Introduction
Fishes of the family Priacanthidae constitute a small group of predatory percomorphs occurring circumtropically with greatest diversity in the Indo-Pacific region (Starnes, Reference Starnes1988). Priacanthids exhibit deep bodies, remarkably large eyes, and rough spinous scales. Because of their large eyes, cryptic habits, and night-time angling results, these fishes are usually regarded as nocturnal, even though evidence from stomach contents seems to indicate that they are also active during the day (see Hiatt and Strasburg, Reference Hiatt and Strasburg1960). Larval and prejuvenile priacanthids are pelagic in the upper layers of the water column (Caldwell, Reference Caldwell1962a, Reference Caldwell1962b), whereas juveniles and adults (standard length >70 mm; Caldwell, Reference Caldwell1962a) are bottom dwellers with a preference for coral reefs and rocky areas. They are very secretive (Caldwell, Reference Caldwell1962a) and usually considered solitary, although some species occasionally occur in loose and undirected aggregations around coral reefs and rock piles (Caldwell and Bullis, Reference Caldwell and Bullis1971). Some species are characterized by a sound-producing mechanism in large part based on extrinsic swimbladder muscles (Salmon and Winn, Reference Salmon and Winn1966).
The phylogenetic position of the Priacanthidae within percomorphs is unclear. These fishes have been traditionally aligned with the Percoidei (e.g., Johnson, Reference Johnson1984; Nelson, Reference Nelson2006), but recent large-scale molecular studies hypothesized a close affinity with the Monodactylidae and acanthuriforms (Betancur et al., Reference Betancur, Broughton, Wiley, Carpenter, López, Li, Holcroft, Arcila, Sanciangco, Cureton, Zhang, Buser, Campbell, Ballesteros, Roa-Varon, Willis, Borden, Rowley, Reneau, Hough, Lu, Grande, Arratia and Ortí2013), or, alternatively, with the Cepolidae, Siganidae, and Scatophagidae (Near et al., Reference Near, Dornburg, Eytan, Keck, Smith, Kuhn, Moore, Price, Burbrink, Friedman and Wainwright2013). However, support for the latter hypotheses is somewhat weak and new detailed focused studies are needed to test such phylogenetic interpretations.
The fossil record of the Priacanthidae is relatively rich, documented by several Eocene, Oligocene, and Neogene articulated skeletal remains and otoliths, primarily from Europe (see Fitch and Crooke, Reference Fitch and Crooke1984; Starnes, Reference Starnes1988; Bannikov, Reference Bannikov2010; Nolf, Reference Nolf2013). The earliest articulated skeletal remains of the group consist of a few well-preserved specimens belonging to the species Pristigenys substriata from the lower Eocene limestone of Monte Bolca, northeastern Italy. The Eocene occurrence of priacanthids documents the remarkable increase of diversity of nocturnal feeders that took place during the earliest part of the Paleogene (see Goatley et al., Reference Goatley, Bellwood and Bellwood2010; Carnevale et al., Reference Carnevale, Bannikov, Marramà, Tyler and Zorzin2014).
Overall, the family Priacanthidae currently includes 19 extant species in four genera, Cookeolus, Heteropriacanthus, Priacanthus, and Pristigenys (Starnes, Reference Starnes1988; Iwatsuki et al., Reference Iwatsuki, Matsuda, Starnes, Nakabo and Yoshino2012). The nomenclatural history of Pristigenys has been controversial (e.g., White, Reference White1936; Myers, Reference Myers1958; Caldwell, Reference Caldwell1962a; Fitch and Lavenberg, Reference Fitch and Lavenberg1975; Fritzsche, Reference Fritzsche1978; Fritzsche and Johnson, Reference Fritzsche and Johnson1981; Fitch and Crooke, Reference Fitch and Crooke1984; Taverne, Reference Taverne1988). In his comprehensive worldwide revision of the family, Starnes (Reference Starnes1988) allied species formerly referred to Pseudopriacanthus to the fossil genus Pristigenys based on a few morphological and meristic features, confirming the hypothesis proposed by White (Reference White1936) and subsequently reiterated by Myers (Reference Myers1958), Fritzsche and Johnson (Reference Fritzsche and Johnson1981), and Taverne (Reference Taverne1988). However, the skeletal morphology of the type species of the genus Pristigenys—the Eocene P. substriata from Monte Bolca—has never been examined in detail in order to conclusively demonstrate its affinity to the extant species formerly referred to Pseudopriacanthus. The goal of this paper is therefore to describe the osteology of Pristigenys substriata in more detail, and to discuss its relationships with the extant members of the Priacanthidae.
