Introduction
The extinct shark Cretodus Sokolov, Reference Sokolov1965, like many other extinct chondrichthyans, was defined based on isolated teeth collected from Upper Cretaceous (Cenomanian–Turonian) marine deposits from all over the world (Cappetta, Reference Cappetta and Schultze2012). However, this large lamniform is also represented by associated skeletal remains recently reported from the middle-upper Turonian Scaglia Rossa of Veneto, northeastern Italy (Amalfitano et al., Reference Amalfitano, Dalla Vecchia, Giusberti, Fornaciari, Luciani and Roghi2017a) and from the middle Turonian Blue Hill Shale Member of the Carlile Shale of Kansas, USA (Shimada and Everhart, Reference Shimada and Everhart2019). The partial skeleton from Kansas was assigned to a new species, Cretodus houghtonorum Shimada and Everhart, Reference Shimada and Everhart2019, whereas the Italian specimen has been regarded since its discovery as close to Cretodus crassidens (Dixon, Reference Dixon1850). Amalfitano et al. (Reference Amalfitano, Dalla Vecchia, Giusberti, Fornaciari, Luciani and Roghi2017a) prudently referred the Italian specimen to Cretodus sp. in a study focused on a pellet-like accumulation of turtle bones alongside the vertebral column of the shark, which was interpreted as its gastric content. Based on dental characters, Shimada and Everhart (Reference Shimada and Everhart2019) considered the specimen discussed by Amalfitano et al. (Reference Amalfitano, Dalla Vecchia, Giusberti, Fornaciari, Luciani and Roghi2017a) to be conspecific with, or closely allied to, Cretodus crassidens. The attribution to Cretodus crassidens is confirmed herein based on a detailed analysis of the Italian specimen and further supported by additional information and comparison with other material, including the holotype, from the English Chalk Group of southern England, UK. A tentative reconstruction of the dentition of this species is also provided here, together with a discussion on several paleobiological traits of the species.
Geological setting
The geological setting of the ‘Lastame’ lithofacies of the Scaglia Rossa of the Lessini Mountains (~30 km N of Verona, Veneto, Italy; Fig. 1), which yielded the Cretodus remains, has been thoroughly described in a series of papers dealing with the remarkable vertebrate assemblage of this Cretaceous Lagerstätte (Amalfitano et al., Reference Amalfitano, Dalla Vecchia, Giusberti, Fornaciari, Luciani and Roghi2017a, Reference Amalfitano, Giusberti, Dalla Vecchia and Kriwetb, Reference Amalfitano, Giusberti, Fornaciari, Dalla Vecchia, Luciani, Kriwet and Carnevale2019; Amadori et al., Reference Amadori, Amalfitano, Giusberti, Fornaciari, Luciani, Carnevale and Kriwet2019, Reference Amadori, Amalfitano, Giusberti, Fornaciari, Carnevale and Kriwet2020a, Reference Amadori, Amalfitano, Giusberti, Fornaciari, Carnevale and Kriwetb; Palci et al., Reference Palci, Caldwell and Papazzoni2013). The 7 m thick nodular/subnodular interval of whitish and pinkish-reddish limestones of the Scaglia Rossa extensively quarried in Verona Province is characterized by abundant echinoids, inoceramids, ammonoids, and rudists, but also by vertebrate remains, e.g., lamniform sharks, sclerorhynchiforms, bony fishes, marine turtles, and mosasaurs. The Lastame spans from Turonian p.p. to Coniacian p.p. (e.g., Cigala-Fulgosi et al., Reference Cigala-Fulgosi, Kotsakis, Massari, Medizza and Sorbini1980; Trevisani and Cestari, Reference Trevisani, Cestari and Scott2007; Walliser and Schöne, Reference Walliser and Schöne2020), but most of the vertebrate skeletons and partial remains so far investigated comes from the middle-upper Turonian interval (e.g., Amalfitano et al., Reference Amalfitano, Dalla Vecchia, Giusberti, Fornaciari, Luciani and Roghi2017a, Reference Amalfitano, Giusberti, Dalla Vecchia and Kriwetb, Reference Amalfitano, Giusberti, Fornaciari, Dalla Vecchia, Luciani, Kriwet and Carnevale2019; Amadori et al., Reference Amadori, Amalfitano, Giusberti, Fornaciari, Carnevale and Kriwet2020a, Reference Amadori, Amalfitano, Giusberti, Fornaciari, Carnevale and Kriwetb).
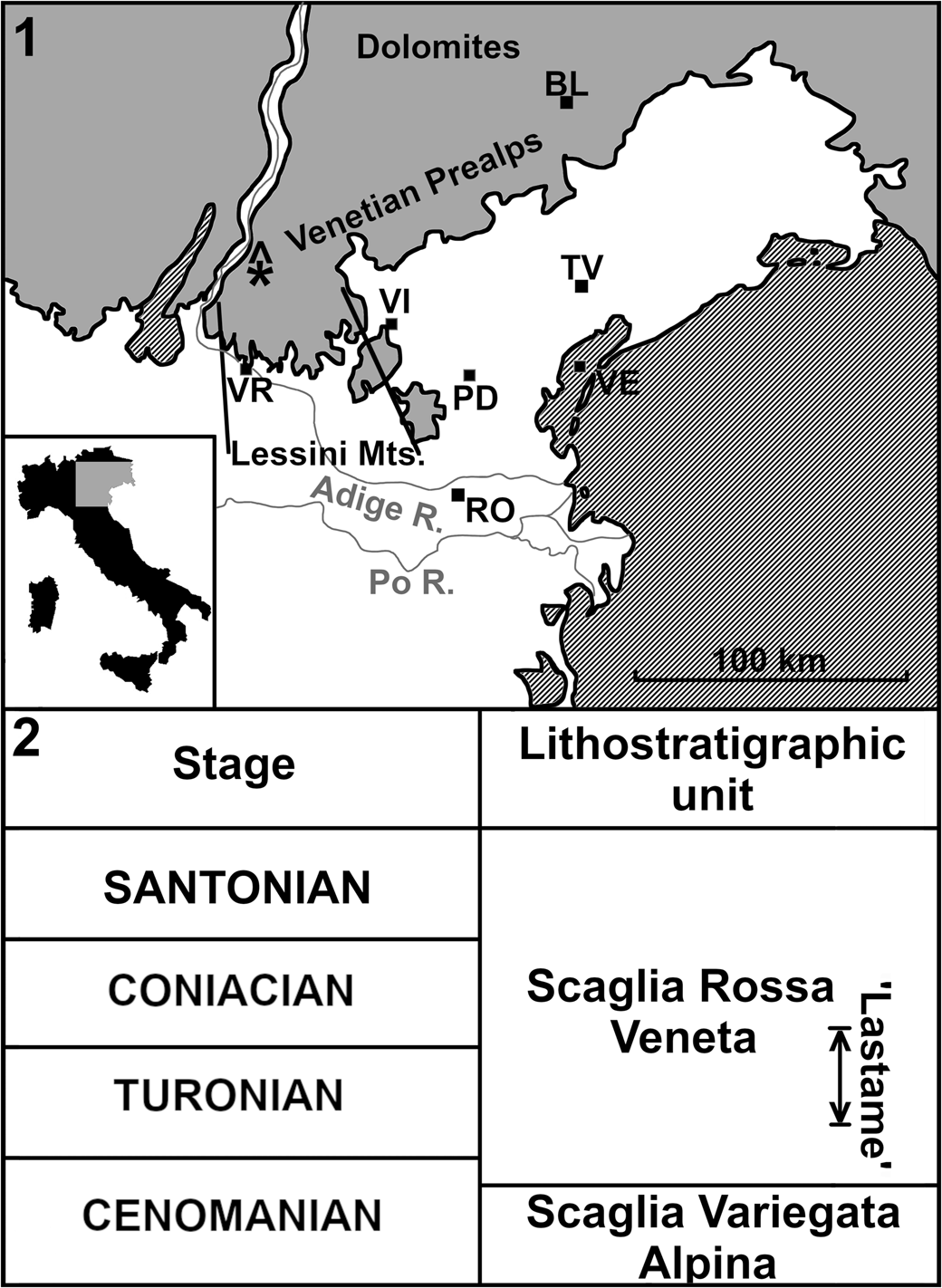
Figure 1. (1) Simplified location map and lithostratigraphic context of the ‘Lastame’ localities. The quarries are located on Mt. Loffa (Lessini Mountains, Verona Province). (2) Lithostratigraphic chart summarizing Cenomanian–Santonian formations of northeastern Italy. ‘Lastame’ spans from Turonian pro parte to Coniacian pro parte. BL = Belluno; PD = Padova; RO = Rovigo; TV = Treviso; VE = Venezia; VI = Vicenza; VR = Verona; * = location of the Benedetti quarry yielding the Italian specimen of Cretodus crassidens; gray = mountain ranges or hills; white = plains and valleys.
The Cretodus remains from southern England (UK) examined herein come from the Upper Cretaceous Chalk of Kent (especially near the town of Lewes; Fig. 2.1) and surrounding counties. The associated remains are referred to the classic ‘Middle Chalk Group’ of southern England (Hopson, Reference Hopson2005). This unit is part of the Chalk Group, specifically the White Chalk Subgroup, deposited in the northwestern part of the Anglo-Paris Basin and equivalent to the lowest part of the Lewes Nodular Chalk Formation, the New Pit Chalk Formation, and the Holywell Nodular Chalk Formation (with the exclusion of the Plenus Marls Member) in the Southern Province (Hopson, Reference Hopson2005; Wilkinson, Reference Wilkinson2011; Gale, Reference Gale2019; Fig. 2.2). The ‘Middle Chalk’ spans from the upper Cenomanian (only the Plenus Marl Member) to the middle Turonian (Wilkinson, Reference Wilkinson2011) and yielded remains of numerous fossil fish taxa, although the number of vertebrates per volume of rock is very low (Mantell, Reference Mantell1822; Woodward, Reference Woodward1902, Reference Woodward1903, Reference Woodward1907, Reference Woodward1908, Reference Woodward1909, Reference Woodward1911, Reference Woodward1912; Kriwet, Reference Kriwet2002; Friedman et al., Reference Friedman, Beckett, Close, Johanson, Johanson, Barrett, Richter and Smith2016); the ichthyofauna therein includes a large variety of bony fishes (e.g., Dixon, Reference Dixon1850; Woodward, Reference Woodward1902, Reference Woodward1903, Reference Woodward1907, Reference Woodward1908, Reference Woodward1909, Reference Woodward1911, Reference Woodward1912; Kriwet, Reference Kriwet2002) and several cartilaginous fishes (e.g., Cretoxyrhina and Ptychodus; Dixon, Reference Dixon1850; Woodward, Reference Woodward1902, Reference Woodward1903, Reference Woodward1907, Reference Woodward1908, Reference Woodward1909, Reference Woodward1911, Reference Woodward1912; Longbottom and Patterson, Reference Longbottom, Patterson, Smith and Batten2002).

