Introduction
The present work identifies and redescribes some of the species of an ostracode fauna that was recovered from sediments of the Hettangian, Lower Jurassic, Whitmore Point Member, Moenave Formation, Glen Canyon Group. We extend the scientific knowledge of ostracode diversity from this formation by addressing their taxonomy, along with major diversification trends of the ostracode suborder Darwinulocopina Sohn, Reference Sohn1988 (darwinulocopines) during the early Mesozoic. The Moenave Formation (Fig. 1) extends from southwestern Utah State into northwestern Arizona State, USA, and consists of sediments deposited under fluvial-lacustrine environments (Tanner and Lucas, Reference Tanner and Lucas2007). Its fossil record is well documented, comprising numerous and diverse fossil fishes (nonmarine sharks, semionotids, ‘palaeoniscoids,’ coelacanths, and lungfishes), stromatolites, ostracodes, spinicaudatans, and a diverse ichnofauna of invertebrates and vertebrates (Milner and Kirkland, Reference Milner and Kirkland2006; Milner et al., Reference Milner, Birthisel, Kirkland, Breithaupt, Matthews, Lockley, Santucci, Gibson, DeBlieux, Hurlbut, Harris and Olsen2012; Kirkland et al., Reference Kirkland, Lockley and Milner2002, Reference Kirkland, Milner, Olsen and Hargrave2014), which were most recently summarized by Harris and Milner (Reference Harris and Milner2015).
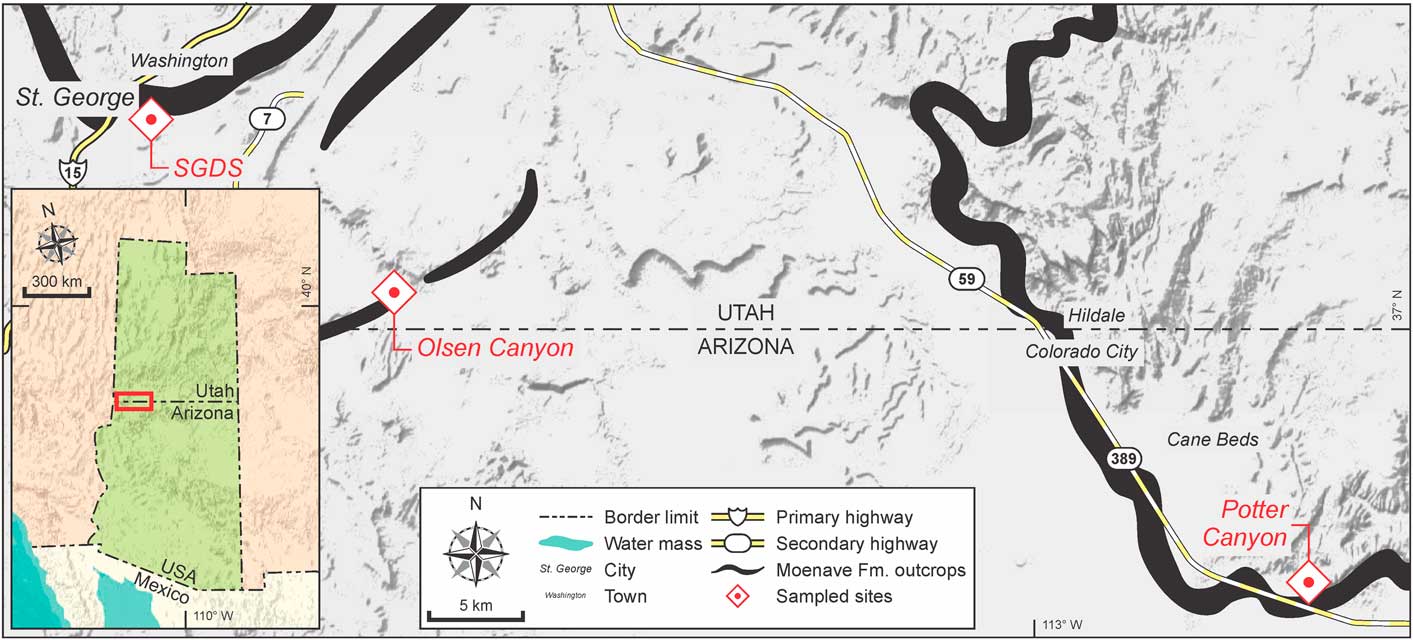
Figure 1 Location of Moenave Formation outcrops and sampling points studied in the present work, Utah and Arizona, USA. Map semiology follows the Federal Geographic Data Committee (2006).
According to Schudack (Reference Schudack2006), the ostracode fauna of the Moenave Formation comprises a restricted number of poorly known species of darwinulocopines (genus Darwinula Brady and Robertson in Jones, Reference Jones1885) and an indeterminate genus of cypridocopines (suborder Cypridocopina Jones in Chapman, Reference Chapman1901). In contrast, the Late Jurassic Morrison Formation, which is widespread through the midwestern USA, presents a diverse fauna of early nonmarine cytherocopines (suborder Cytherocopina Gründel, Reference Gründel1967, e.g., Timiriasevia Mandelstam, Reference Mandelstam1947, and Theriosynoecum Branson, Reference Branson1936). The Morrison is the earliest unit in which cytherocopines and cypridocopines became dominant in US nonmarine faunas, while still associated with the remaining darwinulocopines (Schudack et al., Reference Schudack, Turner and Peterson1998). The faunal composition of the Moenave and Morrison formations is in accordance with major evolutionary trends of these three taxa during the late Paleozoic–early Mesozoic (Whatley, Reference Whatley1988; Sames and Horne, Reference Sames and Horne2012).
The Triassic-Jurassic transition is marked by the end-Triassic extinction event (ETE), one of the five major extinctions in Earth history (Beerling and Berner, Reference Beerling and Berner2002). There are several interpretations of the lithologic position of the ETE in the Glen Canyon Group, ranging from the contact between the Moenave and Kayenta formations (Milner et al., Reference Milner, Birthisel, Kirkland, Breithaupt, Matthews, Lockley, Santucci, Gibson, DeBlieux, Hurlbut, Harris and Olsen2012; Kirkland et al., Reference Kirkland, Milner, Olsen and Hargrave2014) to the transition from the Dinosaur Canyon Member to the Whitmore Point Member of the Moenave Formation (Lucas et al., Reference Lucas, Tanner, Donohoo-Hurley, Geissman, Kozur, Heckert and Weems2011). However, recent chemostratigraphic and detrital zircon studies suggest that the ETE occurred in the lower Dinosaur Canyon Member (Suarez et al., Reference Suarez, Knobbe, Crowley, Kirkland and Milner2017). Considering the evolutionary trend toward drastically diminished diversity of the darwinulocopines at the end of the Triassic, the sediments of the Moenave Formation are very important to the understanding of nonmarine ostracode evolution at a time when the cypridocopines and cytherocopines were supposedly starting to compete for ecological niches with the more ancient darwinulocopines (Sohn, Reference Sohn1988; Whatley, Reference Whatley1988; Horne, Reference Horne2003).
Geological setting
The Moenave Formation is the lowermost unit of the Glen Canyon Group in southwestern Utah and northwestern Arizona (Lucas et al., Reference Lucas, Tanner, Donohoo-Hurley, Geissman, Kozur, Heckert and Weems2011). It was deposited in a retro-arc basin known as the Zuni Sag (Blakey, Reference Blakey1994; Kirkland and Milner, Reference Kirkland and Milner2006; Milner et al., Reference Milner, Birthisel, Kirkland, Breithaupt, Matthews, Lockley, Santucci, Gibson, DeBlieux, Hurlbut, Harris and Olsen2012; Kirkland et al., Reference Kirkland, Milner, Olsen and Hargrave2014), formed on the western edge of the North American craton as a result of its collision with the Cordilleran magmatic arc system that would give rise to the Nevadan orogeny (Renne and Turrin, Reference Renne and Turrin1987; Tanner and Lucas, Reference Tanner and Lucas2009). The basal contact of the Moenave with the underlying Chinle Formation is an abrupt erosive contact in which the conglomerate at the top of the latter is locally eroded (Martz et al., Reference Martz, Kirkland, Milner, Parker and Santucci2017). The overlying Springdale Sandstone Member of the Kayenta Formation also erodes into the top of the Moenave (Lucas and Tanner, Reference Lucas and Tanner2006).