Stratigraphy
The celebrated locality of Monte Bolca lies in the eastern part of Monti Lessini, near Verona, northeastern Italy. This locality includes several productive sites, two of which, the Pesciara and Monte Postale, have provided one of the most important and well-known fossil fish assemblages of the world (see Carnevale et al., Reference Carnevale, Bannikov, Marramà, Tyler and Zorzin2014). The best known of these sites is that of the Pesciara, which is characterized by abundant and exquisitely preserved fossils, particularly fishes (Marramà et al., Reference Marramà, Bannikov, Tyler, Zorzin and Carnevale2016). The fish-bearing deposits of the Pesciara site pertain to the so-called ‘Calcari Nummulitici’, an informal Eocene unit widespread in the surroundings of Monte Bolca, and consist of finely laminated micritic limestone. According to Marramà et al. (Reference Marramà, Bannikov, Tyler, Zorzin and Carnevale2016), the fossiliferous deposits of the Pesciara site accumulated in a shallow intraplatform basin in which anoxic conditions at the bottom and the development of a biofilm promoted the high-quality preservation of its fossils.
The age of the fish-bearing laminated limestone of the Pesciara site has been defined on the basis of their large benthic Foraminifera. These deposits have been referred to the Alveolina dainelli Zone, or to the SBZ 11 Biozone, corresponding to the late Cuisian (late Ypresian, slightly less than 50 Ma; Papazzoni et al., Reference Papazzoni, Carnevale, Fornaciari, Giusberti and Trevisani2014).
Materials and methods
The fossil material documented herein consists of five well-preserved complete to nearly complete articulated skeletons preserved on the surface of laminated micritic limestone. The preservation quality of the examined specimens, as well as the lithology of the associated sediment, suggest that skeletal material belonging to Pristigenys substriata documented herein derive from the excavations carried out at the Pesciara site (see Marramà et al., Reference Marramà, Bannikov, Tyler, Zorzin and Carnevale2016). The material was examined using Wild M5A and Leica M80 stereomicroscopes equipped with camera lucida. Measurements were taken using a dial caliper, to the nearest 0.1 mm. During examination, the specimens were moistened with alcohol to enhance some details of their skeletal anatomy.
Repositories and institutional abbreviations
The fossils are housed in the collections of the Natural History Museum, London (NHM) and the Museum National d’Histoire Naturelle, Paris (MNHN).
Anatomical abbreviations
ach, anterior ceratohyal; ap1, first anal-fin pterygiophore; br, branchiostegals; bsp, basisphenoid; dhh, dorsal hypohyal; dp1, first dorsal-fin pterygiophore; ep, epural; f, frontal; hpu2, haemal spine of the second preural vertebra; hpu3, haemal spine of the third preural vertebra; hs1, first haemal spine; hyp, hypural; io3, third infraorbital bone; lac, lacrimal; le, lateral ethmoid; me, mesethmoid; npu2, neural spine of the second preural vertebra; pas, parasphenoid; pch, posterior ceratohyal; phy, parhypural; pop, preopercle; pp, postpelvic process; SL, standard length; sn, supraneural; soc, supraoccipital; ss, subocular shelf; uh, urohyal; un, uroneural; v, vomer; vhh, ventral hypohyal; vk, ventral keel of the basipterygium.
Systematic paleontology
Percomorphacea sensu Wiley and Johnson, Reference Wiley and Johnson2010
Family Priacanthidae Gill, Reference Gill1872
Genus Pristigenys Agassiz, Reference Agassiz1835
Type species
Chaetodon substriatus Blainville, Reference Blainville1818.
Included species
Type species, by monotypy.
Diagnosis
A deep-bodied priacanthid unique in having the following combination of character-states: orbit moderately large (orbit diameter 14.1–19.6% SL); caudal peduncle short (9.1–11.9% SL) and deep (17.3–20.1% SL); sagittal crest (apparently) present; frontal with smooth supraorbital margin; shelf overlying the preopercular sensory canal smooth; preopercular spine stout; anterior and posterior ceratohyal joined by narrow suture; first haemal spine greatly enlarged and closely associated with anterior anal-fin pterygiophores; single supraneural; dorsal fin containing ten strong and deeply striated spines plus nine to 12 soft rays; dorsal-fin spines and soft rays well developed (longest spine 26.1–32.7% SL; longest ray 27.1–35.6% SL); anal fin containing three strong and deeply striated spines plus nine to 12 rays; anal-fin rays considerably elongate (longest ray 19.7–37.9% SL); pelvic fins remarkably elongate (49.7–57.7% SL); postpelvic processes of basipterygia expanded into lobes; ventral keel of basipterygia narrow; black marginal pigmented band on soft portion of dorsal, anal, caudal, and pelvic fins; caudal fin rounded, with three upper and three lower procurrent rays; spinules absent in all fins; body scales with about 15–25 stout spinules on posterior margin.
Occurrence
As for Pristigenys substriata, the type and only known species.
Pristigenys substriata (Blainville, Reference Blainville1818)

Figure 1 Pristigenys substriata (Blainville, Reference Blainville1818) from the Eocene of Monte Bolca, Italy: holotype, MNHN F.Bol529, right lateral view. Scale bar represents 10 mm.