Figure. 2. (1) Simplified location map of the English Chalk. Cretaceous rocks at outcrop (dark gray) and concealed (light gray) in England. (2) Lithostratigraphic chart summarizing the Cenomanian–Turonian formations of the Chalk Group. BGS = British Geological Survey, Fm. = Formation. Modified after Hopson (Reference Hopson2005) and Wilkinson (Reference Wilkinson2011).
Materials and methods
The material studied herein consists of a partial articulated skeleton (101 teeth, two segments of 86 disconnected and coin-stacked vertebral centra and fragments of cranial mineralized cartilage) from the Scaglia Rossa exhibited at the Paleontological and Prehistorical Museum ‘Don Alberto Benedetti’ (Museo Paleontologico e Preistorico) of Sant'Anna d'Alfaedo, Verona Province, Italy (MPPSA IGVR 91032) and some specimens from the Chalk Group of southern England (UK). The English material includes a disturbed tooth set with a single vertebral centrum housed at the Booth Museum of Natural History of Brighton, England, UK (BMB 007312), and a disarticulated tooth set (NHMUK PV OR 25786) and several isolated teeth that belong to the collections of The Natural History Museum of London (NHMUK PV OR 25823 [holotype of Cretodus crassidens], 41704, 49951, 44623, and NHMUK PV P 4577, 5402, 11144, 12368, 12860, 12870).
The specimens were photographed using a Nikon D810 camera with a 60–90 mm lens and a Canon PowerShot SX720 HS. Measurements were retrieved through the image analysis software ImageJ (https://imagej.nih.gov/ij/, v. 1.6; Schneider et al., Reference Schneider, Rasband and Eliceiri2012). Images and interpretative drawings of the specimens were produced using the free software packages GIMP (GNU Image Manipulation Program, https://www.gimp.org/, v. 2.10.6) and Inkscape (https://inkscape.org/, v. 0.92). The synonymy list follows the standards proposed by Matthews (Reference Matthews1973) and include selected synonyms directly referring to the material described herein. The growth model was reconstructed using the software package Past 3.26 (https://past.en.lo4d.com/windows; Hammer et al., Reference Hammer, Harper and Ryan2001) and plotted with the Desmos graphing software (https://www.desmos.com/).
The reconstruction of the dentition of Cretodus crassidens provided herein is based on the disarticulated dentition of the Italian specimen MPPSA IGVR 91032, therefore might be subject to biases such as taphonomic or preparation loss and interpretation bias. Specimen MPPSA IGVR 91032, in fact, was discovered between 1996 and 1997 by quarry owners Giovanni and Gianfranco Benedetti and was prepared by Giovanni Benedetti in 2003 (Amalfitano et al., Reference Amalfitano, Dalla Vecchia, Giusberti, Fornaciari, Luciani and Roghi2017a). According to the preparator, the two slabs come from the same layer and were separated by a karst fissure; thus, the skeletal remains in the two slabs should belong to the same individual. Nevertheless, the two slabs differ slightly in color and the different sizes between the last vertebral centrum on the main slab and the first centrum on the second slab suggest that several vertebrae are missing between the two segments (Amalfitano et al., Reference Amalfitano, Dalla Vecchia, Giusberti, Fornaciari, Luciani and Roghi2017a). Furthermore, the vertebrae on the second slab are all glued. Most of the teeth (~70%) detached from slab A when it was exposed by quarry works or remained attached to the counterslab (now missing); they were glued onto the slab later and mostly not in their exact original position (some might be lost). However, the glued teeth undoubtedly belong to this specimen because their morphological characters are identical to the in-situ teeth (Amalfitano et al., Reference Amalfitano, Dalla Vecchia, Giusberti, Fornaciari, Luciani and Roghi2017a). The teeth are figured only on the labial side or the lingual side because most of them are still embedded in the sedimentary matrix or were glued after detaching and are exposed mainly on one side. Chemical or physical preparation to separate the teeth from the matrix (e.g., Siversson et al., Reference Siversson, Lindgren and Kelley2007), as well as sectioning the vertebral centra (e.g., Newbrey et al., Reference Newbrey, Siversson, Cook, Fotheringham and Sanchez2015; Shimada and Everhart, Reference Shimada and Everhart2019), was not possible because the specimen is subject to Italian cultural heritage care laws and cannot be altered without special permission. Ammonium chlorite coating of the teeth (e.g., Siversson et al., Reference Siversson, Lindgren and Kelley2007; Amadori et al., Reference Amadori, Amalfitano, Giusberti, Fornaciari, Luciani, Carnevale and Kriwet2019) was also not performed because of technical issues related to the position and size of the specimen in the Museum exhibition. Teeth in the reconstruction are illustrated from the left side of the jaws and missing elements were filled with mirrored images of teeth from the right side. Tooth numbering of MPPSA IGVR 91032 in the text, figures, and tables is consistent with that of Amalfitano et al. (Reference Amalfitano, Dalla Vecchia, Giusberti, Fornaciari, Luciani and Roghi2017a, fig. 6). Some correction of the tooth measurements of MPPSA IGVR 91032 and new tooth measurements of BMB 007312 are provided in Appendices 1 and 2.
Except for the uninformative calcified cartilage fragments, the morphology of each anatomical element is described and figured in detail herein. Terminology and abbreviations follow usual standards for shark teeth, placoid scales, and vertebral centra (e.g., Ridewood, Reference Ridewood1921; Shimada, Reference Shimada1997a, Reference Shimadab, Reference Shimadac; Siversson, Reference Siversson1999; Cappetta, Reference Cappetta and Schultze2012; Newbrey et al., Reference Newbrey, Siversson, Cook, Fotheringham and Sanchez2015; Shimada and Everhart, Reference Shimada and Everhart2019). Nevertheless, the symphyseal teeth sensu Shimada and Everhart (Reference Shimada and Everhart2019) are referred herein as parasymphyseal teeth, and the intermediate teeth from the same paper as third anterior teeth following Siversson's (Reference Siversson1999) terminology. In the literature of fossil lamniforms, teeth near the symphysis have been consistently addressed as symphyseal teeth (e.g., Siversson, Reference Siversson1999; Shimada, Reference Shimada2002). However, Lamniformes all lack symphyseal teeth (Smith et al., Reference Smith, Johanson, Underwood and Diekwisch2013), therefore the erroneous use in dental nomenclature of symphyseal teeth in Lamniformes should be replaced by the term parasymphyseal teeth, already used in other papers (e.g., Cook et al., Reference Cook, Newbrey, Murray, Wilson, Shimada, Takeuchi and Stewart2011; Siversson et al., Reference Siversson, Ward, Lindgren and Kelley2013). The intermediate position sensu Applegate (Reference Applegate, Gilbert, Mathewson and Rail1967) refers to teeth arising from the area (intermediate bar) between the hollows (bullae) where the other teeth accommodate in the odontaspidid dentition, but the majority of lamniforms do not exhibit this condition, having two separate hollows in the upper jaw without teeth on the intermediate bar and a single hollow in the lower jaw (e.g., Siversson, Reference Siversson1999).
A least square linear regression method was applied to the vertebral centrum length data to estimate the original vertebral count of the shark and to provide a length estimate of MPPSA IGVR 91032 (modelled in Past 3.26, Supplemental Data 1; data from Amalfitano et al., Reference Amalfitano, Dalla Vecchia, Giusberti, Fornaciari, Luciani and Roghi2017a, appendix B). Tooth size is usually employed as a parameter to infer total length of a fossil or extant shark, considering both crown or tooth height (e.g., Gottfried et al., Reference Gottfried, Compagno, Bowman, Kimley and Ainley1996; Shimada, Reference Shimada2003, Reference Shimada2019) and tooth width. Tooth width exhibits less variability than crown height (Bass et al., Reference Bass, D'Aubrey and Kistnasamy1975) and thus has been considered more reliable by several authors (e.g., Newbrey et al., Reference Newbrey, Siversson, Cook, Fotheringham and Sanchez2015; Perez et al., Reference Perez, Leder and Badaut2021). In this paper, we considered both methods, despite that the total jaw width method is not directly applicable because of poor preservation of most of the tooth roots in the sample considered herein. Length estimates are provided using the equation by Shimada and Everhart (Reference Shimada and Everhart2019) that exploits the theoretical linear relationship of crown height (CH) and total length (TL) for Cretoxyrhina mantelli (see Shimada, Reference Shimada2008, fig. 5):
However, Shimada et al. (Reference Shimada, Becker and Griffiths2020) provided a more conservative estimate based on the linear function:
with CH of any anterior tooth in any given non-Alopias macrophagous lamniform taxon, although this possibly underestimated the body total length.
Using the definition of bite circumference sensu Lowry et al. (Reference Lowry, de Castro, Mara, Whitenack, Delius, Burgess and Motta2009) to bypass the lack of a reliable total jaw width and applying the reverse of their formula:
for the great white shark Carcharodon carcharias (Linnaeus, Reference Linnaeus1758), it is possible to have a TL estimate of Cretodus crassidens also from the arch-like arrangement of teeth in specimen MPPSA IGVR 91032 (geometrical approximation in Supplemental Data 2).
An attempt to assess the growth pattern of the fossil shark using the von Bertalanffy growth function (VBGF; von Bertalanffy, Reference von Bertalanffy1938) is proposed below. The VBGF has been widely used to describe the growth of fish (Haddon, Reference Haddon2001). This function was specifically used as a quantitative method to describe the growth of extant elasmobranchs based on growth bands on calcified structures such as vertebral centra (e.g., Cailliet and Goldman, Reference Cailliet, Goldman, Carrier, Musick and Heithaus2004; Goldman, Reference Goldman, Musick and Bonfil2004). The method has also been applied to some extinct sharks (e.g., Shimada, Reference Shimada2008; Cook et al., Reference Cook, Newbrey, Murray, Wilson, Shimada, Takeuchi and Stewart2011; Newbrey et al., Reference Newbrey, Siversson, Cook, Fotheringham and Sanchez2015; Shimada and Everhart, Reference Shimada and Everhart2019; Jambura and Kriwet, Reference Jambura and Kriwet2020; Shimada et al., Reference Shimada, Bonnan, Becker and Griffiths2021). The VBGF provides the best fit for species with slow growth and extended longevity (maximum total length >100 cm of total length and 0.02 < k < 0.25 yr–1, where k is the growth coefficient), such as large pelagic sharks (Liu et al., Reference Liu, Wu, Joung, Tsai and Su2021). It must be mentioned that conventional VBGF analyses uses independent measurements from a dataset with many random samples from a population. The VBGF method applied here exploits original and derived measurements (Table 1) from a single but best-preserved specimen, that would be considered dependent measurements. This exploratory method has been recently applied in other papers (e.g., Shimada and Everhart, Reference Shimada and Everhart2019; Shimada et al., Reference Shimada, Bonnan, Becker and Griffiths2021) and has proven to be a viable approach to attempt to explore the growth pattern of extinct elasmobranchs, although with the obvious limits dependent from the restricted sample. Parameters obtained from the VBGF and other derived measurements are applied with equations from Natanson et al. (Reference Natanson, Kohler, Ardizzone, Cailliet, Wintner and Mollet2006) for longevity to discuss and compare the results of the analyses.
Table 1. Raw measurements (BN, CR) and derived measurements (pCR, TL1, TL2, and CH) based on a vertebral centrum of Cretodus crassidens (Dixon, Reference Dixon1850) (MPPSA IGVR 91032; Fig. 11). BN = band pair number; CH = crown height; CR = center radius; pCR = percentage of center radius; TL = total length.