Stratigraphic information
The Moenave Formation is composed of sediments deposited in a mosaic of fluvial, lacustrine, and eolian subenvironments. The Wingate Sandstone Member of the Kayenta Formation—an eolian, sandstone erg sequence—interfingers with the Moenave in the northeastern part of its outcrop belt (Blakey, Reference Blakey1994; Tanner and Lucas, Reference Tanner and Lucas2007; Blakey and Rainey, Reference Blakey and Ranney2008). Stratigraphic nomenclature currently in use subdivides the Moenave Formation into the Dinosaur Canyon and Whitmore Point members (Fig. 2), in ascending order (Biek et al., Reference Biek, Rowley, Hayden, Hacker, Willis, Hintze, Anderson and Brown2009, Reference Biek, Willis, Hylland and Doelling2010). The Dinosaur Canyon Member is a succession of fluvial sandstones and mudstones that become increasingly eolian eastward and represent a northwest-trending alluvial system (Kirkland and Milner, Reference Kirkland and Milner2006; Lucas and Tanner, Reference Lucas and Tanner2007). The Whitmore Point Member sediments were deposited in and along the margins of an extensive lake system—Lake Dixie (Fig. 3)—during a wet climatic interval when an inland delta on the northern end of the Zuni Sag became flooded (Milner et al., Reference Milner, Birthisel, Kirkland, Breithaupt, Matthews, Lockley, Santucci, Gibson, DeBlieux, Hurlbut, Harris and Olsen2012).
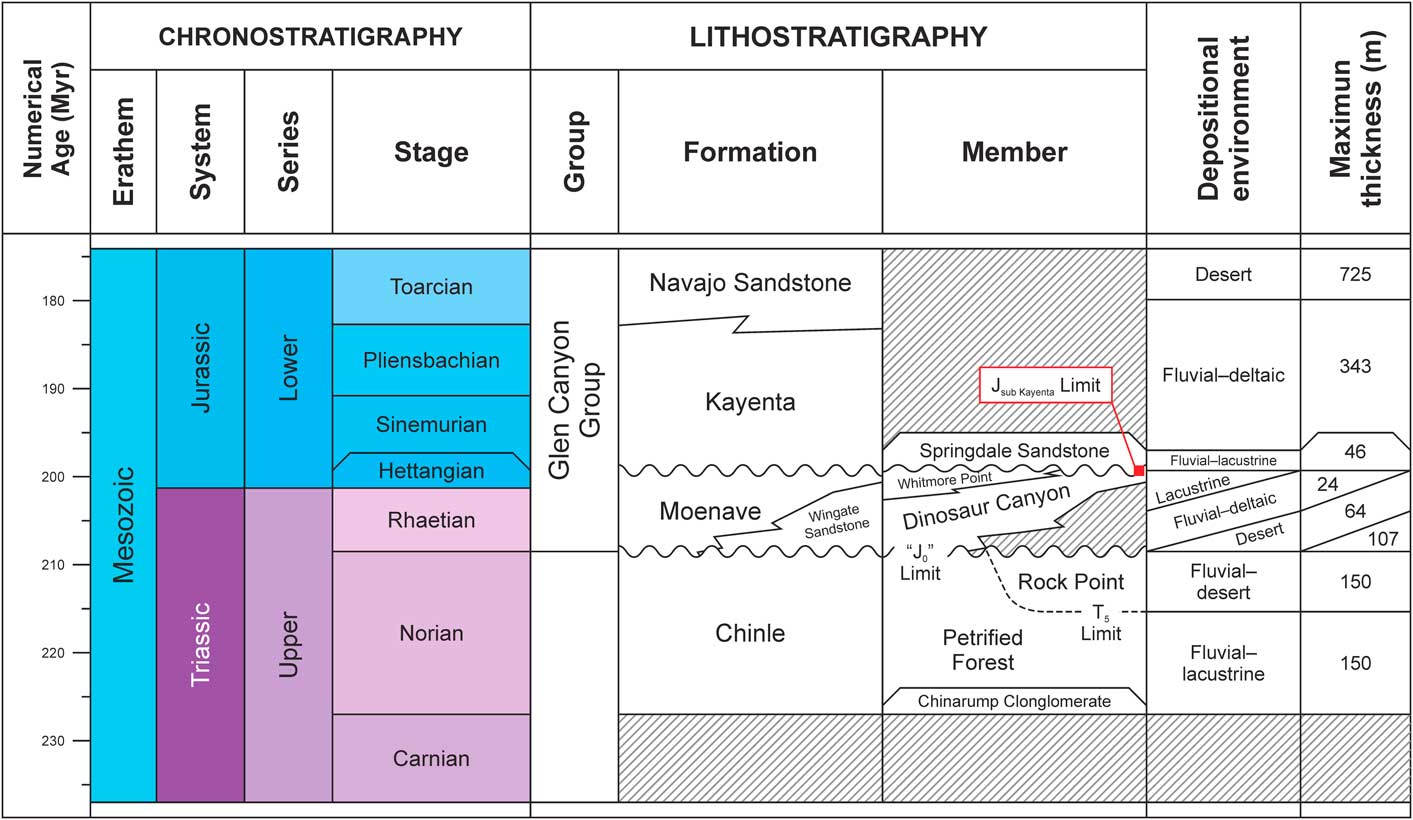
Figure 2 Lithological chart of the Glen Canyon Group in Utah and Arizona, USA, after Lucas et al. (Reference Lucas, Heckert, Estep and Anderson1997), Mathis (Reference Mathis2000), and Tanner and Lucas (Reference Tanner and Lucas2007).