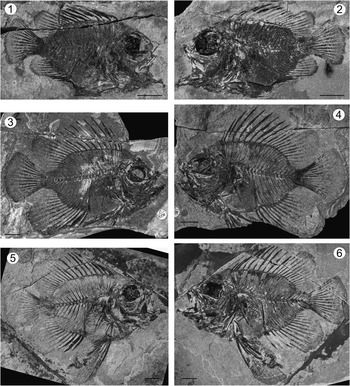
Figure 2 Pristigenys substriata (Blainville, Reference Blainville1818) from the Eocene of Monte Bolca, Italy: (1) NHM P.9941, right lateral view; (2) NHM P.14540 (counterpart of the specimen in 1), left lateral view; (3) NHM P.15370, right lateral view; (4) NHM P.15371 (counterpart of the specimen in 3), left lateral view; (5) NHM P.16127, right lateral view; (6) NHM P.16370 (counterpart of the specimen in 5), left lateral view. All scale bars represent 10 mm.
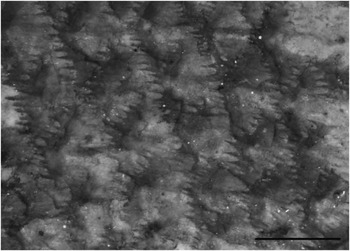
Figure 3 Pristigenys substriata (Blainville, Reference Blainville1818) from the Eocene of Monte Bolca, Italy: MNHN F.Bol529, abdominal scales. Scale bar represents 1 mm.

Figure 4 Pristigenys substriata (Blainville, Reference Blainville1818) from the Eocene of Monte Bolca, Italy: (1) NHM P.15371, reconstruction of the neurocranium, left lateral view; (2) NHM P.16127, reconstruction of the anterior part of the infraorbital series, right lateral view; (3) NHM P.16370, reconstruction of the preopercle and hyoid bar, left lateral view. All scale bars represent 5 mm. See materials and methods section of text for anatomical abbreviations.
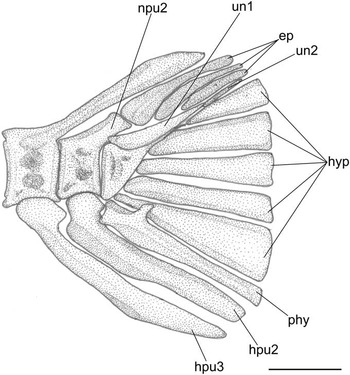
Figure 5 Pristigenys substriata (Blainville, Reference Blainville1818) from the Eocene of Monte Bolca, Italy: NHM P.15371, reconstruction of the caudal skeleton, left lateral view. Scale bar represents 2 mm. See materials and methods section of text for anatomical abbreviations.
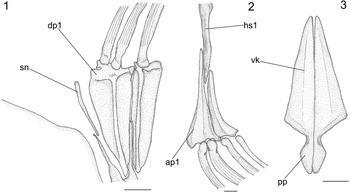
Figure 6 Pristigenys substriata (Blainville, Reference Blainville1818) from the Eocene of Monte Bolca, Italy: (1) NHM P.15371, reconstruction of the nuchal region showing the supraneural and the two anterior dorsal-fin pterygiophores, left lateral view; (2) NHM P.16370, reconstruction of the first haemal spine and anterior anal-fin pterygiophores, left lateral view; (3) NHM P.14540, reconstruction of the pelvic girdle, ventral view. All scale bars represent 2 mm. See materials and methods section of text for anatomical abbreviations.
1796 Chaetodon striatus Reference VoltaVolta, p. 92, pl. 20, fig. 2.
1818 Chaetodon substriatus Reference BlainvilleBlainville, p. 352.
1835 Pristigenys macrophthalmus Reference AgassizAgassiz, p. 313.
1839 Pristigenys macrophthalmus Reference AgassizAgassiz, p. 136, pl. 18, fig. 2.
1874 Pristigenys macrophthalmus Reference de Zignode Zigno, p. 61.
1901 Pristigenys macrophthalmus Reference WoodwardWoodward, p. 415.
1905 Pristigenys substriatus Reference EastmanEastman, p. 21, pl. 3, fig. 3.
1936 Pristigenys substriatus Reference WhiteWhite, p. 49, text-figs. 2, 3.
1958 Pristigenys substriata Reference MyersMyers, p. 41.
1980 Pristigenys substriata Reference BlotBlot, p. 368.
1981 Pristigenys substriata Reference Fritzsche and JohnsonFritzsche and Johnson, p. 490, text-fig. 1.
1984 Pristigenys substriata Reference Fitch and CrookeFitch and Crooke, p. 311, text-fig. 11.
1988 Pristigenys substriata Reference TaverneTaverne, p. 171, text-figs. 1, 2.
2000 Lates gracilis Reference Edwards and RosenEdwards and Rosen, backcover.
2010 Pristigenys substriata Reference Taverne and NolfTaverne and Nolf, p. 187, text-figs. 1, 37.
Holotype
MNHN F.Bol529, partially complete, relatively well-preserved articulated skeleton, 54 mm SL (Fig. 1).