Specimen BMB 007312 is still embedded in Chalk soft matrix. The matrix was hence collected as powdered residual fallen from the specimen after simple handling. The sample was prepared as unprocessed material on a smear slide and examined under a light microscope at 1250X magnification to establish the stratigraphic position of the specimen. The calcareous nannofossil content of the samples was analyzed with semiquantitative methods (three vertical traverses corresponding to 6–7 mm2) following Gardin and Monechi (Reference Gardin and Monechi1998).
Repositories and institutional abbreviations
Types, figured, and other specimens examined in this study are deposited in the following institutions: Booth Museum of Natural History of Brighton, UK (BMB); Sternberg Museum of Natural History, Fort Hays State University, Hays, Kansas, USA (FHSM); Museo Paleontologico e Preistorico di Sant'Anna d'Alfaedo, Verona, Italy (MPPSA IGVR); and The Natural History Museum, London, UK (NHMUK).
Systematic paleontology
Class Chondrichthyes Huxley, Reference Huxley1880
Subclass Elasmobranchii Bonaparte, Reference Bonaparte1838
Cohort Euselachii Hay, Reference Hay1902
Subcohort Neoselachii Compagno, Reference Compagno1977
Order Lamniformes Berg, Reference Berg1958
Family Pseudoscapanorhynchidae Herman, Reference Herman1979 (sensu Siversson and Machalski, Reference Siversson and Machalski2017)
Genus Cretodus Sokolov, Reference Sokolov1965 (sensu Shimada and Everhart, Reference Shimada and Everhart2019)
Type species
Otodus sulcatus Geinitz, Reference Geinitz1843; ‘unterer Pläner (plenus-marl), upper part of upper Cenomanian, Plauen, Saxony, Germany.
Cretodus crassidens (Dixon, Reference Dixon1850)
Figures 3–10
Selected synonymy:
- †Reference Dixon1850
Oxyrhina crassidens Dixon, p. 367, pl. 31, figs. 13, 13A.
- Reference Woodward1889
Oxyrhina crassidens; Woodward, p. 382.
- Reference Woodward1911
Oxyrhina crassidens; Woodward, p. 205, pl. 44, figs. 1, 2.
- Reference Cappetta and Schultze1987
Cretodus crassidens; Cappetta, p. 98.
- Reference Cappetta and Schultze2012
Cretodus crassidens, Cappetta, p. 255.
- Reference Amalfitano, Dalla Vecchia, Giusberti, Fornaciari, Luciani and Roghi2017a
Cretodus sp.; Amalfitano et al., p. 109, figs. 2, 4, 6–9, 15.
- Reference Shimada and Everhart2019
Cretodus crassidens; Shimada and Everhart, p. 4, fig. 9A–P.

Figure 3. Partial articulated skeleton of Cretodus crassidens (Dixon, Reference Dixon1850) from the middle Turonian of the Scaglia Rossa Veneta of northeastern Italy, MPPSA IGVR 91032: (1) Orthophoto of the specimen. The slab embedding the tooth accumulation, the tessellated cartilage elements, the anterior portion of the vertebral column, and the turtle remains is Slab A. The one embedding the caudalmost vertebral centra is Slab B. (2) Interpretative drawing of (1). (3) Interpretative drawing of the tooth accumulation. Teeth in situ are indicated (yellow in electronic version), with tesselated cartilage elements (dark gray) and anteriormost vertebral centra (light gray). Scale bars = 1 m (1, 2), 20 cm (3).

Figure 4. Selection of representative teeth of Cretodus crassidens (Dixon, Reference Dixon1850) from the middle Turonian of the Scaglia Rossa Veneta of northeastern Italy, MPPSA IGVR 91032: (1) first upper parasymphyseal tooth (no. 16), labial view; (2) first lower parasymphyseal tooth (no. 22), labial view; (3) first upper anterior tooth (no. 37), labial view; (4) second lower anterior tooth (no. 3), labial view; (5) second lower anterior tooth (no. 11), lingual view; (6) second upper anterior tooth (no. 13), labial view; (7) third upper anterior tooth (no. 53), labial view; (8) third lower anterior tooth (no. 24), labial view; (9) first lower lateral tooth (no. 62), labial view; (10) third lower lateral tooth (no. 59), labial view; (11) sixth upper lateral tooth (no. 20), lingual view; (12) fourth upper lateral tooth (no. 61), labial view; (13) seventh lower lateral tooth (no. 103), lingual view; (14) eighth lower lateral tooth (no. 94), labial view; (15) ninth lower later tooth (no. 92), lingual view; (16) commissural upper lateral tooth (no. 104), labial view. Numbers match those used by Amalfitano et al. (Reference Amalfitano, Dalla Vecchia, Giusberti, Fornaciari, Luciani and Roghi2017a, fig. 6). Scale bar = 20 mm.

Figure 5. Associated remains of Cretodus crassidens (Dixon, Reference Dixon1850) from the lower Turonian of the Chalk Group of England, BMB 007312: (1, 2) blocks with embedded teeth; (3, 4) complete vertebral centrum in frontal and dorsal views; (5) two fragments of a partial vertebral centrum in frontal view; (6) isolated teeth from the same tooth set (second one from left in labial view, all others in lingual view). Scale bars = 50 mm.

Figure 6. Second lower anterior tooth of Cretodus crassidens (Dixon, Reference Dixon1850), BMB 007312: (1) labial view; (2) mesial view; (3) distal view; (4) lingual view. Arrow indicates a dental malformation (crenulation on the cutting edge between the distal cusplet and the main cusp). Scale bar = 50 mm.

Figure 7. Interpretation of the dentition pattern in Cretodus crassidens (Dixon, Reference Dixon1850), based on specimen MPPSA IGVR 91032. Numbers in gray match those used by Amalfitano et al. (Reference Amalfitano, Dalla Vecchia, Giusberti, Fornaciari, Luciani and Roghi2017a, fig. 6). * = mirrored right teeth; dotted lines = reconstructed portions of the teeth based on other teeth in the sample; ? possibly missing tooth rows; // = gaps in the reconstruction. Scale bar = 50 mm.

Figure 8. Vertebral centra of Cretodus crassidens (Dixon, Reference Dixon1850), MPPSA IGVR 91032: (1) interpretive drawing of slabs, with glued vertebral centra in dark gray; (2) exposed articular surface of corpus calcareum; (3) exposed intermedialia showing pattern of calcification of the vertebral centrum, showing radial and concentric lamellae patterns; (4) lateral side of vertebral centra exhibiting septae. Black and white scale bars in centimeters.

Figure 9. Placoid scales of Cretodus crassidens (Dixon, Reference Dixon1850), MPPSA IGVR 91032: (1) tricuspid placoid scale, in frontal, lateral, and posterior views; (2) single cusp placoid scale, in frontal, lateral, and posterior views; (3) rounded cusp placoid scale, in frontal, lateral, and posterior views. Scale bar = 500 μm.