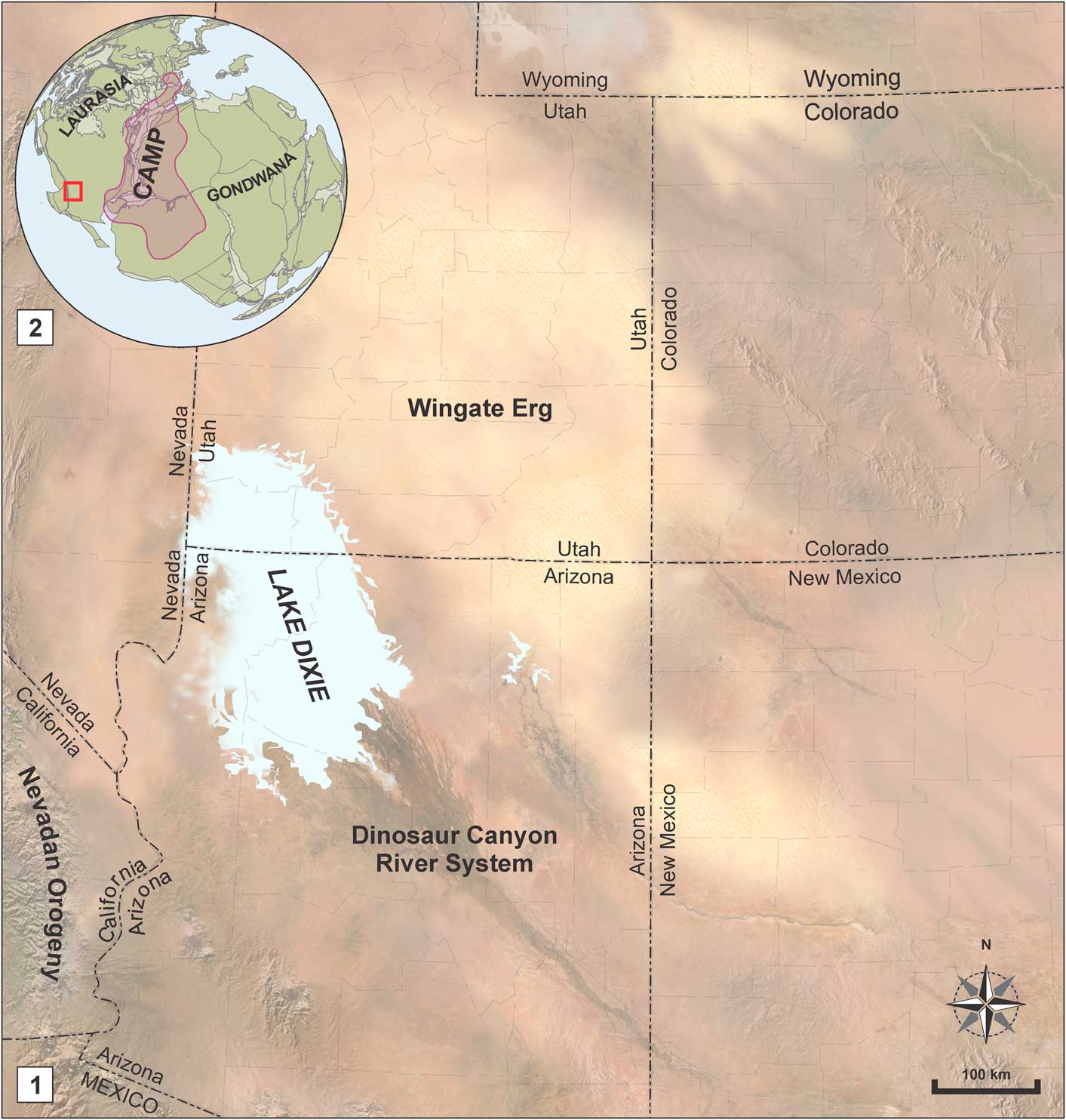
Figure 3 (1) Paleogeographic map of the southwestern USA during Whitmore Point Member time (Blakey and Ranney, Reference Blakey and Ranney2008; Kirkland et al., Reference Kirkland, Milner, Olsen and Hargrave2014), and (2) its position in comparison to Laurasia, Gondwana, and the Central Atlantic Magmatic Province (CAMP) (Suarez et al., Reference Suarez, Knobbe, Crowley, Kirkland and Milner2017). Map semiology follows the Federal Geographic Data Committee (2006).
The upper contact of the Dinosaur Canyon Member with the Whitmore Point Member in southwestern Utah is often placed at the base of a pale green-gray, sometimes stromatolitic limestone unit that is partially replaced by red chert (Wilson, Reference Wilson1967; Kirkland and Milner, Reference Kirkland and Milner2006; Milner et al., Reference Milner, Birthisel, Kirkland, Breithaupt, Matthews, Lockley, Santucci, Gibson, DeBlieux, Hurlbut, Harris and Olsen2012; Kirkland et al., Reference Kirkland, Milner, Olsen and Hargrave2014). Associated with this transition, tracks of the crocodylomorph Batrachopus Hitchcock, Reference Hitchcock1845 and the dinosaurians Eubrontes Hitchcock, Reference Hitchcock1845 and Anomoepus Hitchcock, Reference Hitchcock1848 are thought to be a biostratigraphic indicator of the Triassic-Jurassic boundary in the lower–middle part of the Dinosaur Canyon (Olsen et al., Reference Olsen, Kent, Sues, Koeberl, Huber, Montanari, Rainforth, Fowell, Szajna and Hartline2002; Lucas and Tanner, Reference Lucas and Tanner2007). In the Olsen Canyon area, these track types first appear near the middle of the Dinosaur Canyon (Milner et al., Reference Milner, Birthisel, Kirkland, Breithaupt, Matthews, Lockley, Santucci, Gibson, DeBlieux, Hurlbut, Harris and Olsen2012; Kirkland et al., Reference Kirkland, Milner, Olsen and Hargrave2014). Recent carbon isotope chemostratigraphy, detrital zircon geochronology, and a reassessment of the magnetostratigraphy of Donohoo-Hurley et al. (Reference Donohoo-Hurley, Geissman and Lucas2010) suggests that the ETE is associated with these levels, whereas the Triassic-Jurassic boundary occurs in the upper Dinosaur Canyon to lower Whitmore Point members (Suarez et al., Reference Suarez, Knobbe, Crowley, Kirkland and Milner2017).
Locality information
Sections of the Moenave Formation studied in the present work include those at St. George Discovery Site at Johnson Farm (SGDS) (37°06'05''N, 113°32'08''W) and Olsen Canyon (37°01'02''N, 113°23'22''W), near the city of St. George, Washington County, Utah, and Potter Canyon (36°52'44.4''N, 112°50'31.2''W), Mohave County, Arizona. A full description of the SGDS and Potter Canyon sites was provided by Kirkland et al. (Reference Kirkland, Milner, Olsen and Hargrave2014). A description of the Olsen Canyon section is provided in Supplemental Data 1 of the present work.
Materials and methods
Samples collected at field sites (Fig. 4) were prepared at the Center for Integrative Geosciences of UConn, following the methodology of Antonietto et al. (Reference Antonietto, Do Carmo, Viviers and Adôrno2015, Reference Antonietto, Do Carmo, Viviers, Queiroz Neto and Hunt2016). These samples were disaggregated with 50 mL of 30% hydrogen peroxide (H2O2) in a 1 L tall glass beaker for ~48 h. After disaggregation, 25–50 mL of absolute ethanol was added to each sample/H2O2 solution to interrupt the reaction. The resulting mixtures were individually washed with tap water into 250 mL beakers and dried in a Thermo Fisher Isotemp® 630F furnace at 70ºC. Sediments were then separated using a set of 600, 200, 150 and 75 μm TylerTM sieves and the ostracodes were removed under an Olympus S230 stereoscopic microscope.