Referred material
NHM P.9941, nearly complete, well-preserved articulated skeleton, in part and counterpart (NHM P.14540), 50.4 mm SL (Fig. 2.1, 2.2); NHM P.15370, nearly complete, well-preserved articulated skeleton, in part and counterpart (NHM P.15371), 73.3 mm SL (Fig. 2.3, 2.4), figured in Edwards and Rosen (Reference Edwards and Rosen2000) and erroneously assigned to Lates gracilis; NHM P.16127, nearly complete, well-preserved articulated skeleton, in part and counterpart (NHM P.16370), 83.4 mm SL (Fig. 2.5, 2.6); NHM P.19057, nearly complete and relatively well-preserved articulated skeleton, 60.5 mm SL.
Diagnosis
As for the genus.
Occurrence
Monte Bolca locality, Pesciara site, NE Italy, late early Eocene, late Ypresian, late Cuisian, ca. 50 Ma (see Papazzoni et al., Reference Papazzoni, Carnevale, Fornaciari, Giusberti and Trevisani2014).
Description
General morphology
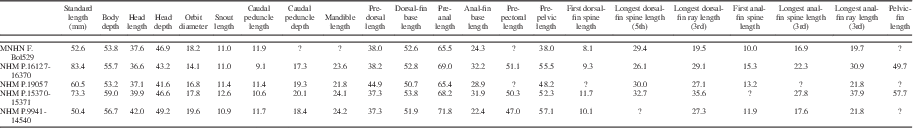
Measurements for Pristigenys substriata are summarized in Table 1. The body is ovoid and deep, its maximum depth contained between 1.69 and 1.85 times SL. The head is moderately large, its length less than a third of SL. The head is characterized by a nearly straight dorsal profile. The snout is relatively blunt, its length contained between 3.1 and 3.8 times in head length. The orbit is rounded and relatively large, its diameter between a fifth and sixth SL. The eyeball is very large and preserved as a thick and conspicuous carbon film. The considerable thickness of this carbon film is likely due to the original remarkable size of the tapetum lucidum in the choroid; the possession of a very large ocular tapetum lucidum consisting of several rows of reflecting cells that underlie the entire retina (e.g., Nicol et al., Reference Nicol, Arnott and Best1973; Wang et al., Reference Wang, Nicol, Thurston and McCants1980) is currently regarded as a priacanthid synapomorphy (Starnes, Reference Starnes1988). The mouth is terminal with an oblique and moderately large gape. The mandible length is contained between 1.5 and 1.7 times in head length. The caudal peduncle is short (caudal peduncle length contained up to more than ten times in SL) and very deep (caudal peduncle depth contained about five times in SL). The dorsal-fin origin is located above the occipital region of the neurocranium. The predorsal length is contained between 2.2 and 2.6 times in SL. The sail-like dorsal fin is continuous with spines that rapidly increase in size posteriorly up to the fifth element; the length of the spines gradually decreases posteriorly in the series. The length of the first spine is contained about three times in that of the fifth spine. The dorsal-fin soft rays are distally bifurcated and gradually increase in length up to the third element, after which their length rapidly decreases posteriorly in the series. The third dorsal-fin soft ray is slightly shorter or slightly longer than the fifth dorsal-fin spine. Overall, both the spinous and soft portions of the dorsal fin have a rounded profile. The anal-fin origin is usually placed below the second caudal vertebra. The preanal length is contained about 1.5 times in SL. The anal-fin spines gradually increase in length posteriorly. The length of the first anal-fin spine is contained between 1.4 and 1.6 times in that of the third spine. The longest (third) anal-fin soft ray is remarkably longer that the longest anal-fin spine. The soft portion of the anal fin has a gently rounded outer profile. The caudal fin is rounded, and its length is contained about four times in SL. The pectoral fins are incomplete in all examined specimens; however, they appear to be rather short. The pelvic fin insertion is located beneath the pectoral-fin base. The prepelvic distance is contained between 1.75 and 2.5 times in SL. The pelvic fin is adnate and remarkably elongate (pelvic-fin length less than half SL), extending posteriorly well beyond the anal-fin insertion (Fig. 2.5, 2.6). The rays of the dorsal, anal, caudal, and pelvic fins are characterized by a remarkably well-preserved black marginal band (Figs. 1,2).
Table 1 Measurements for Pristigenys substriata (Blainville, Reference Blainville1818) from the Eocene of Monte Bolca, Italy. Values are as percentage of SL.