Figure 10. Variability in cusplet number and tooth malformations within the dentition of Cretodus crassidens (Dixon, Reference Dixon1850), MPPSA IGVR 91032: (1) tooth no. 10: the right shoulder of the central cusp has a crenulated cutting edge, whereas the other (arrows in detail view) bears a cusplet with two additional cuspules (a tricuspid cusplet); (2) tooth no. 24: the cutting edge between the main cusp and the cusplet has an accessory papilla (arrow in detail view); (3) tooth no. 33: cutting edge between the main cusp and the left cusplet has an accessory papilla (arrow in detail view); (4) tooth no. 35: smaller accessory cusplet occurs mesial to the mesial cusplet (arrow in detail view); (5) tooth no. 7: smaller accessory cusplet occurs mesial to the mesial cusplet (arrow in detail view); (6) tooth no. 32: left cusplet (arrow in detail view) is much smaller than the right cusplet; (7) tooth no. 10: right shoulder of the main cusp has an irregularly crenulated heel (the cusplet is absent; arrows in detail view), whereas the left shoulder has a cusplet with two additional cuspules (tricuspid cusplet); (8) tooth no. 47: the main cusp is bent lingually and the right cusplet is enlarged, bulky, and recurved lingually (arrow in main view; detail view is from the side); (9) tooth no. 58: right distal cusplet is enlarged and high; a distal slice of the main cusp grew independently and has its own apex (arrow in detail view); (10, 11) tooth no. 66 in labial and lateral view: the main cusp is partially twisted; its upper part is blunt and bears a diminutive and demarcated apex (arrow). Numbers matching those used by Amalfitano et al. (Reference Amalfitano, Dalla Vecchia, Giusberti, Fornaciari, Luciani and Roghi2017a, fig. 6). Scale bars = 10 mm.
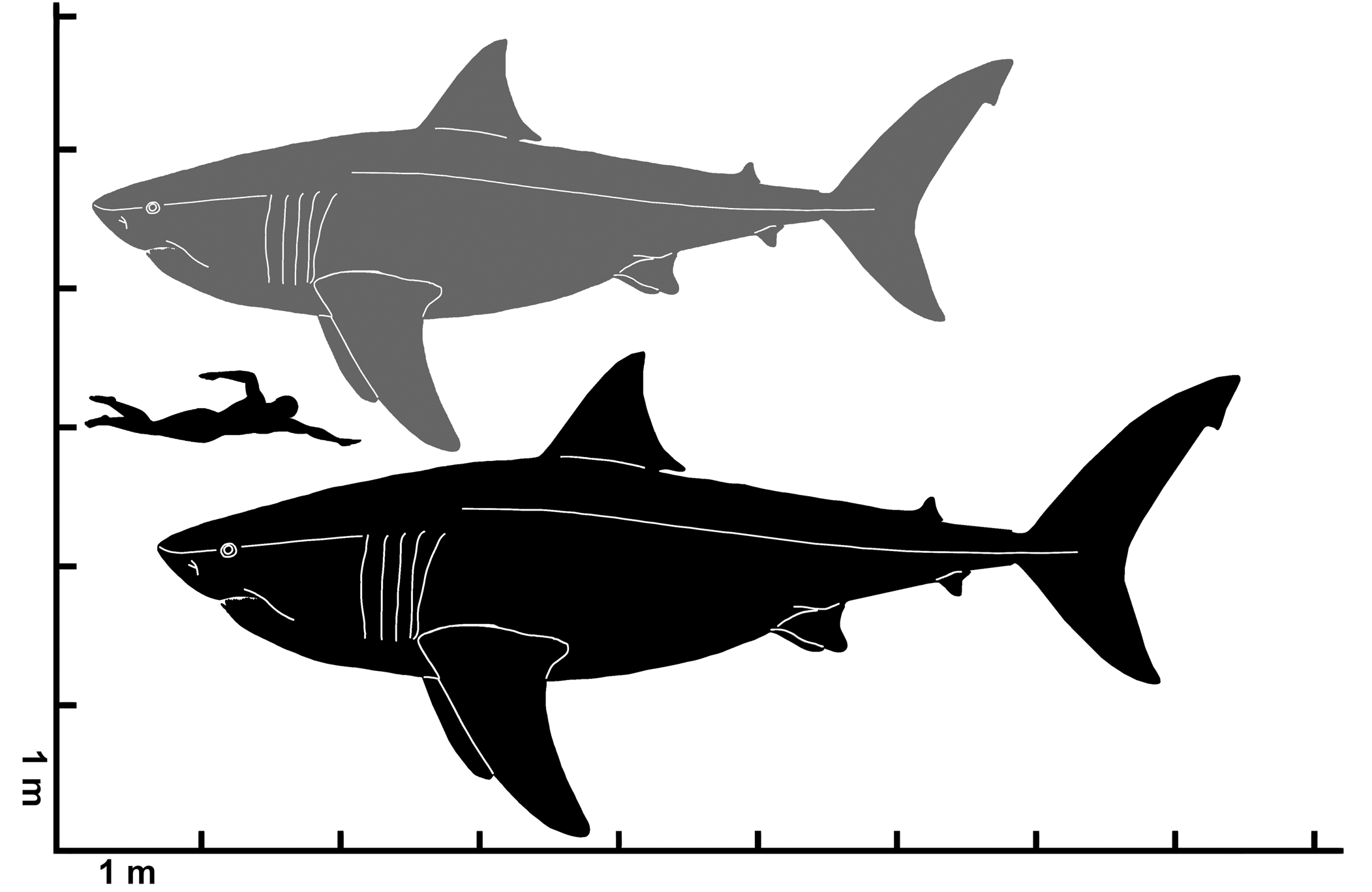
Figure 11. Estimated total length range for Cretodus crassidens (Dixon, Reference Dixon1850), MPPSA IGVR 91032. Gray silhouette indicates the lower limit (660 cm); black silhouette indicates the upper limit (780 cm). Silhouette modified after illustration by O.E. Demuth figured by Cooper et al. (Reference Cooper, Pimiento, Ferrón and Benton2020, fig. 2D).
Holotype
NHMUK PV OR 25823 (isolated tooth).
Diagnosis (emended)
A Cretodus species that differs from all other species of the genus by teeth with mesiodistally broad aspect (crown width to 82% of crown height even in a2), slightly ogival to triangular main cusp, with vertical strong folds and deep grooves on the labial face, weak and well-spaced basal crown ‘costulae’ (or ‘striae’) on labial and lingual faces, robust lateral cusplets (lateral cusplet height ~25–55% of crown height), sinusoid to parabolic crown base and sinusoid to parabolic basal concavity. Aberrant lateral cusplets can be present or replaced by weakly crenulated heels on the shoulders of the main cusp or rounded papillae.
Occurrence
The type locality is Houghton, Sussex (England, UK); the stratigraphic horizon is the ‘Middle Chalk,’ Turonian. All other English specimens come from localities of the ‘Middle Chalk’: Lewes, Sussex, and Whyteleafe, in Surrey (England, UK), although the exact geospatial coordinates are not known. The Italian specimen comes from the ‘Lastame’ lithofacies of Scaglia Rossa Veneta (middle-upper Turonian) of Mt. Loffa, Sant'Anna d'Alfaedo (Lessini Mountains, Veneto, Italy), specifically from Benedetti Quarry (45.6°N, 10.9°E).
The matrix obtained from BMB 007312 contains abundant and well-preserved calcareous nannofossils. The assemblage is well-diversified and contains 13 specimens of Lucianorhabdus maleformis Reinhardt, Reference Reinhardt1966, four specimens of Quadrum gartneri Prins and Perch-Nielsen in Manivit et al., Reference Manivit, Perch-Nielsen, Prins and Verbeek1977 in ~6 mm2, that, together with the absence of Eiffellithus eximius s.s. Huber et al., Reference Huber, Petrizzo, Watkins, Haynes and MacLeod2017 (only one uncertain specimen) and Lucianorhabdus quadrifidus Forchheimer, Reference Forchheimer1972, are indicative of the Biozone UC 7 of Burnett (Reference Burnettp and Bown1999). The corresponding interval would be lower-middle Turonian. Correlation with the foraminiferal zonation of the Chalk Group allows assignment of the sample to the Helvetoglobotruncana helvetica Biozone and possibly the lower part of the Marginotruncana sigali Biozone, corresponding to the British Geological Survey (BGS) Zones 9–10 (Wilkinson, Reference Wilkinson2011; Huber et al., Reference Huber, Petrizzo, Watkins, Haynes and MacLeod2017). Based on biostratigraphic data, the sample is placed between the middle-upper part of the Holywell Nodular Chalk Formation and basal part of the New Pit Chalk Formation.
Description
The English specimens include mainly isolated teeth, previously reported by Woodward (Reference Woodward1911) under various names. There are also several associated specimens: the disarticulated tooth set NHMUK PV 25786 (including seven disarticulated teeth), the disturbed tooth set BMB 007312 and the Italian specimen MPPSA IGVR 91032 (Figs. 3, 4, 8–11). The English specimen BMB 007312 includes 18 teeth, nine still embedded in matrix, associated with two vertebral centra, one fragmentary and the other complete and undeformed, characterized by maximum diameter 95 mm and thickness ~40 mm; this specimen was possibly reported as ‘a small group of associated teeth from Lewes’ by Woodward (Reference Woodward1911, p. 206; Figs. 5, 6). The Italian specimen MPPSA IGVR 91032 is a virtually complete articulated skeleton that includes 120 teeth, 86 vertebral centra, as well as fragments of cranial mineralized cartilage. This specimen also preserves placoid scales retrieved from residues processed from matrix samples, and includes a circular accumulation of bones of a marine turtle alongside the shark vertebral column, interpreted as a gastric pellet (Amalfitano et al., Reference Amalfitano, Dalla Vecchia, Giusberti, Fornaciari, Luciani and Roghi2017a). Dental characters and other morphological features are described in detail below.
Materials
NHMUK PV OR 25786 (disarticulated tooth set, sensu Shimada, Reference Shimada2005), 41704, 49951, 44623; NHMUK PV P 4577, 5402, 11144, 12368, 12860, 12870; BMB 007312 (disturbed tooth set, sensu Shimada, Reference Shimada2005); MPPSA IGVR 91032.
Remarks
The dental characters of the specimens described above are fully consistent with the diagnosis of Cretodus proposed by Shimada and Everhart (Reference Shimada and Everhart2019). In this paper, an emended diagnosis is introduced specifying new details observed on the materials analyzed.
The anatomy of Cretodus crassidens
Teeth
Some of the most representative teeth are illustrated in Figure 4; a reconstruction of the dentition is illustrated in Figure 7. Here follows a description of general dental characters and an interpretation of the tooth pattern within the upper and lower series, considering that the specimen IGVR 91032 preserves a largely disarticulated dentition.
General characters.—Teeth mesiodistally broad (crown width to 82% of crown height even in anterior teeth, specifically a2), with main cusp and a pair of large lateral cusplets, the mesial one divergent and the distal one slightly convergent with respect to main cusp (can be divergent in some teeth). Main cusp is massive, slightly ogival to triangular, with strong vertical folds and two to five deep grooves on labial face. Lateral teeth have triangular main cusp. Lateral cusplets are well separated from main cusp but connected on labial side with basally extended crown base on both root lobes. Numerous, regularly and well-spaced, well-marked, and very short vertical grooves and ridges (‘costulae’ or ‘striae’) are present at crown base on both labial and lingual sides, more marked on labial side. Cutting edges are usually continuous and sharp, connecting main cusp and cusplets. Mesial root lobe is usually slightly pointed, whereas distal lobe is more expanded and rounded. Labial face of root is flat or slightly recessed at crown base. Lingual face of root is overall swollen and bulgy, with lingual protuberance (more evident and protruding in parasymphyseals, anteriors, and mesialmost laterals). Lateral teeth exhibit more splayed root lobes than parasymphyseals and anteriors.
Upper dentition.—Upper teeth are labiolingually thick, massive, and almost straight, with convex labial and lingual faces and tip slightly turned outward in certain specimens. Cutting edges are straight and continuous. Crown base and basal root concavity are deeply sinusoid (especially in anterior teeth). Root-lobes angle is acute in parasymphyseals and anteriors, and is right in laterals. Root lobe apices are directed basally. The upper dentition includes two parasymphyseal rows, three anterior rows, and at least 10 lateral rows, but a large gap is present after L8 (possibly two missing rows). Distinctive characters of tooth rows are:
(1) Upper parasymphyseal teeth (P): Main cusp is rather slender (PCH-PCW ratio 1.4–1.5). P1 exhibits a mesially curved main cusp. P2 almost straight and is the smallest among these teeth. The height of the lateral cusplet represents 32% of crown height.
(2) Upper anterior teeth (A): A1 is reduced (maximum observed height 51 mm) and strongly oblique distally (21° referring to the vertical axis). A2 and A3 have almost upright and apparently symmetrical main cusps; A2 is imperceptibly slanted mesially, and A3 distally. These teeth are rather large (A2 is 61 mm total height, the largest one of upper anteriors) and slender (TH-TW ratio 1.3–1.4). The root lobes of A1 are less splayed with respect to those of the adjacent teeth and U-shaped. The height of the lateral cusplet is ~28–33% of crown height.
(3) Upper lateral teeth (L): Main cusp slightly to strongly distally oblique. Their maximum height ranges from 48 to 16 mm. L1-L3 teeth have an almost upright main cusp, from L4 onward the inclination becomes more evident. L11?-L12? possibly represent the commissural teeth. Teeth are generally larger than high except for the first three, with L2 representing the highest tooth (like in many other lamniforms; Shimada, Reference Shimada2002); lateral cusplet height is 35–54% of crown height, with the ratio increasing toward the commissural rows. Root lobes become generally more divergent distally.
Lower dentition.—Lower teeth have a sigmoid profile (labiolingual direction), nearly flat labial face, and convex lingual face; the tip has a reversed curvature, so that although most of the crown is curved inward toward the mouth cavity, the tip is turned outward (as also observed in other sharks; Frazzetta, Reference Frazzetta1988), which confers a more labiolingually compressed and curved aspect with respect to the upper teeth. Main cusp of lower teeth is generally mesiodistally broader than those of upper teeth. Crown base and root concavity are shallow and more parabolic than those of upper teeth. Cutting edges are sigmoid in profile. Crown base slightly overhangs the upper portion of the root, creating a shallow recess and conferring a slightly inflated aspect. Root-lobes angle is almost right in parasymphyseals and anteriors, obtuse in laterals. Root-lobes apices slightly diverge. Lower dentition includes a single parasymphyseal row, three anterior rows, and at least eight lateral rows, with a gap in the posteriormost positions, including commissural teeth. Distinctive characters of tooth rows are:
(1) Lower parasymphyseal teeth (p): These two teeth have a rather symmetric outline and slender main cusp (PCH-PCW ratio 1.3). Lateral cusplets are strongly divergent. The lateral cusplet height is ~21–26% of crown height.
(2) Lower anterior teeth (a): These are the largest teeth in the dentition (a1 is 67 mm high, whereas a2 is 69 mm high, although incompletely mineralized; in this case it could be higher; 56 mm high in crown height). Tooth a1 is more symmetrical than a2, which is slightly slanted in distal direction. Main cusp of a2 bears four deep enameloid folds on the labial face extending for almost its entire height. Tooth a2 enlarged (crown width to 82% of crown height). Lateral cusplets height is 25–31% of crown height (similar to upper anterior ratio). Tooth a3 is rather large (60 mm TH in functional row), with main cusp slightly bent distally and divergent cusplets. Tooth as high as wide (TH-TW ratio 1.18–1.02). Lateral cusplet height is ~29% of crown height.
(3) Lower lateral teeth (l): Main cusp is almost upright to slightly oblique. The inclination of the cusp increases distally. Total height ranges 32–53 mm, but this range does not include the distalmost rows (which comprises teeth with crown height measuring 22 mm, l7, and 17 mm, l8). Teeth l1–l4 are almost as large as high, with almost upright cusps. Observing the size of l8 compared with those of the corresponding upper laterals, it is possible to suggest that there are at least four missing rows in the commissural part of the lower dentition. Lateral cusplet height represents ~25–53% of crown height, with the ratio increasing toward the distalmost rows.
Vertebral column
MPPSA IGVR 91032 comprises only 86 vertebral centra, 51 on slab A and 35 on slab B. Measurements were provided by Amalfitano et al. (Reference Amalfitano, Dalla Vecchia, Giusberti, Fornaciari, Luciani and Roghi2017a, appendix B). Centra on slab A are partially articulated and represent the anterior part of the vertebral column (Figs. 3.1–3.2, 8.1). Centra on slab B are artificially aligned and decrease in size posteriorly (Figs. 3.1–3.2, 8.1). Vertebral centra are round, with height equal to width (Fig. 8.2). The sizes of the last centrum on slab A and the first centrum on slab B differ by ~20 mm, suggesting that a portion of the vertebral column between the two segments is missing (Amalfitano et al., Reference Amalfitano, Dalla Vecchia, Giusberti, Fornaciari, Luciani and Roghi2017a). This is also indicated by the low vertebral count (86). The diameter of the centra on slab A ranges from 115 mm (vertebra 16) to 53 mm (vertebra 1); that on slab B ranges from 79 mm (first centrum of the slab) to 28 mm (last three centra) (Amalfitano et al., Reference Amalfitano, Dalla Vecchia, Giusberti, Fornaciari, Luciani and Roghi2017a, appendix B). The mean length of the vertebral centra is ~32.5 ± 6.22 mm (Amalfitano et al., Reference Amalfitano, Dalla Vecchia, Giusberti, Fornaciari, Luciani and Roghi2017a, appendix B). The centra are well-calcified and structurally match the definition of ‘lamnoid vertebrae' (sensu Applegate, Reference Applegate, Gilbert, Mathewson and Rail1967, p. 62; Fig. 8.2–8.4). Many centra of MPPSA IGVR 91032 suffer slight taphonomic distortion and some are incomplete, whereas a single one of BMB 007312 is nearly intact. The articular surface of the corpus calcareum is devoid of any kind of ornamentation and generally exhibits ~20 prominent concentric rings that are interpreted to be annual incremental growth bands. These bands are not easily discernable on the vertebral centra of BMB 007312, probably due to erosion. In MPPSA IGVR 91032, a vertebral centrum deprived of the articular surface of the corpus calcareum due to biostratinomic processes (Fig. 8.3) presents concentric lamellae in the intermedialia and radial lamellae that are moderately thick (~1 mm). The radial lamellae tend to branch near the half of the radius length, enlarging and merging into composite and thick longitudinal septae toward the centrum periphery. The longitudinal septae, visible in lateral view in both MPPSA IGVR 91032 and BMB 007312 (Figs. 5.4, 8.4), are well-spaced with low density along the lateral surface of the centrum, separated by large fossae (~20 mm wide), except for the dorsal and ventral sides of the centrum, which exhibit a higher density of septae (three or four in an interval of ~20 mm). Diagonal or transverse septae are absent. Articular foramina are visible on some centra from MPPSA IGVR 91032 but are better visible on the complete vertebral centrum of BMB 007312, being more oval and larger than adjacent fossae (Figs. 5.4, 8.4).
Tessellated cartilage elements
Seven main fragments of tessellated calcified cartilage are present on slab A of MPPSA IGVR 91032. Four occur close to each other at one extremity of the tooth accumulation and three are glued within the turtle remains. These tessellated cartilage elements probably split away from the slab during removal of the counterslab and have erroneously been glued to the turtle remains (Amalfitano et al., Reference Amalfitano, Dalla Vecchia, Giusberti, Fornaciari, Luciani and Roghi2017a). The fragments are flat and range 55–130 mm in length and 40–85 mm in width. Their exposed surface shows the striated texture corresponding to the underlying polygonal prisms (tesserae; Dingerkus et al., Reference Dingerkus, Séret and Guilbert1991; Dean and Summers, Reference Dean and Summers2006), which are well recognizable in the damaged areas. One of the fragments close to the tooth accumulation still has a lateral tooth associated (Fig. 3.3). The presence of a tooth rooted into one of the fragments further indicates that the fragment is part of the palatoquadrate or Meckel's cartilage, possibly a commissural portion considering the tooth size.
Placoid scales
Placoid scales (or dermal denticles) that covered the body of Cretodus crassidens are common in the reddish calcareous marly limestone embedding MPPSA IGVR 91032. They appear as whitish submillimetric prisms in the reddish rock. They are usually composed of a base, with a nutrient foramen at the bottom, and a crown (Fig. 9). The base is often missing because it is delicate and is easily damaged by the action of the acid used to dissolve the limestone to isolate them (Amalfitano et al., Reference Amalfitano, Dalla Vecchia, Giusberti, Fornaciari, Luciani and Roghi2017a). Placoid scale height ranges 1–0.3 mm and width 0.6–0.3 mm. It was not possible to determine how the placoid scales were originally distributed. All scales are ornamented with strong parallel basoapical ridges and plications on the convex anterior face of their crown; ridges and plications do not extend posteriorly and have different sizes (from broad to slender) and general shapes (e.g., rhomboid, Fig. 9.1, 9.2; rounded or drop-like, Fig. 9.3). Except for some tricuspid scales (Fig. 9.1), the cusp is usually single, varying usually from pointed to rounded in shape (Fig. 9.2, 9.3).
Discussion
Taxonomy
Genus-level taxonomy was recently commented upon by Siversson and Machalski (Reference Siversson and Machalski2017, p. 453, 454) and Shimada and Everhart (Reference Shimada and Everhart2019). The types of the first two described species of Cretodus—Cretodus semiplicatus (Münster in Agassiz, Reference Agassiz1843) from the Turonian of Germany and Cretodus crassidens from the Turonian of the UK—are each based on isolated and poorly preserved teeth (Shimada and Everhart, Reference Shimada and Everhart2019). The syntypes of Cretodus semiplicatus are considered marginally diagnostic for their poor preservation state and position (lateroposterior tooth, generally conservative in morphology among lamniforms and thus less taxonomically informative; Siversson and Machalski, Reference Siversson and Machalski2017; Shimada and Everhart, Reference Shimada and Everhart2019). Furthermore, being almost coeval, they could be conspecific with the type specimen of Cretodus crassidens (see Siversson and Machalski, Reference Siversson and Machalski2017). Differences in morphology are addressed as dependent on different tooth position (syntype of Cretodus semiplicatus interpreted as a lower lateroposterior tooth, thus a more posterior position) and ontogenetic stage (Siversson and Machalski, Reference Siversson and Machalski2017). The type specimen of Cretodus crassidens, a large, robust tooth with crenulated heels and lateral cusplet loss, might indicate a senile, female morphotype (Siversson and Machalski, Reference Siversson and Machalski2017).
Shimada and Everhart (Reference Shimada and Everhart2019) clearly distinguished five species of the genus—Cretodus crassidens, Cretodus giganteus (Case, Reference Case2001), Cretodus houghtonorum, Cretodus longiplicatus Werner, Reference Werner1989, and Cretodus semiplicatus—and proposed a phylogenetic hypothesis recognizing three categories based on similarities between the species (‘longiplicatus/semiplicatus-grade,’ ‘giganteus/houghtonorum-grade,’ and ‘crassidens-grade’). Based on the original illustration of the holotype of Cretodus sulcatus (Geinitz, Reference Geinitz1843), it is possible that Cretodus longiplicatus is a junior synonym of Cretodus sulcatus (see Siversson and Machalski, Reference Siversson and Machalski2017). Cretodus crassidens is easily distinguished from its congenerics by its mesiodistally broad teeth with large main cusp, robust lateral cusplets, and long vertical folds (to two-thirds of CH) and grooves on the labial face, especially in anteriormost rows. A rather large main cusp is also present in Cretodus giganteus, but this species seems to be more related to Cretodus houghtonorum for other characters and displays a thinner mesiodistal aspect (Shimada and Everhart, Reference Shimada and Everhart2019). Basal crown ‘costulae’ (or ‘striae’; Shimada and Everhart, Reference Shimada and Everhart2019) are stronger and wider spaced than those of the ‘giganteus/houghtonorum-grade’ (sensu Shimada and Everhart, Reference Shimada and Everhart2019) but weaker and less dense than those of the ‘semiplicatus/longiplicatus-grade’ (sensu Shimada and Everhart, Reference Shimada and Everhart2019) and less evident in large teeth, but the difference is well recognizable in lateral teeth. Lateral cusplets of Cretodus crassidens also differ from those of Cretodus giganteus by having a more robust aspect and divergent mesial and convergent distal cusplets, whereas both the cusplets of Cretodus giganteus are divergent (Shimada and Everhart, Reference Shimada and Everhart2019).
