 patients (cohort one). Quantitative analyses were performed on multiple choice questions using a Likert scale and were used to give a general overview of the participants’ experiences. Qualitative analyses were performed on the open-ended questions to gain insight into the aspects of the MR simulation that affected patient experiences. Common response themes were identified, and the recurrence of themes was quantified for both positive and negative responses.
Potential changes to the MRI scan protocol to improve patient experience, based on the results of cohort one, were discussed with MRI and radiotherapy radiographers to identify feasible changes. Discussions focused on simple practical solutions to the raised experience issues and changes were confirmed where all staff groups had consensus that the solution was achievable and had potential to be beneficial. The identified changes were implemented, and 13 patients (cohort two) were asked to complete the experience questionnaire. The questionnaire results from cohort two were analysed with the same method as for cohort one with the aim of assessing whether the implemented changes affected patient experience. No direct comparison between cohorts was undertaken due to the limited number of cohort two questionnaire responses.
Results
Cohort one
The questionnaire response rate for cohort one participants was 28/33 (85%). The mean examination time was 29 min and 40 s (range: 18 min 20 s to 42 min 8 s). Two patient MR simulations were deliberately terminated prior to completion due to departmental delays and so were excluded from the examination time analysis.
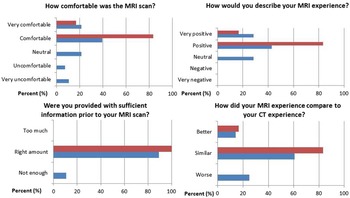
Figure 1 shows the quantitative questionnaire responses (questions 1, 2, 3 and 5). Key findings include 75% of respondents found the MRI experience to be similar or better than the CT experience, while 25% of respondents found the MRI experience to be worse than the CT experience; 18% of respondents described the MRI scan as uncomfortable or very uncomfortable and 11% of respondents indicated there was not enough information prior to the MRI scan.

Figure 1. Quantitative analysis of the multiple choice responses to the questionnaire from cohort one (blue) and cohort 2 (red), where the percentage is of questionnaire responses.
Seven themes, including scanner noise, information, music, scan length, room temperature, staff feedback and claustrophobia, were identified from the qualitative questionnaire responses (questions 4, 5 and 6). Table 3 shows the common themes observed from patient responses, the number of responses per theme and examples of a patient quotes regarding these themes.
Table 3. Thematic structure, number of respondents who address the stated theme and direct quotes

* Negative comments were from patients who went head first, positive comments were from patient who went feet first.
Changes to MR simulation protocol
The following achievable changes were identified and implemented for cohort two participants:
-
Use of both earplugs and headphones for all patients
-
Use of music for all patients as default unless specifically rejected by patients, ensuring that the volume is sufficient
-
Use of blankets below and above coil bridges
-
Patients scanned feet first as standard for MR simulation
-
Reduction of the scanning time by reducing in the number of scans—removing the T2 sequence from the scan protocol as other phases of the exploratory research study identified it was no longer required
-
Extra staff focus on ensuring information regarding the MRI scan details, particularly the length of examination, had been explained fully directly prior to MR simulation
Cohort two
The questionnaire response rate for cohort two participants was 7/13 (54%). The mean total scan time was 20 min and 53 s (range: 16 min 33 s to 28 min 8 s). No scanning sessions were ended early.
Figure 1 shows the quantitative questionnaire responses (questions 1, 2, 3 and 5). Key findings include all respondents described the MRI scan as comfortable or very comfortable; all respondents indicated they had the appropriate amount of information prior to the MRI scan and all respondents found the MRI experience to be better or similar to the CT experience. The qualitative questionnaire responses were uniformly positive, and no common themes were identified.
Discussion
Here we aimed to compare the patient experience of MR simulation compared to CT simulation, where in both cases patients were positioned using radiotherapy immobilisation devices. Our findings showed that MR simulation for guiding radiotherapy treatment planning can be a comfortable and positive experience that is comparable in experience to standard radiotherapy CT simulation. This is an important finding as it provides confidence that MR simulation can be implemented into widespread use within radiotherapy without the fundamental barrier of unacceptable patient experience.
However, we also found that following a CT simulation protocol without alterations to account for the change in modality led to a significant number of patients (25%) having experiences that were worse than CT simulation. The analysis of our qualitative responses highlighted a number of areas that affected patient experience, and the challenge was whether it was possible to address these in a practical way that did not impact the quality of the data collection, which has precise requirements as it is for radiotherapy purposes.
MRI scanning takes place in a noisy, enclosed position for a substantial length of time. These features are a requirement for MRI scanners which use large superconducting magnets in the acquisition of their images—the noise is a bi-product of movement (gradient coils) within the scanner as images are acquired, the enclosed position allows the magnetic field to be uniform within the scanner which is necessary for geometric accuracy and the length of time is required for producing good image quality. Reference Vlaardingerbroek and Boer10 In addition, MRI scanning rooms are often deliberately cold to help prevent patients from overheating as MRI scans cause patient body temperatures to increase due to radiofrequency energy being deposited in patient tissues as images are acquired. Reference Vlaardingerbroek and Boer10 However, none of these features are required for CT simulation, and as a consequence, it is not surprising that these MRI specific features dominated the experience feedback from patients in cohort one.