Squamation
The body is covered by thick, adherent spinoid scales (type 1 of Roberts, Reference Roberts1993) (Fig. 3). The scales are polygonal in outline with a nearly straight anterior margin and a weakly pointed or convex posterior margin. The spines are stout, variable in size, their number ranging from 15 to ~25. The scales appear to be smaller, irregularly arranged, and structurally modified on the nape, top of the head, gular (including the branchiostegals) and prepectoral areas, opercle, chin, and cheek; although their precise morphology is unclear, at least those of the cheek and some of those of the chin seem to bear spines emerging from their outer surfaces. The circuli of the body scales are characterized by a rough texture, ostensibly related to the presence of microscopic denticles (Starnes, Reference Starnes1988). The lateral line runs very high on the body flank, following the dorsal profile of the body up to the posterior end of the caudal peduncle. The lateral-line scales bear a single tube.
Neurocranium
Overall, the skeletal morphology is consistent with that of other priacanthid fishes (see Starnes, Reference Starnes1988; Taverne and Nolf, Reference Taverne and Nolf2010). The skeletal structure of the skull is only partially recognizable due to the extensive fragmentation of most of the bones. The neurocranium is robust, compact, and relatively deep, about 1.5 times as long as deep (Fig. 4.1). Due to the presence of a large orbit, both the ethmoid and postorbital portions of the neurocranium are anteroposteriorly compressed. The outer margin of the skull roof is nearly straight, whereas that of the ethmoid region appears to be convex. The frontals are the largest bones of the skull roof. A low sagittal crest appears to be present. The supraorbital margin of the frontal is smooth. The mesethmoid is rather thick. The lateral ethmoids are characterized by a large laminar lateral flange with a rounded outer margin. The supraoccipital crest is relatively large, reaching its maximum height just above the midlength of the orbit. The parasphenoid is robust and nearly straight for most of its length, forming a shallow angle at the level of the posterior orbital wall. The basisphenoid is columnar and nearly perpendicular to the parasphenoid.
Infraorbital series
The bones of the infraorbital series are in general moderately well preserved (Fig. 4.2). A partially preserved and articulated series is exposed in the holotype. The lacrimal has a serrated ventral margin with a large stout spine emerging from its midlength. The third infraorbital is characterized by a relatively large subocular shelf (see Smith and Bailey, Reference Smith and Bailey1962). The fourth, fifth, and sixth infraorbitals are preserved in specimen NHM P.15371; their ventral margin is serrated (Fig. 4.2). The nasal and supratemporal bones are not recognizable in the examined material.
Jaws
The premaxilla has robust ascending and articular processes. A postmaxillary process is not preserved, although it was possibly present originally. The alveolar process is elongate and rather thick, bearing numerous irregularly arranged small conical teeth with slightly recurved tips. The maxilla has an expanded and spatulate distal end. The lower jaw is upturned and projects strongly. The craniomandibular articulation is located just below the anterior margin of the orbit. The dentary is stout and very thick at the symphysis, where it bears a short ventral process. There are numerous teeth along the dorsal margin of this bone, very similar to those of the premaxilla. The anguloarticular and retroarticular are very robust.
Suspensorium
The morphology of the bones of the suspensorium is consistent with that of other priacanthid fishes (Starnes, Reference Starnes1988). The endopterygoid and ectopterygoid are well preserved in specimens NHM P.16370 and NHM P.14540. The endopterygoid is large and ovoid in outline. The palatine bears a strong finger-like maxillary process, which is well exposed in specimen NHM P.16127. The quadrate is fan-shaped. The symplectic is relatively short. The hyomandibular is always fragmented and inadequately preserved.
Opercular series
The bones of the opercular series are poorly preserved due to their extensive fragmentation. The crescent-shaped preopercle has finely serrated posterior and ventral margins, and a moderately developed, finely serrated spine at the posteroventral angle (Fig. 4.3). The bony shelf overlying the preopercular sensory canal is smooth. The interopercle is partially preserved in the specimen NHM P.16127; it is oblong with a pointed anterior end and a finely serrated ventral margin.
Hyoid apparatus and gill arches
The hyoid bar is robust and strongly ossified (Fig. 4.3). The dorsal and ventral hypohyals are subquadrangular. The anterior and posterior ceratohyals are sutured to each other by a narrow suture formed by deep longitudinal interdigitations. The anterior ceratohyal is constricted at its midlength; a vertical process emerges at its anterodorsal corner; there is no beryciform foramen. The posterior ceratohyal is approximately triangular in outline. There are six strong and relatively short saber-like branchiostegals, of which the posterior two articulate with the posterior ceratohyal. Fragments of the urohyal are recognizable in specimen NHM P.16370.
The gill-arch skeleton and pharyngeal teeth are not exposed in the available specimens.
Vertebral column
The vertebral column is compact and contains 23 (10+13) vertebrae, including the urostylar element. The abdominal portion of the vertebral column is arched with the concave side oriented toward the venter of the fish, whereas the caudal portion is linear. Except for the first five that are anteroposteriorly compressed, the centra are subrectangular and longer than high. The structure of the first neural arch and spine is difficult to interpret. The neural spines of the second to seventh vertebrae are relatively short, anteroposteriorly expanded, and obliquely oriented. Those of the successive abdominal vertebrae are more elongate, slender, and nearly vertical. The first haemal spine is strong and anteroposteriorly expanded. Most of the centra bear deep and large lateral fossae. The dorsal prezygapophyses are well developed. In the caudal region, the neural spines and their haemal antimeres emerge from the anterior half of the vertebrae and are thick and distally pointed. There are eight pairs of thick ribs articulating with the third to tenth vertebrae. Epineural fragments can be recognized.