The reconstruction of the dentition of Cretodus crassidens differs from that of the congeneric Cretodus houghtonorum in its mesiodistally broad morphology and strong vertical enameloid folds; the number of parasymphyseal teeth (‘symphyseal’ of Shimada and Everhart, Reference Shimada and Everhart2019), three in the upper dentition and one in the lower one in Cretodus houghtonorum, two in both dentitions in Cretodus crassidens; and the presence of a reduced first upper anterior in Cretodus crassidens, although Shimada and Everhart (Reference Shimada and Everhart2019) interpreted it as an upper ‘intermediate’ (third upper anterior) tooth. The lateral teeth have similarly upright cusp, becoming more oblique distally, in both Cretodus crassidens and Cretodus houghtonorum. The number of lateral tooth rows is different: Cretodus crassidens has at least 10 upper laterals and eight lower laterals, whereas Cretodus houghtonorum has 11 upper laterals and eight lower laterals. Therefore, Cretodus crassidens has the following dental formula:
It must be noted that ontogenetic variation could strongly affect the taxonomy of Cretodus. Tooth size has been variously addressed as dependent on ontogenetic stage and dietary shifts during ontogeny, and care must be taken when using it as taxonomic character (Adnet, Reference Adnet2006; Purdy and Francis, Reference Purdy and Francis2007; Belben et al., Reference Belben, Underwood, Johanson and Twitchett2017; Marramà and Kriwet, Reference Marramà and Kriwet2017).
Comparing the dentition pattern presented herein with that of other Cretaceous lamniform sharks, the lack of specialized intermediate teeth combined with the presence of at least one upper parasymphyseal file is commonly found in Cretaceous taxa other than Cretodus (e.g., Archaeolamna Silversson, Reference Siversson1992, Cardabiodon Siversson, Reference Siversson1999, Cretalamna Glickman, Reference Glickman1958, Cretoxyrhina Glickman, Reference Glickman1958, Haimrichia Vullo, Guinot, and Barbe, Reference Vullo, Guinot and Barbe2016; Shimada, Reference Shimada1997c, Reference Shimada2007; Siverson, Reference Siversson1999; Cook et al., Reference Cook, Newbrey, Murray, Wilson, Shimada, Takeuchi and Stewart2011; Dickerson et al., Reference Dickerson, Shimada, Reilly and Rigsby2013; Siversson et al., Reference Siversson, Ward, Lindgren and Kelley2013, Reference Siversson, Lindgren, Newbrey, Cederström and Cook2015; Vullo et al., Reference Vullo, Guinot and Barbe2016). The presence of a reduced first upper anterior has been reported in other Cretaceous and modern lamniforms (e.g., Alopias Rafinesque, Reference Rafinesque1810, Carcharias Rafinesque, Reference Rafinesque1810, Cardabiodon, Haimrichia, Odontaspis Agassiz, Reference Agassiz1838; Applegate, Reference Applegate1965; Shimada, Reference Shimada2002; Siversson, Reference Siversson1999; Vullo et al., Reference Vullo, Guinot and Barbe2016).
Vertebral centra are considered poor in diagnostic characters in lamniforms, except for a few cases in which their morphology has been observed in detail (e.g., Newbrey et al., Reference Newbrey, Siversson, Cook, Fotheringham and Sanchez2015). Comparing vertebral centrum morphology in Cretodus with that of other neoselachians (Newbrey et al., Reference Newbrey, Siversson, Cook, Fotheringham and Sanchez2015), Cretodus is characterized by distinctive radial lamellae branching and organizing in composite and thick septae toward the periphery of the centrum, divided by large fossae. These structures are different from those observed in other extant and extinct lamniform sharks and could represent a genus- or family-level diagnostic character. However, a family-level taxonomic discussion is well beyond the scope of this paper and needs further evidence from additional complete or associated remains of closely related genera.
Teratologic remarks
A certain variability in the morphological pattern of the cusplets that could be interpreted as malformations (Gudger, Reference Gudger1937; Becker et al., Reference Becker, Chamberlain and Stoffer2000) can be observed in MPPSA IGVR 91032 (Figs. 10, 11; Amalfitano et al., Reference Amalfitano, Dalla Vecchia, Giusberti, Fornaciari, Luciani and Roghi2017a, fig. 6). In tooth number 10, one of the shoulders of the central cusp bears a cusplet with two further cuspules, i.e., a tricuspid cusplet (Fig. 10.1). The other shoulder has a crenulated cutting edge (Fig. 10.1). In three teeth (nos. 22, 24, and 33), a papilla is present on the cutting edge between the central cusp and the normally developed cusplet. In two cases (nos. 24 and 33; Fig. 10.2, 10.3), this occurs in the mesial half of the crown. A small supplementary cusplet is found in three teeth (nos. 2, 7, and 35; Fig. 10.4, 10.5) mesial to the mesial cusplet. In another tooth (no. 9), it is unclear whether the small supplementary cusplet is mesial to the mesial cusplet or distal to the distal cusplet. In two cases (nos. 2 and 35), it is triangular in outline and pointed, whereas in others it is papilla-like. In one tooth (no. 32; Fig. 10.6), one cusplet (the distal one) is smaller than the other.
Besides those with additional or smaller cusplets, the sample also contains malformed teeth (Fig. 10.7–10.11). Tooth number 10 has a weakly and irregularly crenulated heel on the shoulder of the main cusp instead of a well-formed cusplet (Fig. 10.7). This kind of malformation also occurs in three other teeth (nos. 52, 81, and 96). The main cusp of tooth number 47 (Fig. 10.8) is bent lingually and one cusplet is overgrown, bulky, and also recurved lingually. Tooth number 58 (Fig. 10.9) has the central cusp divided into two parts by a deep notch; the distal slice grew independently with its own apex, like a shark tooth figured by Welton and Farish (Reference Welton and Farish1993, fig. 17K). Furthermore, the distal cusplet is overgrown and tall. Tooth number 66 (Fig. 10.10, 10.11) has a main cusp that is partially twisted, and its apical part is blunt with a demarcated, diminutive apex.
Malformed teeth also occur in other Cretodus crassidens specimens from the Chalk Group of England discussed herein. The most common malformation is the loss of lateral cusplet and replacement by a weakly, irregularly crenulated heel on the shoulder of the main cusp. This kind of malformed tooth is present in specimens with associated tooth sets, e.g., NHMUK PV OR 25786 and BMB 007312, but also in isolated teeth, namely the holotype NHMUK PV OR 25823, NHMUK PV OR 49951, and NHMUK PV P 12870.
Malformed teeth have been object of several studies and reports on both fossil and living sharks. Gudger (Reference Gudger1937) provided the probably first methodic report of malformed teeth in extant sharks. Later, some authors focused on tooth pattern reversal (Compagno, Reference Compagno1967; Reif, Reference Reif1980), which also contributed to the development of subsequent studies on pattern formation in development of chondrichthyan dentitions from an evolutionary perspective (Smith et al., Reference Smith, Johanson, Underwood and Diekwisch2013). Other studies focused on feeding-related malformations in early growth stages (Becker et al., Reference Becker, Chamberlain and Stoffer2000; Becker and Chamberlain, Reference Becker and Chamberlain2012). Tooth anomalies in fossil and extant sharks consist mainly of curved or twisted crowns, punctures or notches, deformed or missing cusps, fusion of teeth of the same tooth family, excessive growth of dentine, or abnormal root morphology (Becker et al., Reference Becker, Chamberlain and Stoffer2000; Witzmann et al., Reference Witzmann, Haridy, Hilger, Manke and Asbach2021). Because damaged shark teeth cannot heal, Johnson (Reference Johnson1987) and Welton and Farish (Reference Welton and Farish1993) regarded all shark tooth deformities as developmental in origin, i.e., the result of mutation or damage at an early growth stage (Wiztmann et al., Reference Witzmann, Haridy, Hilger, Manke and Asbach2021). Based on comparisons with extant sharks, Becker et al. (Reference Becker, Chamberlain and Stoffer2000) noted that many of the observed tooth anomalies in extant and fossil sharks were likely from feeding-related injury to the dental lamina of the jaws, particularly by impaction of chondrichthyan and teleost fin and tail spines. Furthermore, these authors explicitly stated that at least some malformed teeth could be caused by disease or mutation. However, the original cause of any dental anomaly could be virtually impossible to determine in a fossil shark (Shimada, Reference Shimada1997c; Wiztmann et al., Reference Witzmann, Haridy, Hilger, Manke and Asbach2021).
Teeth of the sample described above are affected by several malformations that do not differ from malformations listed above and reported in other sharks. Fifteen teeth out of 120 total (including those with atypical size or number of cusplets) exhibit malformations. Despite the incompleteness of the sample, the incidence of malformations in only one specimen is rather high, if compared with other Cretaceous species numbers with larger datasets (e.g., from ~0.015% in Squalicorax kaupi (Agassiz, Reference Agassiz1843) to ~0.36% in Paranomotodon sp.; Becker et al., Reference Becker, Chamberlain and Stoffer2000). This difference is certainly due to a sampling bias, but the high incidence could be due to the peculiar trophic preferences of Cretodus crassidens, as evidenced by the association with marine turtle remains, or to the ontogenetic stage of the individual. Thus, the dental malformations could be interpreted as feeding-related injuries to the dental lamina (e.g., Becker et al., Reference Becker, Chamberlain and Stoffer2000) or as senile characters (especially the lateral cusplet loss and replacement with crenulated heel; Siversson and Machalski, Reference Siversson and Machalski2017).
Body size and body form: paleoecological remarks
The Italian specimen MPPSA IGVR 91032 allows some suppositions on the overall morphology and size of Cretodus crassidens. The two segments preserved measure ~244 cm and 182 cm (although the latter was completely reworked by the preparator). The sum is ~426 cm, but many vertebral centra are missing and this could be an underestimation of the TL of the individual. Applying the least square liner regression method (Supplemental Data 1; r2 = 0.86115), it is possible to estimate an original vertebral count of 169 vertebral centra, similar to the vertebral count of other extant and extinct large lamniform sharks (Springer and Garrick, Reference Springer and Garrick1964; Shimada et al., Reference Shimada, Cumbaa and van Rooyen2006; Natanson et al., Reference Natanson, Skomal, Hoffmann, Porter, Goldman and Serra2018). The mean length of the vertebral centra is ~ 32.5 ± 6.22 mm, therefore the estimated articulated vertebral column is ~549 cm long. Considering the intervertebral disc length (+10%; Newbrey et al., Reference Newbrey, Siversson, Cook, Fotheringham and Sanchez2015), taphonomic compression (+20%; Newbrey et al., Reference Newbrey, Siversson, Cook, Fotheringham and Sanchez2015), and skull length (+50 cm; approximation based on the Cretoxyrhina skull length, 60 cm, from Shimada, Reference Shimada1997c and Newbrey et al., Reference Newbrey, Siversson, Cook, Fotheringham and Sanchez2015, and presuming a shorter and more laterally expanded skull in Cretodus), the estimated total length of the Italian specimen is ~764 cm. The body size estimates reported by Amalfitano et al. (Reference Amalfitano, Dalla Vecchia, Giusberti, Fornaciari, Luciani and Roghi2017a) suggested a TL ranging 661–776 cm based on the size of the vertebral centra. The new estimate, based on the vertebral centrum length and approximate vertebral count, falls within the previous estimated range. This estimate can be compared also to TL estimates based on tooth size. The largest CH measured in the Italian specimen is ~56 mm (Appendix 1) and if the CH of 56 mm is applied to the equation used by Shimada (Reference Shimada2008), the calculation provides a TL of 700 cm. On the other hand, the linear function from Shimada et al. (Reference Shimada, Becker and Griffiths2020) provides an estimated TL of ~659.6 cm, which, if applied to the largest teeth of the Cretodus crassidens dentition, is very close to the previous value of 661 cm based on vertebral centra diameter. Shimada and Everhart (Reference Shimada and Everhart2019) also suggested that Cretodus had a stouter body than Cretoxyrhina, observing the arrangement of the teeth in a 120 cm wide arch (assuming that they are not dislodged) and the 156 cm long, 108 cm wide elliptical accumulation of turtle bones interpreted as gastric content. These indicate a rather stout body, with an abdominal width of at least 108 cm, and a wide, gently curved, laterally expanded mouth aperture, almost semielliptical, more like Galeocerdo Müller and Henle, Reference Müller and Henle1837 (Randall, Reference Randall1992) or, compared to any other lamniform shark, Squalicorax Whitley, Reference Whitley1939 (Shimada and Cicimurri, Reference Shimada and Cicimurri2005). Accordingly, the head would also be laterally expanded. The shape of vertebral centra, almost perfectly circular and rostrocaudally short, is like many other Cretaceous lamniform sharks (e.g., Cretoxyrhina, Shimada, Reference Shimada1997c; Cardabiodon, Newbrey et al., Reference Newbrey, Siversson, Cook, Fotheringham and Sanchez2015; Squalicorax, Shimada and Cicimurri, Reference Shimada and Cicimurri2005). Thus, it could be inferred that this shark had a fusiform body with a circular girth at the trunk region, with a great vertebral column elasticity that allowed carangiform swimming behavior (Newbrey et al., Reference Newbrey, Siversson, Cook, Fotheringham and Sanchez2015). The length of the entire semielliptical dental arch, calculated with a geometric approximation (Supplemental Data 2), is ~137 cm. Using, then, this new value applied to the relation between ‘bite circumference’ and TL by Lowry et al. (Reference Lowry, de Castro, Mara, Whitenack, Delius, Burgess and Motta2009), the estimated TL of the individual is ~813 cm. All of these estimates are more or less consistent, and it is reasonable to define an estimated range of TL between ~660 and ~ 780 cm (Fig. 11), based on the vertebral centrum diameter, which is apparently the least biased proxy.
Another parameter useful to infer body form and paleoecology of a shark is the morphology of the placoid scales (Reif, Reference Reif1982, Reference Reif1985; Reif and Dinkelacker, Reference Reif and Dinkelacker1982), which are preserved in MPPSA IGVR 91032. Amalfitano et al. (Reference Amalfitano, Dalla Vecchia, Giusberti, Fornaciari, Luciani and Roghi2017a) and Shimada and Everhart (Reference Shimada and Everhart2019) discussed the morphology of the placoid scales to infer the swimming behavior of Cretodus. Shimada and Everhart (Reference Shimada and Everhart2019) observed, contra Amalfitano et al. (Reference Amalfitano, Dalla Vecchia, Giusberti, Fornaciari, Luciani and Roghi2017a), that the strong ridges and plications ornamenting the placoid scale crown do not extend posteriorly on the exterior crown face, unlike those of typical fast-swimming lamniforms, including fossil taxa interpreted as fast pelagic-hunting sharks, e.g., Cretoxyrhina (Shimada, Reference Shimada1997b, Reference Shimadac; Shimada et al., Reference Shimada, Cumbaa and van Rooyen2006; Amalfitano et al., Reference Amalfitano, Giusberti, Fornaciari, Dalla Vecchia, Luciani, Kriwet and Carnevale2019) and Cardabiodon (Dickerson et al., Reference Dickerson, Shimada, Reilly and Rigsby2013; Newbrey et al., Reference Newbrey, Siversson, Cook, Fotheringham and Sanchez2015). This morphological condition is also present in scales of the holotype of Cretodus houghtonoum (FHSM VP 17575; Shimada and Everhart, Reference Shimada and Everhart2019). For this reason, the ridge spacing and the crown width from the original sample of Amalfitano et al. (Reference Amalfitano, Dalla Vecchia, Giusberti, Fornaciari, Luciani and Roghi2017a) were measured (Appendix 3). The mean scale crown width is 562 ± 103.31 μm, whereas the mean ridge spacing is 87.32 ± 30.65 μm. Plotting these values with those of extant sharks and Cretoxyrhina mantelli Agassiz, Reference Agassiz1835 (data retrieved from Reif, Reference Reif1985, Shimada, Reference Shimada1997a, and Amalfitano et al., Reference Amalfitano, Giusberti, Fornaciari, Dalla Vecchia, Luciani, Kriwet and Carnevale2019; Fig. 12), the Cretodus crassidens value falls close to the group of large nearshore predators and moderate-speed pelagic predators (sensu Reif, Reference Reif1985). This confirms the assumption made by Shimada and Everhart (Reference Shimada and Everhart2019) that considers Cretodus a more sluggish swimmer with higher maneuverability (as also evidenced by vertebral centra morphology) than thunniform fast-cruising swimmers, e.g., Cretoxyrhina. This assumption is corroborated by association with the turtle remains, which suggests a trophic preference of Cretodus crassidens toward these reptiles, similar to that of the extant tiger shark, Galeocerdo cuvier (Péron and Lesueur in Lesueur, 1922), which displays very similar placoid scale ornamentation combined with the ecological niche of a large nearshore predator (Reif, Reference Reif1985). Shimada and Everhart (Reference Shimada and Everhart2019), in their discussion of the ecology of the genus Cretodus, reported that during excavation of the holotype of Cretodus houghtonoum (FHSM VP 17575) that remains of two additional species of sharks were identified from the same stratigraphic horizon in immediate association with FHSM VP 17575, namely two teeth of Squalicorax cf. S. falcatus (Agassiz, Reference Agassiz1843) (FHSM VP 19273 and 19274) and two partial dorsal fin spines of a hybodontid shark (FHSM VP 19272) comingled with remains of Cretodus houghtonorum. This fossil association is interpreted as another case of a ‘vertebrate three-level trophic chain’ (Kriwet et al., Reference Kriwet, Witzmann, Klug and Heidtke2008, p. 183), but involving three species of sharks in this instance (Shimada and Everhart, Reference Shimada and Everhart2019). The individual of Cretodus houghtonorum must have died shortly after ingesting the hybodont because of the absence of acid-etching alteration on the hybodont remains; the Cretodus houghtonorum carcass was scavenged by S. cf. S. falcatus before or during the decay, followed by disarticulation and scattering of the skeletal and dental elements of Cretodus houghtonorum due to the presence of weak water currents on the seafloor (Shimada and Everhart, Reference Shimada and Everhart2019). Hybodont remains are more common in shallow-nearshore environments, if not in fresh or brackish water environments (e.g., Underwood and Rees, Reference Underwood and Rees2002; Underwood, Reference Underwood2004; Sweetman and Underwood, Reference Sweetman and Underwood2006), and the presumed predator-prey relationship between Cretodus houghtonorum and the hybodontid shark suggests that Cretodus houghtonorum dwelled, at least sometimes, in shallow-nearshore environments. This assumption is supported by the fact that although Cretodus houghtonorum and Cretoxyrhina mantelli lived contemporaneously (Shimada, Reference Shimada2006), the distribution of the two taxa indicates that they likely practiced resource partitioning within the North American Western Interior Sea, because Cretodus houghtonorum teeth are more commonly found in nearshore deposits whereas those of Cretoxyrhina mantelli are common in offshore deposits (Shimada and Everhart, Reference Shimada and Everhart2019). The occurrence of Cretodus crassidens in the pelagic deposits of the Scaglia Rossa and Chalk Group, however, implies that this species preferentially dwelled in the offshore setting. It must be remarked, however, that Cretodus crassidens is represented by a single specimen in the Scaglia Rossa to date, whereas Cretoxyrhina mantelli and Ptychodus spp. remains are much more common (Amadori et al., Reference Amadori, Amalfitano, Giusberti, Fornaciari, Luciani, Carnevale and Kriwet2019, Reference Amadori, Amalfitano, Giusberti, Fornaciari, Carnevale and Kriwet2020a, Reference Amadori, Amalfitano, Giusberti, Fornaciari, Carnevale and Kriwetb; Amalfitano et al., Reference Amalfitano, Giusberti, Fornaciari, Dalla Vecchia, Luciani, Kriwet and Carnevale2019).