The mean examination time for cohort one was 29 min and 40 s minutes, for cohort two this was reduced to 20 min and 53 s. This reduction in was due to a combination of reducing the number of scans acquired (removing the T2 sequence saved 5 min 28 s) and improving the efficiency of the scanning session, where staff became more proficient and quicker at managing and acquiring the required sequences as they scanned more patients with this new protocol. It was fortunate that the T2 sequence could be removed from the protocol, but this was only made possible by findings in from a different phase of the wider Manta-ray study which meant that the T2 sequence was not required for further patients. The length of scanning time is a fundamental difference between the imaging modalities that accentuates the other differences in the environment. It is easy to attempt to compare MR simulation to diagnostic MRI scans in terms of acquisition time and consider MR simulation to be similar in length; however, an obvious difference is the patient position required for radiotherapy that can be uncomfortable due to the necessary immobilisation. In addition, our assessment only included time spent during image acquisition, in practice patients will be in an uncomfortable position for longer than this due to set up times. Our findings provide evidence that highlights the importance of optimising MR simulation protocols such that the time on the MRI couch is minimised. Particularly this is important for MR simulation which can often be, as it was in this case, a new intervention and so experience within radiotherapy of MRI protocol optimisation is limited.
The majority of changes to the pathway were simple solutions; the default use of earplugs and headphones with music to reduce noise and provide distraction, blankets to ensure warmth, being scanned feet first rather than head first as standard to prevent patient’s heads from entering the scanner bore, and therefore reduce claustrophobia, and minimising time being scanned to limit discomfort. However, the results of the questionnaires suggest that it is these small adjustments that could make a substantial improvement to the patient experience. It is notable that in some MR unit’s interventions such as these for diagnostic MR scans are common practice; however, it is important to recognise that when MR simulation is undertaken, even in a diagnostic setting as here, often its radiotherapy staff who are responsible for patient set up due to the precise requirements of radiotherapy patient positioning. Therefore, these learning points (that these pathway changes are suitable and beneficial for radiotherapy MR simulation) are important as they highlight the challenges of MR simulation to radiotherapy centres and also the benefit of working closely with radiology departments to fully understand our pathway differences.
Our findings from cohort two suggested that our changes were successful in improving patient experience of MR simulation as all cohort two patients found their experience to be as good as their CT simulation, unlike cohort one. However, this finding is only suggestive due to the low cohort two size, which has prevented a more rigorous analysis. Cohort two was originally aiming to recruiting 30 patients rather than 13 until the global COVID-19 pandemic caused the study to close early. Compounding this issue is the lower response rate for cohort two of 54% vs. 85% for cohort one which was unexpected. This drop in response rate is not easily explainable as the only changes to the patient pathway between cohort one and two were those to improve patient experience and the patient demographics (Table 1) of the two cohorts show no clear bias which may impact response.
A small number of patients in cohort one felt not enough information were provided regarding the MRI scan in terms of its how long it would take and there was an isolated misunderstanding regarding patient set up which negatively affected patient experience. Patients were provided with written information sheets explaining what to expect from the MR simulation at the time of entering the study as well as being verbally informed on what to expect by the study recruitment team and radiotherapy and MRI radiographers prior to undertaking the scan. However, our findings suggest that it is challenging to always ensure the correct level of information is provided, and it is plausible to suggest this would improve as radiotherapy staff become more experienced at preparing patients for MR simulation. Interestingly, although patients were not asked about staff in the questionnaire, six responses in the free text boxes also praised staff and this is a tribute to their professional, kind and positive attitudes. This should not be overlooked as a key factor in positive patient experiences.
Only one other study Reference Bellhouse, Brown and Dubec9 to our knowledge has assessed the patient experience of MR simulation, in the context of lung radiotherapy treatments. These scans were acquired in a significantly different patient position to the ano-rectal cancer patients in this study; however, the environment is comparable. The main findings of claustrophobia and noise being limiting factors were similar to those seen here. Their conclusion that two-thirds of patients tolerated additional MRI scans Reference Bellhouse, Brown and Dubec9 with minimal adverse anxiety levels is similar to our cohort one findings, where 75% of patients felt the experience was similar to their standard radiotherapy CT scan. It was interesting to note that their implications for clinical practice were that comfort and patient position ought to be considered when introducing MRI into the radiotherapy pathway, as the identification of practical options for improving patient comfort was one of our aims.
Conclusions
In this study, we assessed the patient experience of dedicated radiotherapy MR simulation in the context of pelvic radiotherapy treatments. We found that MR simulation can be comfortable and a positive experience that is comparable to standard radiotherapy CT simulation. Our findings also highlight the importance of taking into account the differences in scanning environment between CT and MRI to ensure comparable experience. Here we described simple changes to the MR simulation pathway that removed or mitigated the causes of worse patient experience including; the routine use of blankets, earplugs and headphones, music, feet-first positioning and ensuring an optimised MRI protocol in terms of acquisition time. Our findings also showed the importance of staff to good patient experience.
Acknowledgements
Mr David Bird is funded by a National Institute for Health Research (NIHR) Clinical Doctoral Research Fellowship for this research. Dr Richard Speight is supported by a Cancer Research UK Centres Network Accelerator Award Grant (A21993) to the ART-NET consortium. The assistance with data collection and transfer from the following was greatly appreciated: radiotherapy CT and diagnostic MR radiographers, physicists; Dan Wilson, David Broadbent, Sarah Wright and Marcus Tyyger (funded by the Sir John Fisher Foundation), research radiographers; Pam Shuttleworth and Louise Loughman and clinical oncologists; Paul Hatfield, Michelle Kwok-Williams and Mohan Hingorani.
Funding
Mr David Bird is funded by a National Institute for Health Research (NIHR) Clinical Doctoral Research Fellowship for this research. This publication presents independent research funded by the National Institute for Health Research (NIHR). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. Dr Richard Speight is supported by a Cancer Research UK Centres Network Accelerator Award Grant (A21993) to the ART-NET consortium.
Conflict of Interest
No conflicts of interest to declare.
Data Availability Statement
Research data are not available at this time.