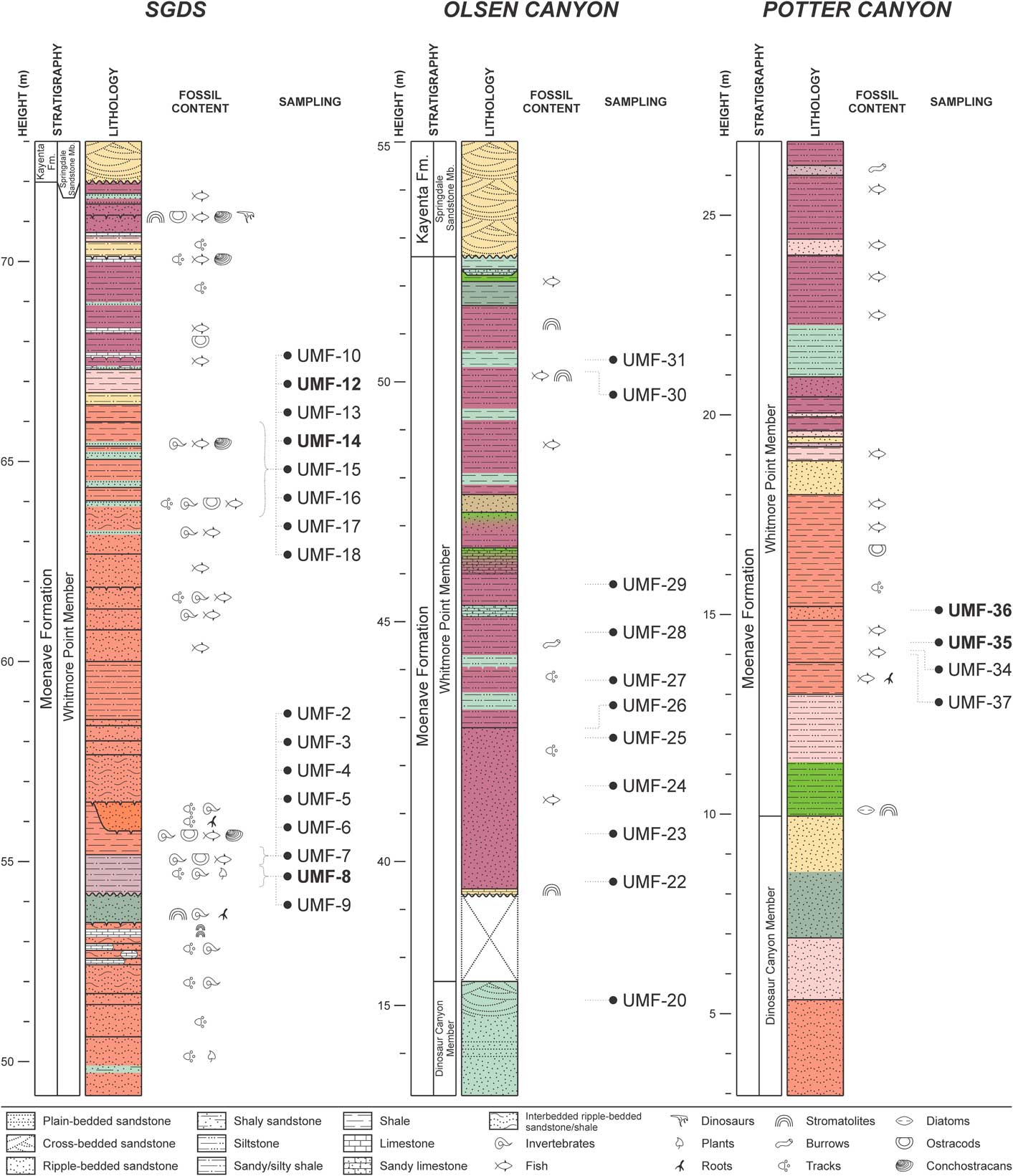
Figure 4 Lithology of sampled Moenave Formation outcrops in the Utah and Arizona, USA: St. George’s Discovery Site at Johnson Farm (SGDS), Olsen Canyon, and Potter Canyon. Sample numbers in bold indicate those that yielded specimens in the present work. Semiology follows the Federal Geographic Data Committee (2006).
Carapaces and valves of the identified species were photographed at the Old World Archaeobotany Laboratory of the University of Connecticut (UConn) Anthropology Department, Mansfield, USA. The scanning electron microscope (SEM) used was a JCM-6000PLUS NeoScope Benchtop, at low-vacuum mode and 10kV filament voltage, with no contrast-enhancing coating. Additional images of rock samples preserving ostracode extinction levels were taken with a Macropod Pro 3D device coupled with Zerene Stacker ‘focus stacking’ software. Specimens were measured with Adobe Acrobat Pro software, by converting photographs into portable document format (PDF) files and then using the software measuring tool. Abbreviations used in the text include: H=height; L=length, W = width.
Repositories and institutional abbreviations
The materials photographed for this study (13 specimens of four species from two families) are housed in the collections of the Natural History Museum of Utah (NHMU) in Salt Lake City, USA, under the prefix UMNH (Utah Natural History Museum, former name of the NHMU), combined with the second prefix IP (invertebrate paleospecimens and localities) and numbers 5292−5304. Samples collected at the SGDS are housed at the SGDS museum (Washington County, Utah), whereas those from Olsen and Potter canyons are currently at the Center for Integrative Geosciences, UConn. Other repositories include The Natural History Museum, London (BMNH).
Systematic paleontology
The classification above family level used herein follows Liebau (Reference Liebau2005). The taxonomy of lower ranks is based on Molostovskaya (Reference Molostovskaya2000), although with reservations that will be further discussed in this paper. Morphological terminology is that used by Moore (Reference Moore1961). Four species recovered from the present samples are listed below. Two were left in open nomenclature, Whipplella? sp. 1 and W.? sp. 2, whereas the other two were identified as Suchonellina globosa (Jones, Reference Jones1862) and S. stricta (Jones, Reference Jones1894). These species were previously assigned to the genus Darwinula, but are herein transferred to Suchonellina Spizharsky, Reference Spizharsky1937. The type species of Suchonellina, S. inornata Molostovskaya, Reference Molostovskaya1980, is properly established.
Current status of the taxonomy of fossil and living darwinulocopines
Previous studies on Paleozoic ostracodes (Spizharsky, Reference Spizharsky1937; Mandelstam, Reference Mandelstam1956; Belousova, Reference Belousova1961; Molostovskaya, Reference Molostovskaya1979, Reference Molostovskaya1980, Reference Molostovskaya1982, Reference Molostovskaya1990, Reference Molostovskaya1996; Kashevarova and Neustrueva, Reference Kashevarova and Neustrueva1982; Kukhtinov, Reference Kukhtinov1985) described several families and genera to accommodate the diversity of darwinulocopines in strata from eastern European Russia. These classification systems relied heavily on features such as the number and format of the individual scars on the central muscle scar field. Other characters utilized were mostly related to the general layout of the carapace, such as the shapes of the dorsal and ventral margins, overlap of the valves along their free margin, the magnitude of the cardinal angles and overall shape of the carapace in dorsal view.
While reviewing Recent and Mesozoic darwinulocopines from Scotland, Wakefield (Reference Wakefield1996) demonstrated how highly variable central muscle scars could be, even within darwinulocopine species, i.e., Darwinula stevensoni (Brady and Robertson, Reference Brady and Robertson1870) and D. cicatricosa Wakefield, Reference Wakefield1994. This variability, along with those of the shape of dorsal and ventral margins, layout in dorsal view and the magnitude of the cardinal angles, were also observed at the generic level by Rossetti and Martens (Reference Rossetti and Martens1998) while reviewing species of Darwinula, Alicenula Rossetti and Martens, Reference Rossetti and Martens1998, Penthesilenula Rossetti and Martens, Reference Rossetti and Martens1998, and Vestalenula Rossetti and Martens, Reference Rossetti and Martens1998. To separate genera, these authors relied on carapace length/height/width (size) ratios and the presence, shape, and position of marginal teeth on the internal surface and keels along the free margin of the valves, but most of all on an analysis of soft parts.
Until now, no attempts have been carried out to integrate taxonomic approaches for fossil and living darwinulocopine species. Aside from carapace size ratios, the presence of morphological characters such as marginal inner teeth, keels along the free margin, and, obviously, soft parts has never been evaluated in fossil taxa. At the same time, there is no assessment of the rates of overlap of the valves along their free margins for the Recent taxa. A wide morphological analysis of key specimens from several families and superfamilies, especially those from the Paleozoic and Cenozoic, will lead to improvements on that topic. Such effort, however, is not in the scope of the present work.
Subclass Ostracoda Latreille, Reference Latreille1802
Superorder Podocopomorpha Kozur, Reference Kozur1972
Order Podocopida Sars, Reference Sars1866
Suborder Darwinulocopina Sohn, Reference Sohn1988
Superfamily Darwinuloidea Brady and Norman, Reference Brady and Norman1889
Family Darwinulidae Brady and Norman, Reference Brady and Norman1889
Genus Suchonellina Spizharsky, Reference Spizharsky1937
Type species
Suchonellina inornata Molostovskaya, Reference Molostovskaya1980, by subsequent designation (herein).