Figure 12. Correlation diagram of mean scale crown width (x axis) and mean ridge distance (y axis). Taxa in the light gray cloud are from the group of fast pelagic hunting sharks, whereas those in the dark gray cloud are from the group of large nearshore predators/moderate speed pelagic predators. Note that the Cretodus crassidens mean (star) falls within the cloud of correlation of large nearshore predators and moderate pelagic predators. Diagram modified after Reif (Reference Reif1985). Taxa not otherwise mentioned in the text include: Carcharhinus amblyrhynchos (Bleeker, Reference Bleeker1856), Carcharhinus falciformis (Müller and Henle, Reference Müller and Henle1841), Carcharhinus galapagensis (Snodgrass and Heller, Reference Snodgrass and Heller1905), Carcharhinus melanopterus (Quoy and Gaimard, Reference Quoy, Gaimard and de Freycinet1824), Carcharhinus plumbeus (Nardo, Reference Nardo1827), Isurus oxyrinchus Rafinesque, Reference Rafinesque1810, Lamna nasus (Bonnaterre, Reference Bonnaterre1788), Prionace glauca (Linnaeus, Reference Linnaeus1758), and Sphyrna tudes (Valenciennes, Reference Valenciennes1822).
Age estimate and growth model
One of the vertebral centra of MPPSA IGVR 91032 exhibits a total of 23 pairs of growth bands on the articular surface of the corpus calcareum (Fig. 13, Table 1). Each pair of growth bands is traditionally interpreted to have been deposited annually (Cailliet, Reference Cailliet, Pratt, Gruber and Taniuchi1990; Cailliet and Goldman, Reference Cailliet, Goldman, Carrier, Musick and Heithaus2004), with the total band pair number (BN) indicating the age at death. Such growth band pairs do not necessarily record age or time but rather are related simply to growth or vertebral size (Harry, Reference Harry2018; Natanson et al., Reference Natanson, Skomal, Hoffmann, Porter, Goldman and Serra2018; Natanson and Deacy, Reference Natanson and Deacy2019). Hence, the relationship of BN with time or age is loosely correlated (Natanson et al., Reference Natanson, Skomal, Hoffmann, Porter, Goldman and Serra2018) and generally tends to retrieve an underestimation or an overestimation, because not all growth bands are necessarily consistent with aging for the entire lifespan (Passerotti et al., Reference Passerotti, Andrews, Carlson, Wintner, Goldman and Natanson2014; Harry, Reference Harry2018, Natanson et al., Reference Natanson, Skomal, Hoffmann, Porter, Goldman and Serra2018), especially in older individuals or when later growth bands are not annual. However, it is possible to hypothesize that the individual of Cretodus crassidens MPPSA IGVR 91032 was at least 23 years old at the time of its death if the deposition of each pair of growth bands is annual. The hypothetical von Bertalanffy growth function (VBGF) is here reconstructed based on the individual MPPSA IGVR 91032 and following the same estimates made by Shimada and Everhart (Reference Shimada and Everhart2019), with the aim to compare the ontogenetic growth of the two species Cretodus crassidens and Cretodus houghtonorum (Figs. 14, 15). The VBGF is applied to the size estimates discussed above, namely ~660 cm and ~780 cm. The VBGF fitted to the BN-TL (660 cm) (Fig. 14.1) data gives the following growth parameter estimates: L∞ (maximum TL) = 955.22 cm; L0 (total length at birth) = 141.9 cm; k = 0.045 yr-1; estimated longevity (after Natanson et al., Reference Natanson, Kohler, Ardizzone, Cailliet, Wintner and Mollet2006) = 64.429 yr. The BN-TL (780 cm) data (Fig. 14.2), on the other hand, retrieve the following parameters: L∞ = 1128.8 cm; L0 = 167.7 cm. The longevity estimate of ~64 yr is consistent with those for extant large lamniform sharks (Camhi et al., Reference Camhi, Pikitch and Babcock2008) and that of Cretodus houghtonorum (~55 years; Shimada and Everhart, Reference Shimada and Everhart2019).