Median fins and support
The caudal skeleton is consistent with that of other priacanthids (Fujita, Reference Fujita1990; Fig. 5). The urostylar vertebra consists of fused first and second ural and first preural centra. There are five nearly triangular autogenous hypurals. The autogenous parhypural is very thick and bears a well-developed parhypurapophysis. There are two uroneurals and three epurals. The neural spine of the second preural vertebra is very short and reduced to a spatulate crest. The haemal spines of the second and third preural vertebrae are autogenous. The caudal fin consists of 16 principal rays (I,7-7,I) plus three upper and three lower procurrent rays; there is no procurrent spur.
There is a single thin, slightly curved, and obliquely oriented supraneural. This slender and delicate bone extends parallel to the anterior margin of the first dorsal-fin pterygiophore and its dorsal end does not reach the dorsal border of the body (Fig. 6.1). The predorsal formula (Ahlstrom et al., Reference Ahlstrom, Butler and Sumida1976) is /0+2/1/1/1. The dorsal fin contains 10 spines plus nine to 12 distally branched rays, supported by 17–20 pterygiophores. The spines are strong and pointed, ornamented with numerous deep striations throughout their length (Fig. 6.1). The membrane that connected the dorsal-fin spines is preserved as a very thin, marginal dark band. The first two dorsal-fin spines are in supernumerary association with the first dorsal-fin pterygiophore (Fig. 6.1). The dorsal-fin pterygiophores basically consist of a long and narrow main shaft supporting anterior and posterior laterally flattened bony laminae. The pterygiophores of the soft portion of the dorsal fin are distally bent. The interneural spaces underlying the dorsal fin are occupied by the vertical shafts of one (in the spiny and anteriormost soft portion of the series) or two (in most of the soft portion of the series) pterygiophores.
The anal fin consists of three strong, pointed, and deeply striated spines plus nine to 12 soft rays supported by 10–13 pterygiophores. The first two anal-fin spines are in supernumerary association with the first anal-fin pterygiophore (Fig. 6.2). The large first anal-fin pterygiophore inserts in front of the first haemal spine, to which it appears to be closely bound together with the second anal-fin pterygiophore, which inserts posterior to that haemal spine (Fig. 6.2). The interhaemal spaces overlying the anal fin are occupied by the shafts of two anal-fin pterygiophores. The overall structure and orientation of the anal-fin pterygiophores are similar to those of their dorsal counterparts. Their size gradually decreases posteriorly in the series. There are no spinules along the anterolateral surfaces of the dorsal- and anal-fin spines and rays.
Paired fins and girdles
The pectoral girdle is difficult to interpret due to inadequate preservation in all the available specimens. What appears to be a strongly fragmented posttemporal is recognizable in specimen NHM P.16370. The supracleithrum is oblong, laminar, and apparently devoid of the dorsal posterior process typical of most extant members of the Priacanthidae. The cleithrum is elongate and slightly curved. The coracoid has a thick and nearly horizontal ventral process that, together with the posteroventral margin of the cleithrum, bounds a large fenestra. There is a single postcleithrum characterized by a moderately expanded posterodorsal lobe. Four pectoral-fin radials appear to be present. The exact number of pectoral-fin rays is difficult to evaluate. About 12 rays are recognizable in the specimen NHM P.14540; a similar count was provided by White (Reference White1936).
The basipterygia are approximately triangular and characterized by a narrow ventral keel. The postpelvic processes are rather elongate and expanded laterally into lobes (Fig. 6.3). The pelvic fin consists of a single strong, pointed and laterally striated spine plus five remarkably elongate rays.