Remarks
The type species for Suchonellina was not assigned in the original description of the genus (Spikharsky, Reference Spizharsky1937), something that was tentatively corrected by Benson et al. (Reference Benson, Berdan, Bold, Hanai, Hessland, Howe, Kesling, Levinson, Reyment, Moore, Scott, Shaver, Sohn, Stover, Swain and Sylvester-Bradley1961), who identified Cythere (Cytherella?) inornata McCoy in King, Reference King1850 as Suchonellina cf. S. inornata Spikharsky, Reference Spizharsky1937. However, it was unnoticed to them that T.N. Spizharsky named this species assuming that it was similar to but not the same as Cythere (Cytherella?) inornata (hence the ‘cf.’, or conferre, from the Latin=compare to). Considering that Cythere (Cytherella?) inornata and S. inornata are not the same species, and that the binomial name S. inornata was first coined as such by Molostovskaya (Reference Molostovskaya1980), the present authors establish S. inornata as the type species of Suchonellina.
Suchonellina globosa (Jones, Reference Jones1862)

Figure 5 Ostracodes of the Whitmore Point Member, Moenave Formation, Hettangian, Lower Jurassic, Utah and Arizona, USA: (1–13) Suchonellina globosa (Jones, Reference Jones1862): (1, 3, 4, 5) adult carapace (UMNH.IP 5293): (1) right lateral view; (3) dorsal view; (4) ventral view; (5) frontal view; (2) adult carapace (UMNH.IP 5303), left lateral view; (6, 8, 9) adult left valve (UMNH.IP 5296): (6) dorsal view; (8) internal view; (9) detail of the anterior inner lamella; (7) adult right valve (UMNH.IP 5297), dorsal view; (10) A-1? juvenile carapace (UMNH.IP 5301), right lateral view; (11) A-2 juvenile carapace (UMNH.IP 5292), right lateral view; (12) A-3 juvenile carapace (UMNH.IP 5302), right lateral view; (13) A-4 juvenile carapace (UMNH.IP 5304), right lateral view; (14–18) Suchonellina stricta (Jones, Reference Jones1894): (14, 16, 17) adult carapace (UMNH.IP 5299): (14) right lateral view; (16) dorsal view; (17) frontal view; (15) adult carapace (UMNH.IP 5300), left lateral view; (18) A-2? right valve (UMNH.IP 5298), internal view; (19–22) Whipplella? sp. 1 (UMNH.IP 5294), right valve: (19) lateral view; (20) internal view; (21) dorsal view; (22) detail of the anterior inner lamella; (23–25) Whipplella? sp. 2 (UMNH.IP 5295), right valve: (23) lateral view; (24) internal view; (25) dorsal view. Scale bar = 100 μm.
1862 Candona (?) globosa Duff, Reference Duff1842; Reference JonesJones, p. 126, pl. 5, figs. 23, 24.
1894 Darwinula globosa; Reference JonesJones, p. 163, pl. 9, figs. 3, 4a, b.
non 1894 Darwinula globosa var. stricta Reference JonesJones, p. 164, pl. 9, fig. 5.
1951 Darwinula sp. (803); Reference WicherWicher, p. 757, pl. 1, fig. 17a, b.
?1962 Darwinula (102); Reference ChristensenChristensen, p. 94, pl. 3, fig. 3a–g.
non 1963 Darwinula globosa; Reference Dadlez and KopikDadlez and Kopik, p. 163, pl. 1, fig. 9.
1963 Notocythere media excelsa Will, Reference Will1953; Reference Dadlez and KopikDadlez and Kopik, p. 139, pl. 1, fig. 10.
1964 ‘Darwinula’ globosa (Jones, Reference Jones1862); Reference AndersonAnderson, p. 135, pl. 15, fig. 128.
?1979 Darwinula spp.; Reference Sohn and ChatterjeeSohn and Chatterjee, p. 584, pl. 1, fig. 6, pl. 2, figs. 1–3, 5–9, 14–16.
?1983 Darwinula liulingchuanensis Zhong, Reference Zhong1964; Reference Wei, Li and JiangWei et al., p. 172, pl. 53, fig. 5D, R.
1983 Darwinula longovata Wei; Reference Wei, Li and JiangWei et al., p. 172, pl. 53, fig. 6D, R.
1984 Darwinula longovata; Reference WeiWei, p. 350, pl. 2, figs. 14–17.
1987 Darwinula sp.; Reference KietzkeKietzke, p. 123, fig. 2J–N.
1988 Darwinula subovatiformis Su et al., Reference Su, Li, Pang and Chen1980; Reference XuXu, p. 1286, pl. 2, fig. 4.
1995 Darwinula sarytirmensis (sic) Sharapova [in Mandelstam], Reference Mandelstam1947; Reference Kietzke and LucasKietzke and Lucas, p. 27, fig. 2I–L.
2002 Darwinula maanshanensis Hou; Reference Hou, Gou and ChenHou et al., p. 753, pl. 303, figs. 1–4.
2006 Darwinula sp.; Reference SchudackSchudack, p. 428, fig. 2A–F.
Lectotype
BMNH I 6086, designated by Anderson (Reference Anderson1964).
Occurrence
Rhaetian, Upper Triassic, Inner Moray Firth Basin, Linksfield, Elgin, Morayshire County, Scotland, UK (type locality) (Jones, Reference Jones1862, Reference Jones1894; Anderson, Reference Anderson1964). Upper Triassic, Wayaobu Formation, Shaanxi Province (Xu, Reference Xu1988); Lower Jurassic, Ziliujing Formation, Sichuan Province, and Xialufeng Formation, Yunnan Province, China (Wei et al., Reference Wei, Li and Jiang1983; Wei, Reference Wei1984; Hou et al., Reference Hou, Gou and Chen2002). Lower Rhaetian, Upper Triassic, Germany (Wicher, Reference Wicher1951). Lower Rhaetian, ‘Zbąszynecka Series,’ Upper Triassic, Poland (Dadlez and Kopik, Reference Dadlez and Kopik1963). Upper Triassic, Upper Shale and Redonda members, Chinle Formation, and Sloan Canyon Formation, New Mexico, USA (Kietzke, Reference Kietzke1987). Sinemurian to Toarcian, Lower Jurassic, Kayenta Formation, Glenn Canyon Group, Arizona, USA (Kietzke and Lucas, Reference Kietzke and Lucas1995). In the present work, extended to the Hettangian stage, Lower Jurassic, Whitmore Point Member, Moenave Formation, Arizona and Utah, USA.
Materials
Carapaces: UMNH.IP 5303 (adult; 0.88 mm L, 0.40 mm H, 0.35 mm W), UMNH.IP 5293 (adult; 0.94 mm L, 0.47 mm H, 0.39 mm W), UMNH.IP 5301 (A-1 juvenile?; 0.80 mm L, 0.41 mm H, 0.34 mm W), UMNH.IP 5292 (A-2 juvenile; 0.67 mm L, 0.35 mm H, 0.25 mm W), UMNH.IP 5302 (A-3 juvenile; 0.63 mm L, 0.31 mm H, 0.20 mm W), and UMNH.IP 5304 (A-4 juvenile; 0.50 mm L, 0.27 mm H, 0.17 mm W). Valves: UMNH.IP 5296 (adult, left; 0.95 mm L, 0.49 mm H, 0.24 mm W) and UMNH.IP 5297 (adult?, right; 0.84 mm L, 0.43 mm H, 0.18 mm W).