Figure 13. Growth band pairs count of Cretodus crassidens (Dixon, Reference Dixon1850), MPPSA IGVR 91032. Vertebral centrum shows 23 incremental growth band pairs presumably formed annually (arrow indicates the center of the centrum; ‘0’ indicates vertebral size at birth). Scale bar = 50 mm.
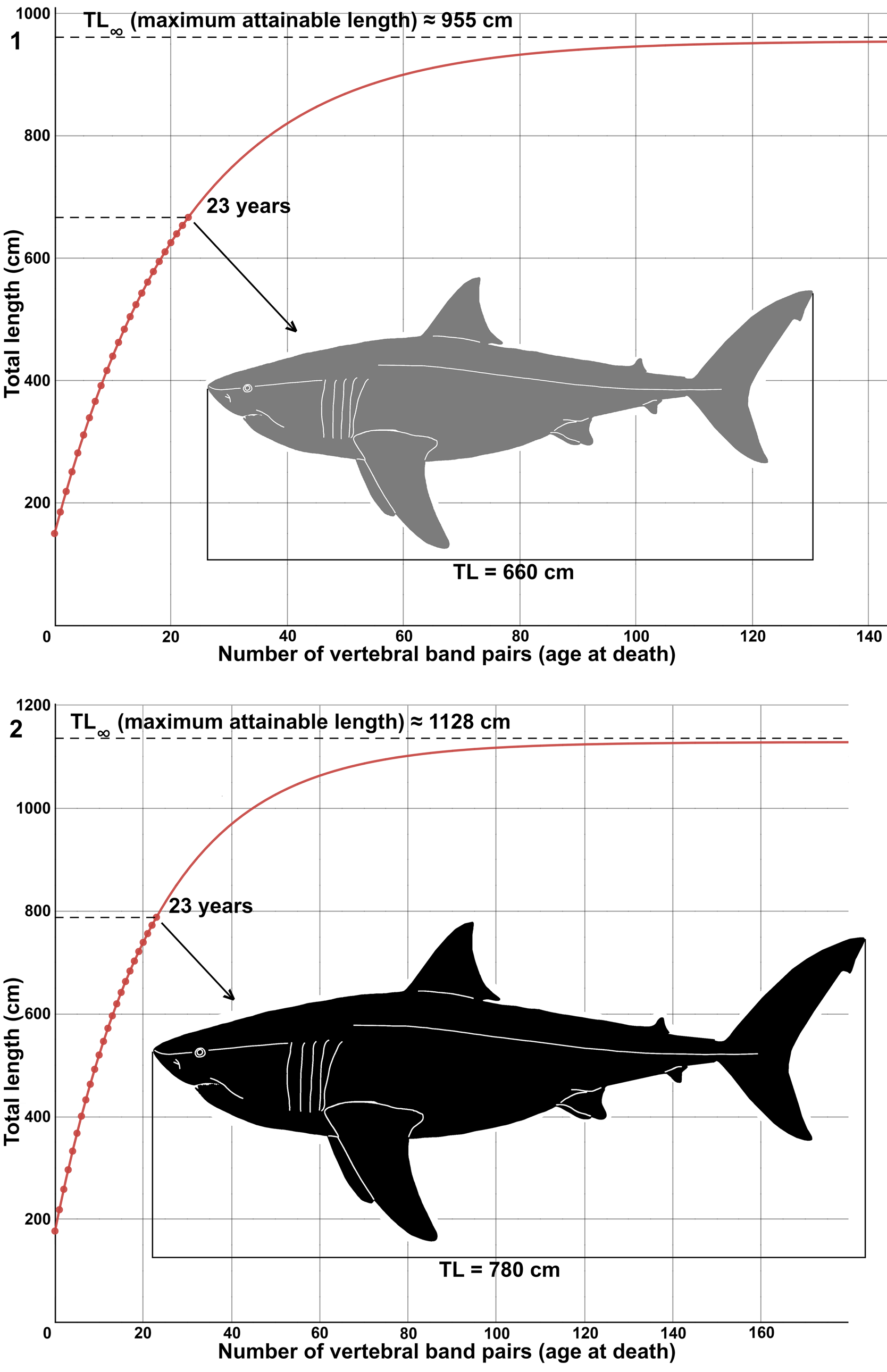
Figure 14. Suggested growth models of Cretodus crassidens (Dixon, Reference Dixon1850) based on MPPSA IGVR 91032 (see text; Table 1): (1) von Bertalanffy growth function fitted to data points that show the relationship of number of vertebral growth band pairs with estimated total body length of 660 cm (TL1); (2) von Bertalanffy growth function fitted to data points that show relationship of number of vertebral growth band pairs with estimated total body length of 780 cm (TL2). Gray silhouette indicates the lower limit (660 cm); black silhouette indicates the upper limit (780 cm). Silhouette modified after illustration by O.E. Demuth figured by Cooper et al. (Reference Cooper, Pimiento, Ferrón and Benton2020, fig. 2D).