Discussion
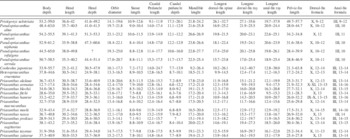
In his description of the holotype included in the fourth volume of the “Recherches sur les Poissons Fossiles,” Agassiz (Reference Agassiz1839) considered Pristigenys a close relative of Beryx, reinterpreting the identification by Volta (Reference Volta1796) and Blainville (Reference Blainville1818) who considered it a member of the butterflyfish genus Chaetodon. It is interesting to note, however, that Agassiz (Reference Agassiz1839) convincingly pointed out the similarities between Pristigenys and the Oligocene Acanus, the latter currently regarded as a subjective junior synonym of Priacanthus (Bannikov, Reference Bannikov2010). Based on Agassiz’s comments, Woodward (Reference Woodward1901) listed Pristigenys among the fossil representatives of the Berycidae, an opinion subsequently followed by Eastman (Reference Eastman1905). Finally, White (Reference White1936) described new specimens in the collection of the British Museum (Natural History) (now The Natural History Museum, London) and, for the first time, referred this Eocene taxon to the family Priacanthidae, a placement supported by our morphological analysis. Numerous features, other than overall physiognomy of the head and body, unquestionably demonstrate that Pristigenys substriata is a priacanthid (Fitch and Crooke, Reference Fitch and Crooke1984; Starnes, Reference Starnes1988), including spinoid scales, scales covering the branchiostegals, infraorbital bones with serrated ventral margins, vertebrae 23 (10+13), principal caudal-fin rays 16 (8+8), fin spines deeply striated, single postcleithrum, and adnate pelvic fins. The relationships of Pristigenys substriata within the Priacanthidae were discussed by White (Reference White1936), who considered it almost indistinguishable from Pseudopriacanthus, pointing out that the differences between the Eocene and extant genera are inadequate to justify a separation above the species level. Myers (Reference Myers1958) reiterated the arguments discussed by White (Reference White1936) and indicated the correct generic name for Eocene and extant species would be Pristigenys. Since the publication by White (Reference White1936), the skeletal morphology of Eocene priacanthids from Monte Bolca has been examined only superficially (Fritzsche and Johnson, Reference Fritzsche and Johnson1981; Taverne, Reference Taverne1988), primarily to corroborate White’s conclusion that Pristigenys is the subjective senior synonym of Pseudopriacanthus. Although the synonymy of Pristigenys and Pseudopriacanthus has been accepted by many authors (e.g., Smith, Reference Smith1966; Fritzsche and Johnson, Reference Fritzsche and Johnson1981; Starnes, Reference Starnes1988; Taverne, Reference Taverne1988; Taverne and Nolf, Reference Taverne and Nolf2010; Iwatsuki et al., Reference Iwatsuki, Matsuda, Starnes, Nakabo and Yoshino2012), several studies noted that there is no substantial evidence to support such a taxonomic assessment, suggesting that both genera should be retained as valid (e.g., Caldwell, Reference Caldwell1962a, Reference Caldwell1962b; Fritzsche, Reference Fritzsche1978; Fitch and Crooke, Reference Fitch and Crooke1984). A close affinity between Pristigenys substriata and its (presumed) extant congenerics is supported by several shared features, including a deep, robust body (Figs. 1, 2), anterior and posterior ceratohyals joined by a narrow suture (Fig. 4.3), three upper plus three lower procurrent caudal-fin rays, a single supraneural (Fig. 6.1), an oblong supracleithrum, postpelvic process of the basipterygium expanded into a lobe (Fig. 6.3), a narrow ventral keel of the basipterygium (Fig. 6.3), and a black marginal band on the soft portions of the dorsal, anal, pelvic, and caudal fins (Fig. 2). However, the Eocene taxon exhibits a unique set of features that have not been observed in any of the extant or fossil species currently referred to Pristigenys (Starnes, Reference Starnes1988), suggesting generic separation. In particular, Pristigenys substriata is characterized by having a moderately developed orbit (Tables 1, 2), a short, deep caudal peduncle (Tables 1, 2), very elongate dorsal, anal, and pelvic fins (Table 1), spinules completely absent on median and paired fins (Figs. 1, 2), soft portions of dorsal and anal fins gently rounded with nine to 12 rays (Tables 1, 2), frontals bearing a shallow sagittal crest and smooth supraorbital margins (Fig. 4.1), a stout spine at the posteroventral angle of the preopercle (Fig. 4.3), a scaleless preopercular shelf (Figs. 1, 2), and a strong and expanded first haemal spine closely bound to the two anterior anal-fin pterygiophores (Fig. 6.2). We believe that these features (Starnes, Reference Starnes1988; Kon and Yoshino, Reference Kon and Yoshino1997) provide substantial morphological evidence to justify the retention of Pristigenys and Pseudopriacanthus as valid genera. Some of the features shared by these two genera are not plesiomorphic, and thus may be considered as evidence for their sister-group relationship, e.g., the reduction of the number of procurrent caudal-fin rays, and presence of a black marginal band on dorsal, anal, pelvic and caudal fins (Johnson, Reference Johnson1984; Starnes, Reference Starnes1988). In particular, the possession of three procurrent caudal-fin rays in these genera is regarded as derived, considering that other priacanthids have four or five elements (Johnson, Reference Johnson1984; Starnes, Reference Starnes1988) and the primitive number of procurrent caudal-fin rays is higher in percoid percomorphs (see Johnson, Reference Johnson1984).
Table 2 Synopsis of selected morphometric (as percentage of SL) and meristic values of fossil and extant species of the family Priacanthidae. Includes new data and data from Starnes (Reference Starnes1988) and Iwatsuki et al. (Reference Iwatsuki, Matsuda, Starnes, Nakabo and Yoshino2012).