Remarks
The diagnosis of this species follows Jones (Reference Jones1862) and Anderson (Reference Anderson1964). The original proposal of the species, however, is from Duff (Reference Duff1842), although it was not formally described or illustrated in that monograph. Its generic placement was changed to Suchonellina, as proposed by Spizharsky (Reference Spizharsky1937) to accommodate Permo-Triassic darwinulocopine species from Russia. According to Rossetti and Martens (Reference Rossetti and Martens1998), the genus Darwinula presents different valve overlap and general carapace size ratios in comparison to S. globosa. Other than that, some morphological characters attributed to Suchonellina by Molostovskaya (Reference Molostovskaya1990) seem to be observable in specimens of the present work: (1) in dorsal view, it is hinted that their hinge displays an arrangement of two marginal, enlarged protuberances separated by a sulcus in the right valve, and (2) the frontal view indicates a shifting upward of the right valve along the overlapping in the ventral margin. Darwinula maanshanensis Hou in Hou et al. (Reference Hou, Gou and Chen2002) (nom. nov. for the homonym D. longovata Wei in Wei, Li, and Jiang, Reference Wei, Li and Jiang1983) also displays the aforementioned morphological characters and is herein synonymized with S. globosa. Specimens of ‘S. globosa’ were misfigured by Dadlez and Kopik (Reference Dadlez and Kopik1963) as Notocythere media excelsa Will, Reference Will1953, and vice versa. Specimens identified as A-2 juveniles of D. sarytirmenensis Sharapova in Mandelstam, Reference Mandelstam1947 (misspelled as ‘Darwinula sarytirmensis’) by Kieztke and Lucas (Reference Kietzke and Lucas1995) are actually adult individuals of S. globosa, based on the presence of a posterior brood pouch, clearly apparent in dorsal view. Synonymy with the present species is tentative for: (1) Darwinula (102) of Christensen (Reference Christensen1962), because it is a similar, but slightly smaller, species; and (2) Darwinula spp. of Sohn and Chatterjee (Reference Sohn and Chatterjee1979) and (3) D. liulingchuanensis Zhong, Reference Zhong1964 of Wei et al. (Reference Wei, Li and Jiang1983), due to poor preservation of specimens affecting general layout observations. The size ratios between instars of S. globosa is like that found between ontogenetic stages of D. stevensoni and Vestalenula sp. by Smith and Kamiya (Reference Smith and Kamiya2008). However, Smith et al. (Reference Smith, Kamiya and Horne2006) noted that adult males of V. cornelia Smith, Kamiya, and Horne, Reference Smith, Kamiya and Horne2006 are also similar in size ratios to A-1 juveniles. Therefore the present authors, while figuring some of the instars of S. globosa, attributed a doubtful identification to specimens identified as A-1 juveniles.
Suchonellina stricta (Jones, Reference Jones1894)
1894 Darwinula globosa (Duff, Reference Duff1842) var. stricta Reference JonesJones, p. 164, pl. 9, fig. 5.
?1963 Darwinula liassica; Reference Dadlez and KopikDadlez and Kopik, p. 138, pl. 1, fig. 8.
1964 Darwinula stricta Jones, Reference Jones1894; Reference AndersonAnderson, p. 136, pl. 15, figs. 129–132.
1977 Darwinula cf. liassica (Brodie, Reference Brodie1843); Reference Ye, Gou, Hou and CaoYe et al., p. 263, pl. 21, fig. 3a, b.
1983 Darwinula xinpingensis Jiang; Reference Wei, Li and JiangWei et al., p. 176, pl. 54, fig. 6D, R.
?1989a Gerdalia sp.; Reference KietzkeKietzke, p. 186, fig. 4F.
1991 Gerdalia sp.; Reference Kietzke and LucasKietzke and Lucas, p. 193, fig. 3A.
1999 Gerdalia sp.; Reference SwainSwain, p. 168, pl. 17, figs. 43, 44.
2002 Darwinula xinpingensis; Reference Hou, Gou and ChenHou et al., p. 773, pl. 310, figs. 7, 8.
Lectotype
BMNH I 6089, designated by Anderson (Reference Anderson1964).
Occurrence
Rhaetian, Upper Triassic, Penarth Group, Pylle hill, Bristol, Bristol County, England, UK (type locality); also at Glamorgan, Gloucestershire, Shropshire, and Warwickshire counties (Jones, Reference Jones1894; Anderson, Reference Anderson1964). Rhaetian, Upper Triassic, Inner Moray Firth Basin, Linksfield, Elgin, Morayshire, Scotland, UK (Jones, Reference Jones1894). Upper Triassic, Baijizu Formation, Yunnan, China (Ye et al., Reference Ye, Gou, Hou and Cao1977). Uppermost Triassic, Shezi Formation, Yunnan, China (Wei et al., Reference Wei, Li and Jiang1983). Carnian, Upper Triassic, Tecovas Formation, Texas, USA (Kieztke and Lucas, Reference Kietzke and Lucas1991). In the present work, extended to the Hettangian, Lower Jurassic, Whitmore Point Member, Moenave Formation, Arizona and Utah, USA.
Materials
Carapaces: UMNH.IP 5299 (adult; 0.91 mm L, 0.41 mm H, 0.30 mm W), UMNH.IP 5300 (adult; 0.84 mm L, 0.38 mm H, 0.31 mm W). Valves: UMNH.IP 5298 (incomplete A-2 juvenile?, left; 0.34 mm H, 0.13 mm W).
Remarks
For a discussion on the generic placement of this species in Suchonellina, see the Remarks section under S. globosa (above). The diagnosis of S. stricta follows Jones (Reference Jones1894) and Anderson (Reference Anderson1964). The original proposal of the species, however, is from Brodie (Reference Brodie1843), although the species was not formally described or illustrated in that monograph. Due to major morphological similarity between the type specimens of S. stricta and Darwinula xinpingensis Jiang in Wei et al. (Reference Wei, Li and Jiang1983), these are herein synonymized, as also are specimens of D. cf. D. liassica of Ye et al. (Reference Ye, Gou, Hou and Cao1977). Synonymy with the present species is tentative for: (1) D. liassica of Dadlez and Kopik (Reference Dadlez and Kopik1963), because no size measurements for the illustrated specimens were given; and (2) Gerdalia sp. of Kietzke (Reference Kietzke1989a), also figured by Swain (Reference Swain1999), for having a very similar, but not equal, general layout.
Superfamily Darwinuloidoidea Molostovskaya, Reference Molostovskaya1979
Family Darwinuloididae Molostovskaya, Reference Molostovskaya1979
Genus Whipplella Holland, Reference Holland1934
Type species
Whipplella cuneiformis Holland, Reference Holland1934.
Whipplella? sp. 1
?2006 Cypridoidea indet.; Reference SchudackSchudack, p. 428, fig. 2H.
Occurrence
Hettangian, Lower Jurassic, Whitmore Point Member, Moenave Formation, Arizona and Utah, USA.
Material
UMNH.IP 5294, valve (0.79 mm L, 0.42 mm H, 0.18 mm W).
Remarks
The generic diagnosis follows Holland (Reference Holland1934) and Kukhtinov (Reference Kukhtinov2004). The single specimen of Whipplella? sp. 1 is badly preserved, which allows only a tentative placement in the genus Whipplella, based on the general shape of the valves in lateral and dorsal view. Whipplella? sp. 1 and Cypridoidea indet. by Schudack (Reference Schudack2006) could be the same species, but the specimens of the latter are badly preserved, and the attribution is at best doubtful.
Whipplella? sp. 2
Occurrence
Hettangian, Lower Jurassic, Whitmore Point Member, Moenave Formation, Arizona and Utah, USA.
Material
UMNH.IP 5295, valve (0.90 mm L, 0.49 mm H, 0.16 mm W).
Remarks
The generic diagnosis follows Holland (Reference Holland1934) and Kukhtinov (Reference Kukhtinov2004). The single specimen of Whipplella? sp. 2 is badly preserved, which allows only a tentative placement in the genus Whipplella, based on the general shape of the valves in lateral and dorsal view.