Figure 15. Plot of percentage increment of the vertebral radius of Cretodus crassidens (Dixon, Reference Dixon1850) (MPPSA IGVR 91032) and Cretodus houghtonorum Shimada and Everhart, Reference Shimada and Everhart2019 (FHSM VP 17575). Arrows indicate peaks interpreted as maturity onset for Cretodus crassidens (black) and Cretodus houghtonorum (gray).
The percentage increment of the centrum radius of Cretodus crassidens and Cretodus houghtonorum was also considered to find any significant variation in growth rate and for further comparison (Fig. 15). The percentage increment of the two species is almost identical, except for two delayed peaks, which could correspond to the maturity onset at 12–17 years and 10–15 years, respectively. The maturity onset is consistent with those of other large macropredatory lamniform sharks (Camhi et al., Reference Camhi, Pikitch and Babcock2008). The delayed growth peaks could alternatively be caused by sexual dimorphism, with females maturing later than males (Camhi et al., Reference Camhi, Pikitch and Babcock2008). However, despite the strong similarities between the two species, there is no evidence to support Cretodus crassidens and Cretodus houghtonorum dentitions as gynandric heterodont variants, but rather they were vicariant species dwelling in different environments (Cretodus crassidens in offshore settings, Cretodus houghtonorum in nearshore settings) or geographically separated (Cretodus crassidens in the European Tethys and Boreal seas, Cretodus houghtonorum in the Western Interior Seaway; see also Guinot and Cavin, Reference Guinot and Cavin2016 for vicariances related to the Cenomanian diversification event). After attaining maturity, the growth rate in the plot generally tends to become asymptotic, as evidenced after the peaks at 17 and 15 yr, respectively.
The growth model provided herein allows calculation of the possible TL of other specimens. The growth bands are not well preserved on the two vertebral centra of BMB 007312. The specimen also includes the lower second anterior tooth, the CH of which measures 47 mm (Appendix B). If the linear functions CH-TL extrapolated from MPPSA IGVR 91032 is applied, i.e.,
the estimated TL of BMB 007312 results in a range between ~554 cm and ~654 cm (versus an estimated TL range of 546–573 cm when applying the equations used by Amalfitano et al. [Reference Amalfitano, Dalla Vecchia, Giusberti, Fornaciari, Luciani and Roghi2017a] based on vertebral centrum diameter). The age at death was ~16–17 years if the CH is considered, comparing this specimen to MPPSA IGVR 91032 (Table 1).
Conclusions
The specimen MPPSA IGVR 91032 and others described herein provide new morphological and paleobiological information about the Late Cretaceous large-sized shark genus Cretodus. The specimen is assigned to Cretodus crassidens and reconstruction of its dentition based on the Italian specimen reveals the peculiarities of this species with respect to other species of the genus. Cretodus crassidens likely represents a separate lineage within Cretodus (see the phylogenetic hypothesis by Shimada and Everhart, Reference Shimada and Everhart2019). The body form and size estimates are indicative of a large-sized macropredatory shark, reaching a size over the limit that defines gigantic elasmobranch species (> 6 m; Pimiento et al., Reference Pimiento, Cantalapiedra, Shimada, Field and Smaers2019). The maximum estimated total length (9–11 m) and length at birth are comparable to those of the giant Cenozoic genus Otodus Agassiz, Reference Agassiz1838 (Shimada et al., Reference Shimada, Bonnan, Becker and Griffiths2021). Cretodus crassidens was probably characterized by a moderate-speed swimming behavior, suggested by the morphology of the vertebral centra and the placoid scales. This shark was a large predator feeding on, among others, large protostegid turtles, as evidenced by the gastric content preserved within the individual from the basinal high settings of the ‘Lastame.’ Estimated age at death (23 yr) and longevity (64 yr) are consistent with those of extant large lamniform shark populations not subject to anthropic pressure. Interestingly, this study notes that Cretodus crassidens fossils occur both in Boreal and Tethyan domains at the same interval, implying a broad paleobiogeographic distribution. Moreover, Cretodus crassidens exhibits a likely preference toward offshore settings, in contrast with other species of the genus found in nearshore settings, indicating a possible vicariance scenario. Another conceivable hypothesis could be age- (thus size-) related partitioning with very large individuals less suited to hunt in nearshore environments; however, further evidence is required to fully resolve that scenario.
Acknowledgments
R. Zorzin (Museo Civico di Storia Naturale di Verona), E. Bernard (NHMUK), and L. Ismail and J. Cooper (BMB) are deeply thanked for access to the specimens and to information about the collections under their care. Copyright of the NHMUK photos is reserved by K. Webb and The Natural History Museum (NHMUK). S. Castelli (Dipartimento di Geoscienze, Università degli Studi di Padova) is also acknowledged for their valuable contribution with photos and postproduction for the Italian material. Funding for this research was provided by University of Padova (Progetto di Ateneo CPDA159701/2015, titled ‘Reappraisal of two key Fossil-Lagerstätten in Scaglia deposits of northeastern Italy in the context of Late Cretaceous climatic variability: a multidisciplinary approach,’ assigned to E. Fornaciari and Dotazione Ordinaria Ricerca (DOR) funds assigned to L. Giusberti). The reviewers M. Siversson, C. Underwood, and D.J. Ward, and the Editor H.-D. Sues are deeply thanked for helpful and valuable suggestions on an earlier draft of this paper.
Data availability statement
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.31zcrjdnk.
Appendix 1. Corrections to tooth measurements of MPPSA IGVR 91032 (in mm). The numbers of teeth are those reported by Amalfitano et al. (2017a, fig. 6). CH = crown height; CT = crown thickness (labiolingual); CW = crown width; DCL = distal cutting-edge length; LCH = cusplet height; MCL = mesial cutting-edge length; PCH = central cusp height; PCW = central cusp width; TH = tooth height; TT = tooth thickness (labiolingual); TW = tooth width. Gray-shaded cells are those corrected.

Appendix 2. Tooth measurements of BMB 007312 (in mm). The table includes measurements of some of the best-preserved teeth. CH = crown height; CT = crown thickness (labiolingual); CW = crown width; DCL = distal cutting-edge length; LCH = cusplet height; MCL = mesial cutting-edge length; PCH = central cusp height; PCW = central cusp width; TH = tooth height; TT = tooth thickness (labiolingual); TW = tooth width; * = tooth isolated from the matrix.

Appendix 3. Placoid scale measurements of Cretodus crassidens (Dixon, 1850).






