Summarizing, the detailed reinterpretation of the skeletal morphology of Pristigenys substriata suggests that this Eocene taxon forms a clade with the extant Pseudopriacanthus, and that this grouping can be considered the sister-group to all remaining extant priacanthid genera (Cookeolus, Heteropriacanthus, Priacanthus) (Fig. 7). According to Starnes (Reference Starnes1988), Cookeolus represents the sister-group to Heteropriacanthus plus Priacanthus. As a final remark, we note that the skeletal morphology of Pristigenys substriata and its putative sister-group relationship indicate that the evolutionary significance of certain phylogenetically relevant features used by Starnes (Reference Starnes1988) should be reconsidered. In particular, the median sagittal crest of the frontals and the massive anterior haemal spines closely associated with robust anal-fin pterygiophores, which were interpreted as derived in Cookeolus, Heteropriacanthus, and Priacanthus by Starnes (Reference Starnes1988), should be regarded as plesiomorphic for the family and their absence in Pseudopriacanthus as derived.
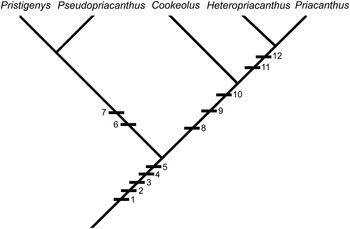
Figure 7 Cladogram showing hypothetical relationships of Pristigenys within the Priacanthidae. Character states are: (1) 16 (8+8) principal caudal-fin rays; (2) 23 (10+13) vertebrae; (3) scales on branchiostegals; (4) adnate pelvic fins; (5) single supraneural; (6) black marginal fin bands; (7) 3+3 procurrent rays; (8) supraneural absent; (9) broad suturing of ceratohyals; (10) enlarged ventral keel of the basipterygium; (11) lateral foramina at the base of the haemal spines; and (12) prominent anterior interradial projections on scales (see Starnes [Reference Starnes1988] for a detailed explanation).
A number of Eocene, Oligocene, and Miocene priacanthid species have been referred to the genus Pristigenys (e.g., Arambourg, Reference Arambourg1967; Danil’chenko, Reference Danil’chenko1980; Fitch and Crooke, Reference Fitch and Crooke1984; Pharisat, Reference Pharisat1991; Micklich and Parin, Reference Micklich and Parin1996; Taverne and Nolf, Reference Taverne and Nolf2010; Prokofiev, Reference Prokofiev2013). Of these, the Oligocene and Miocene species are currently assigned to Priacanthus (Bannikov, Reference Bannikov2010), or, in certain cases (e.g., Pristigenys macropus; Arambourg, Reference Arambourg1967), cannot be assigned to the family Priacanthidae (Prokofiev, Reference Prokofiev2013). As for the other Eocene taxa, they are exclusively based on partially articulated or isolated bones from Belgium and England, representing two species, Pristigenys rutoti and P. hermani (Stinton, Reference Stinton1980; Taverne and Nolf, Reference Taverne and Nolf2010). As discussed above, many of the diagnostic features of the genus Pristigenys refer to the overall physiognomy and proportions of the body, meristics, and pigmentation, all features that cannot be observed in partially articulated or isolated skeletal remains (including otoliths; Taverne and Nolf, Reference Taverne and Nolf2010). Furthermore, Pristigenys substriata differs from these two species from Belgium and England in having a shallow (vs. absent) sagittal crest on the frontals, smooth (vs. serrate) shelf overlying the preopercular sensory canal, a single stout spine at the posteroventral angle of the preopercle (vs. spine absent in P. rutoti and two spines present in P. hermani) and spinules absent on all fin rays. Accordingly, these two Eocene species cannot be assigned to the genus Pristigenys.
Slightly fewer than ten otolith-based species are currently included within the genus Pristigenys (see Taverne and Nolf, Reference Taverne and Nolf2010; Nolf, Reference Nolf2013). However, as previously pointed out by Fitch and Crooke (Reference Fitch and Crooke1984), because there are no otoliths associated with the skeletal remains of Pristigenys substriata, there is no robust support for the generic assignment of these otolith-based species.
Acknowledgments
We are particularly obliged to E. Bernard (The Natural History Museum, London) and G. Clement (Muséum National d’Histoire Naturelle, Paris) for access to fossil material in their care. The manuscript benefited from the critical reviews and comments by W.F. Smith-Vaniz (Florida Museum of Natural History, University of Florida, Gainesville), W.C. Starnes (North Carolina Museum of Natural Science, Raleigh), J.C. Tyler (National Museum of Natural History, Smithsonian Institution, Washington), and an anonymous reviewer. This work was supported by the SYNTHESYS grants of the Natural History Museum, London (GB-TAF-5800) and Museum National d’Histoire Naturelle, Paris (FR-TAF-5419), and grants (ex-60% 2015 and 2016) to one of the authors (G.C.) from the Università degli Studi di Torino. The research of A.F.B. was supported by the Russian Foundation for Basic Research, projects no. 14-04-00005 and 17-04-00212.