Results and discussion
In the present taxonomic review, four ostracode species were identified from sediments of the Whitmore Point Member, deposited in and along the margins of a lacustrine environment, referred throughout the region as Lake Dixie, and associated/interbedded mudflat environments during the earliest Jurassic. The composition and stratigraphic position of this ostracode fauna makes it an interesting case to understand the demise of darwinulocopines as the main nonmarine ostracodes, both in terms of abundance and diversity, during early to middle Mesozoic times.
Evolutionary history of late Paleozoic–early Mesozoic darwinulocopines
Darwinulocopines are known in the fossil record since the late Paleozoic (Carbonel et al., Reference Carbonel, Colin, Danielopol, Löffler and Neustrueva1988). The group flourished during the Carboniferous–Triassic, an interval from which the majority of darwinulocopine families, genera, and species has been so far described. During that time, the group presented greater morphologic variety (Wang, Reference Wang1980; Kashevarova and Neustrueva, Reference Kashevarova and Neustrueva1982; Sohn, Reference Sohn1987; Molostovskaya, Reference Molostovskaya1990) than shown by darwinulocopine flocks in earlier strata. Nonmarine faunas of the Carboniferous–Triassic also contained forms likely more closely related to the Carbonitidae Sohn, Reference Sohn1985 and Geisinidae Sohn in Benson et al., Reference Benson, Berdan, Bold, Hanai, Hessland, Howe, Kesling, Levinson, Reyment, Moore, Scott, Shaver, Sohn, Stover, Swain and Sylvester-Bradley1961 of the suborder Metacopina Sylvester-Bradley in Benson et al., Reference Benson, Berdan, Bold, Hanai, Hessland, Howe, Kesling, Levinson, Reyment, Moore, Scott, Shaver, Sohn, Stover, Swain and Sylvester-Bradley1961 (Martens et al., Reference Martens, Horne and Griffiths1998; Tibert et al., Reference Tibert, Rygel, Sanders, Elrick and Nelson2013). Cytherocopines were also present in these environments, especially during the Permian, represented by orders such as the Permianoidea Sharapova in Schneider, Reference Schneider1948 and the Cytheroidea Baird, Reference Baird1850 (Kashevarova, Reference Kashevarova1990).
Late Permian darwinulocopines were highly diverse in terms of morphology and taxa, and three superfamilies are present in the fossil record (Darwinuloidea, Suchonelloidea Mishina, Reference Mishina1972, and Darwinuloidoidea), each with its distinctive evolutionary history. They inhabited large, shallow lakes in which the variety of habitats was favorable to high diversity (Neustrueva, Reference Neustrueva1990). This apparently coincided with the presence of sexuality in at least some groups in this lineage, and Permian darwinulocopines commonly exhibited sexual dimorphism (Sohn, Reference Sohn1988; Molostovskaya, Reference Molostovskaya2000). On the other hand, all modern darwinulocopines, except possibly one species (Smith et al., Reference Smith, Kamiya and Horne2006), are exclusively parthenogenetic, and have apparently been so since late in the Mesozoic (Schön et al., Reference Schön, Rossetti and Martens2009).
The Permian-Triassic (P-Tr) extinction event greatly reduced darwinulocopine diversity in nonmarine environments in ways similar to those observed in marine ones (Jin et al., Reference Jin, Wang, Wang, Shang, Cao and Erwin2000; Liu et al., Reference Liu, Wang, Yuan, Yang, Song and Zhang2010; Forel, Reference Forel2013). It is still poorly known how these nonmarine faunas rebounded from such event during the Triassic, as they would soon be hit by another major extinction event, the ETE, which was probably caused by a disruption in the global carbon cycle due to emplacement of the Central Atlantic Magmatic Province (CAMP) (Beerling and Berner, Reference Beerling and Berner2002). The Upper Triassic also possibly witnessed the origin of the cytherocopine family Limnocytheridae Klie, Reference Klie1938, which would become widespread in the Upper Jurassic−lower Cretaceous (Zhong, Reference Zhong1964; Zheng, Reference Zheng1976; Fang and Xu, Reference Fang and Xu1978). The diversity of these faunas was maintained during the Lower Jurassic (Wakefield, Reference Wakefield1994; Ballent and Díaz, Reference Ballent and Díaz2012), only to be halved by the remainder of the period, before the explosive radiation of nonmarine cypridocopines, notably of the family Cyprideidae Bosquet, Reference Bosquet1852, and the limnocytherids in the Middle–Late Jurassic (Whatley, Reference Whatley1988; Sames and Horne, Reference Sames and Horne2012).
Proposed scenarios for the widespread dispersal of nonmarine darwinulocopines in the late Paleozoic range from transportation by early tetrapods, either in wet mud adhering to their feet or in the intestines of aquatic plant-feeding animals, to humid winds in warm, subtropical environments, considering that monsoons and hurricanes can carry wet particles for long distances (Lethiers and Damotte, Reference Lethiers and Damotte1993; Horne, Reference Horne2003). Evidence suggests that darwinulocopines were also tolerant of stressed environments such as shallow, warm, hypersaline lagoons and saline lakes (Gramann, Reference Gramann1971; McKenzie, Reference McKenzie1981; Molostovskaya, Reference Molostovskaya2000). Certain morphophysiological traits that enabled their rapid spread through these and other habitats were present in darwinulocopine nonmarine faunas since at least the Permian (retaining eggs and juveniles in brood pouches) (Molostovskaya, Reference Molostovskaya1990; Neustrueva, Reference Neustrueva1990) or the Early Jurassic (asexual reproduction by parthenogenesis). The record of parthenogenesis in darwinulocopines is a particularly remarkable one; despite being common in nonmarine ostracodes from several ages, it has not been the sole mechanism of reproduction in any other group for so long in the fossil record (Butlin et al., Reference Butlin, Schön and Martens1998; Martens et al., Reference Martens, Horne and Griffiths1998; Schön et al., Reference Schön, Rossetti and Martens2009). Exactly how long, however, is still a topic of discussion, although it is currently estimated to have persisted since 208 Ma ago (Martens et al., Reference Martens, Rossetti and Horne2003).
Lake Dixie as the ‘Last Hurrah of the Reigning Darwinulocopines’
The strata of the Whitmore Point Member were deposited in Lake Dixie, soon after the ETE and the establishment of CAMP. The ostracode fossil record of this unit is marked by several mass mortality events, as evidenced by strata in the Olsen Canyon containing numerous, piled-up ostracode carapaces and valves that were deposited during what might be an up-and-down trend in local carbon isotope records (Suarez et al., Reference Suarez, Knobbe, Crowley, Kirkland and Milner2017) (Fig. 6). These values are like those observed during the P-Tr extinction event, which was also associated with volcanic activity on a large igneous province in Siberia, Russia (Korte and Kozur, Reference Korte and Kozur2010).
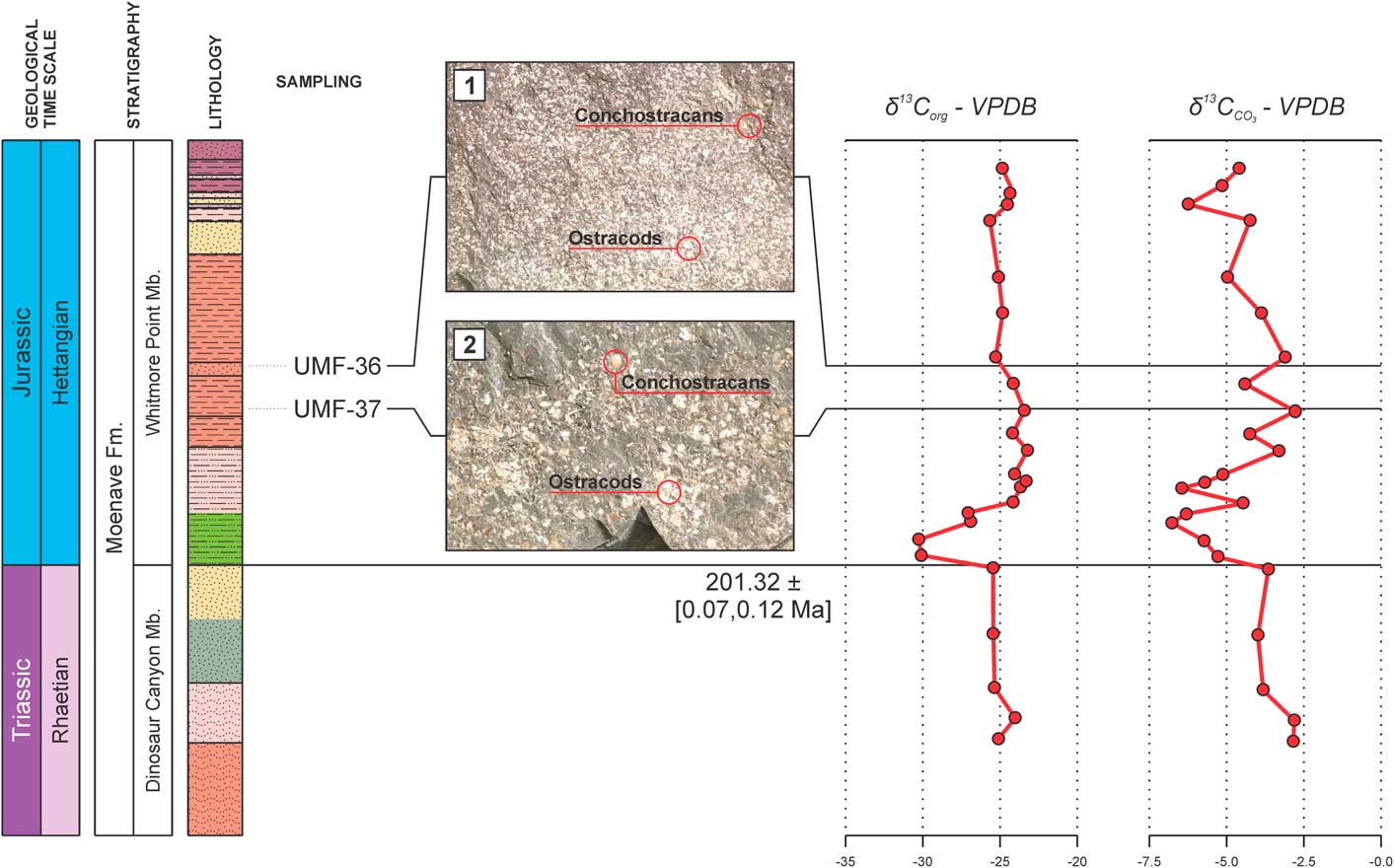
Figure 6 Ostracode extinction levels observed in the present work (1, 2) in comparison with the inorganic and organic carbon isotope curves (δ13C) of the Potter Canyon section (Suarez et al., Reference Suarez, Knobbe, Crowley, Kirkland and Milner2017), Mohave County, Arizona, USA. Note the up-and-down trend of the inorganic (CO3) curve (VPDB = Vienna Pee Dee Belemnite standard).
Several horizons preserving mud cracks, triclinic sulfate crystal casts and algal mats indicate that Lake Dixie, although large, was relatively shallow, with sporadically fluctuating base levels (Kirkland and Milner, Reference Kirkland and Milner2006; Kirkland et al., Reference Kirkland, Milner, Olsen and Hargrave2014). Sandstones with dinosaur tracks at the SGDS are interpreted as the shoreline area of the lake, and salt crystals were formed in the sediment of these shores during times of drought (Kirkland et al., Reference Kirkland, Lockley and Milner2002; Milner et al., Reference Milner, Lockley and Johnson2006). Lake Dixie was bounded southeastward by coastal and fluvial deposits of the upper Dinosaur Canyon Member, and further east by the eolian, sand-dune desert deposits known as the Wingate Sandstone (Blakey, Reference Blakey1994). Westward it was limited by the first stages of what would become the Nevadan orogeny by the end of the Jurassic (Renne and Turrin, Reference Renne and Turrin1987).
The diversity and composition of the Whitmore Point Member ostracode fauna agree with previous interpretations of the paleoenvironments of Lake Dixie. After the ETE, a very diversity-depleted fauna populated the member, comprising few species, one of which (Suchonellina globosa) was dominant in all of the currently analyzed samples containing sizable ostracode faunas. The majority of specimens recorded in the present work are females, and the very few that could be males are also interpretable as A-1 juveniles, according to the morphological parameters of Martens et al. (Reference Martens, Rossetti and Horne2003).
Similar faunas, both in terms of diversity and population composition, were also found in several formations that are coeval to the Moenave in Arizona, New Mexico, and Texas (Kietzke, Reference Kietzke1987, Reference Kietzke1989a, Reference Kietzkeb; Kietzke and Lucas, Reference Kietzke and Lucas1991, Reference Kietzke and Lucas1995; Lucas and Kietzke, Reference Lucas and Kietzke1993). Lake Dixie was a shallow, occasionally saline lake, located at the center of a network of similar paleoenvironments in the southwestern USA. Along these regions, depauperated darwinulocopine faunas mostly had to overcome the effects of CAMP by deploying metabolic, but more probably reproductive (parthenogenesis), strategies that allowed them to occupy previously stressed environments after their recovery.
Conclusions
Four ostracode species of darwinulocopines were recovered from sediments of the Hettangian, Lower Jurassic, Whitmore Point Member of the Moenave Formation. These sediments were deposited in and along the margins of Lake Dixie and associated/interbedded mudflat environments in the counties of Washington and Mohave, Utah and Arizona, USA. The diversity and composition of the Whitmore Point Member ostracode fauna agree with previous interpretations of Lake Dixie as a paleoenvironment where depauperated darwinulocopine faunas survived by deploying parthenogenesis as a strategy to recolonize stressed environments soon after their recovery from the establishment of CAMP and subsequent ETE.
The darwinulocopine ostracodes had their origin in the late Paleozoic, but were significantly decimated by the P-Tr extinction event and the ETE, never having re-established similar diversities since these extinctions. Lake Dixie has one of the earliest records so far known of an exclusively darwinulocopine fauna, and could represent the last episode of darwinulocopine dominance in nonmarine environments before the Late Jurassic−Early Cretaceous diversification of the modern cypridocopine/cytherocopine ostracodefaunas. It is possible that the geographic isolation of Lake Dixie, and perhaps other nearby lakes, behind the Wingate Erg desert and the early stages of the Nevadan orogeny played a major role in isolating these environments from the explosive radiation of the cypridocopines and cytherocopines that ended the ‘Last Hurrah of the Reigning Darwinulocopines.’
Acknowledgments
The authors wish to first acknowledge the late N.E. Tibert (1996–2015) for his efforts on the research of ostracode samples throughout the terrestrial Mesozoic sequences of Utah, which served as an important basis for the present work. We thank A. Smith (Department of Anthropology, UConn) for use of the SEM in taking the photographs for the present work. Also many thanks go to M. Suarez (Department of Geological Sciences, University of Texas at San Antonio) for support of field trips to Potter and Olsen canyons. LSA acknowledges J. Krishna for assistance with the cataloging of specimens deposited at NHMU. CAS wishes to thank G. McDonald and R. Hunt-Foster (Bureau of Land Management, Washington, D.C., USA) for support in obtaining field permit #UT16-017S, which allowed for collection of samples in the Olsen Canyon area.
Accessibility of supplemental data
Data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.sh45f








